Abstract
Background
Concise, accurate measures of maternal prenatal alcohol use are needed to better understand fetal alcohol spectrum disorders (FASD).
Methods
Measures of drinking by mothers of children with specific FASD diagnoses and mothers of randomly-selected controls are compared and also correlated with physical and cognitive/behavioral outcomes.
Results
Measures of maternal alcohol use can differentiate maternal drinking associated with FASD from that of controls and some from mothers of alcohol-exposed normals. Six variables that combine quantity and frequency concepts distinguish mothers of FASD children from normal controls. Alcohol use variables, when applied to each trimester and three months prior to pregnancy, provide insight on critical timing of exposure as well. Measures of drinking, especially bingeing, correlate significantly with increased child dysmorphology and negative cognitive/behavioral outcomes in children, especially low non-verbal IQ, poor attention, and behavioral problems. Logistic regression links (p<.001) first trimester drinking (vs. no drinking) with FASD, elevating FASD likelihood 12 times; first and second trimester drinking increases FASD outcomes 61 times; and drinking in all trimesters 65 times. Conversely, a similar regression (p=.008) indicates that drinking only in the first trimester makes the birth of a child with an FASD 5 times less likely than drinking in all trimesters.
Conclusions
There is significant variation in alcohol consumption both within and between diagnostic groupings of mothers bearing children diagnosed within the FASD continuum. Drinking measures are empirically identified and correlated with specific child outcomes. Alcohol use, especially heavy use, should be avoided throughout pregnancy.
Keywords: alcohol use and abuse, women, prenatal alcohol use, fetal alcohol spectrum disorders, South Africa, dysmorphology, cognition
1. INTRODUCTION
1.1 Background
Evidence linking multiple maternal traits to fetal alcohol spectrum disorders (FASD) is substantial (Abel and Hannigan, 1995; May and Gossage, 2011). However, much of the evidence links only summary measures of alcohol consumption (e.g., mothers drank/did not drink or binged occasionally) to outcomes often limited to particular behavioral traits such as IQ, attention, and memory (Bailey et al., 2005; Nulman et al., 2004; Sayal et al., 2009). Also, summary drinking measures are infrequently linked to specific diagnoses within the FASD continuum, only to “FASD.” Utilizing specific diagnoses combines physical dsymorphology and cognitive/behavioral traits for a more complete outcome indicator. Overall, more empirical evidence is needed to clarify and define the quantity, frequency, and timing (QFT) of alcohol usage that produce risk for a specific diagnosis within FASD.
1.2 Specific measures of alcohol consumption in pregnancy
The search for specific measures of prenatal alcohol use levels and patterns that produce fetal alcohol syndrome (FAS) and other diagnoses within the FASD continuum has been carried out in clinic settings (Alvik et al., 2006a, 2006b, 2006c; Day et al., 1999), drinking surveys (Floyd et al., 1999), epidemiologic studies of various methods (Kvigne et al., 2003; May et al., 2005, 2008a; Petković and Barišić, 2010), and drawn from data in existing populations studies. Few large case control samples exist with mothers who drank heavily during pregnancy giving birth to large numbers of children with an FASD. Such samples provide an opportunity to identify specific alcohol use measures and link them with specific diagnoses in the FASD continuum (Alvik et al., 2006c; Colvin et al., 2007; Kristjanson et al., 2007; May et al., 2009).
Prenatal drinking varies among and within populations of the world (Abel, 1998). In the United States, England, and Canada, 20 – 32% of pregnant women drink, and in some European countries the rate may exceed 50% (Alvik et al., 2006c; Bonati and Fellin, 1991; Centers for Disease Control and Prevention, 1997; Primatesta et al., 1993; Waterson and Murray-Lyon, 1989). In South Africa (ZA), 34 to 51% of women report prenatal drinking (Croxford and Viljoen, 1999; May et al., 2008a).
Some individual children escape diagnoses within the FASD continuum in spite of substantial prenatal alcohol exposure (Abel, 1998; May et al., 2013a; Skogerbø et al., 2012; Underbjerg et al., 2012). Maternal risk to the fetus involves the interaction of biological, familial, historical, social, and psychological influences (Gomberg, 1993), and the relative importance of these co-factors to FAS or other diagnoses within the FASD continuum has been demonstrated elsewhere (Abel and Hannigan, 1995; May et al., 2011, in press). This paper focuses on isolating particular measures of alcohol use, for alcohol use is by far the primary risk factor for the physical characteristics and behavioral deficits which define FASD (May et al., 2011, 2013b). In the study population, alcohol is virtually the sole drug used. Alcohol is the teratogen and other variables are predisposing conditions as we currently understand them (Abel and Hannigan, 1995; May and Gossage, 2011).
1.3 Prevalence of FASD and the study population
Recent estimates are that FAS affects 2 to 7 per 1,000 children in the Western world (May et al., 2006, 2009, 2011; Petković and Barišić, 2010). All levels of FASD may affect 2 to 5% (May et al., 2009). More prevalent in ZA (May et al., 2000, 2007; Urban et al., 2008; Viljoen et al., 2005), total FASD for this study community is 13.6 to 20.9%, the highest ever reported (May et al., 2013a).
This paper focuses on alcohol use data reported by mothers of children with a diagnosis within the FASD continuum. Understanding alcohol use by QFT provides insight into the etiology of FASD in humans, adds to diagnostic rigor, and facilitates intervention and prevention strategies by identifying dangerous: consumption patterns, levels of use, and times for fetal exposure (Kvigne et al., 2003; May, 1995).
2. METHODS
2.1 Sample
Alcohol use characteristics of mothers of children with one of three specific Institute of Medicine (IOM) FASD diagnoses are contrasted with mothers of randomly-selected, normal children (both alcohol-exposed and unexposed). These diagnoses are: FAS, partial fetal alcohol syndrome (PFAS), and alcohol-related neurodevelopmental disability (ARND; Stratton et al., 1996). All mothers and children are from the same community in ZA. A fourth study of FASD in this community, all actively consented, 1st grade school children were screened for height, weight, and head circumference. Children ≤ 25th centile on height and weight and/or head circumference and children randomly-selected as potential controls were advanced to a diagnostic physical exam by blinded dysmorphologists. Clinical characteristics and measurements for each child were recorded on a quantified dysmorphology checklist where a high score indicates more features of FASD (Hoyme et al., 2005). Children suspected of an FASD, and the randomly-selected children, were administered developmental tests by psychometricians, their teachers completed behavior checklists, and their mothers were interviewed about maternal risk factors. All final child diagnoses were made in case conferences using revised IOM criteria (Hoyme et al., 2005). Paired mother/child cases constitute the sample (n = 250): mothers of children with FAS (n=63), with PFAS (n=48), with ARND (n=32), and 107 randomly-selected controls, found to neither have an FASD nor suffer from another disability. Twenty-six controls were alcohol-exposed in utero, and 81 were not. Mirroring the population of this community, virtually all study participants were mixed-race (“Coloured”) individuals, and <10% were Blacks or Whites. The children with an FASD were on average 83.7 months (SD=8.9), 50.3% male, and a birth order of 2.4 (SD=1.2). Control children were 80.1 months old (SD=6.4), 56.1% male, with an average birth order of 2.0 (SD=1.0). Mothers of children with FASD averaged 33.5 years of age (SD=6.1) with gravidity of 3.6 (SD=1.5) and parity of 3.2 (SD=1.3). Mothers of normal controls were 34 years old (SD=6.6) with gravidity of 2.9 (SD=1.3) and parity of 2.7 (SD=0.9; see May et al., 2013a for more sample details).
2.2 Questionnaire and interview sequence
All mothers were administered identical questionnaires and received incentive grocery gift cards. The questionnaire was developed and refined to gather information in this population (May et al., 2005, 2008a; Viljoen et al., 2002) to quantify maternal risk factors ranging from general health and nutrition, child bearing, and socioeconomic status before, during, and after gestation of the index child. When interviewed, the maternal respondents were unaware if the index child had an FASD. Interviewers were aware in only some instances (due to logistical duties in the physical exam clinics) that any provisional diagnosis of possible FASD existed. Non-threatening questions on general health and childbearing were asked first, and then nutrition, for alcohol consumption responses are more accurate in this format (King, 1994). Then the QFT of the mother’s current drinking were introduced and explored via a 1-week, day-by-day log and other questions.
2.3 Measures
Drinks containing alcohol were measured in standard, American ethanol units equaling 0.5 ounces of absolute alcohol: 340 ml can/bottle of beer (5% ethanol), 120 ml of wine (11% ethanol), 95 ml of wine (13.5% ethanol), or 44 ml of distilled spirits (43% ethanol). Respondents viewed pictures of standard containers of local brands to calibrate exact amounts consumed (Kaskutas and Graves, 2000, 2001; Kaskutas and Kerr, 2008). Questions on current drinking establish the method and calibration of reporting, and refresh recall before the timeline follow-back questions (Sobell et al., 1988, 2001) about the index pregnancy. Retrospective, post-natal reports of gestational alcohol use have proven to be accurate (Alvik, 2006a; Czarnecki et al., 1990; Hannigan et al., 2010).
Blood alcohol concentrations (BAC) were estimated via the BACCuS for a single drinking episode based on the individual’s sex, weight, number of drinks consumed, and duration of consumption (Markham et al., 1993). Because of individual differences in alcohol metabolism, these calculations are estimations.
This population uses alcohol rather exclusively, and alcohol consumption is the sole focus of this paper. Use of other drugs (prescription or illicit) was reported by less than 1% in this study sample (May et al., 2013a), and has always been <2% in all studies in this community (May et al., 2005, 2008a; Viljoen et al., 2002). The major recreational drug used is daga (marijuana), although methamphetamine is emerging in urban areas. Smoking behavior as it impacts FASD has been described in detail elsewhere for this community (May et al., 2005, 2008a; Viljoen et al., 2002). Cigarette smoking is a high prevalence behavior (30 – 60% of mothers), but a low frequency behavior; smoking mothers reported using an average of 33 to 62 cigarettes a week, a low quantity by US standards (Centers for Disease Control and Prevention, 2005).
Development and behavior were assessed with: Tests of the Reception of Grammar (TROG), a measure of verbal IQ (Bishop, 1989); Colored Progressive Matrices (Raven, 1981) for non-verbal IQ; the WISC-IV Digit-Span Scaled Score (Wechsler, 2003) for executive functioning; and the Teacher Report Form for problem behaviors (Achenbach and Rescorla, 2001). Each test represents a domain of functioning that discriminates children with FASD from controls.
2.4 Data analysis
Data were entered with Epi Info software (Dean et al., 1994), and analyses performed with SPSS, version 19 (SPSS, 2010). For maternal drinking during pregnancy by diagnostic group, categorical variables were analyzed by chi square and continuous variables by one way analysis of variance, shown to be robust to differing frequency distributions (Tabachnick and Fidell, 2013). Alpha levels for statistical significance for these analyses are adjusted with Bonferroni corrections as noted in the tables. ANOVAs that were statistically significant by the Bonferroni criterion were followed by pairwise comparisons of groups using an additional Dunnett’s correction, which corrects for the post hoc multiple testing of all combinations of two groups. Bonferroni corrections were used for all bivariate and partial correlations. Binary logistic regression analyses were performed to specifically compare odds of having a child with FASD for mothers who drank during different trimesters.
3. RESULTS
3.1 Quantity and frequency of drinking before pregnancy
Maternal drinking results (Table 1) show many significant differences across groups. The number of standard drinks consumed three months before pregnancy is significantly different (F = 23.31, p <.001), with Dunnett’s C post hoc analyses indicating differences between the unexposed controls (x̄ = 0.3, SD = 1.3) and all three other FASD diagnostic groups, FAS (x̄ = 11.0), PFAS (x̄ = 14.0), ARND (x̄ = 16.9), and exposed controls (x̄ = 8.2). More mothers of children with ARND (67.9%) reported drinking more (than current levels at interview) prior to the index pregnancy than did mothers of children FAS (62.5%), PFAS (52.4%), and exposed controls (44.4%).
Table 1.
Maternal Drinking Behavior during Pregnancy by Diagnostic Group within FASD: Means and Standard Deviations
| Mothers of Children with FAS (n = 63) | Mothers of Children with PFAS (n = 48) | Mothers of Children with ARND (n = 32) | Mothers of Exposed Control Children (n = 26) | Mothers of Unexposed Control Children (n = 81) | Test Score | p | |
|---|---|---|---|---|---|---|---|
| Quantity/Frequency | |||||||
| Total # of standard drinks per week, 3 months before pregnancy, Mean (SD) | 11.0 (9.0) | 14.0 (14.7) | 16.9 (16.9) | 8.2 (5.4) | 0.3 (1.3) | F = 23.31 | <.001* |
| Percent drinking more (than currently at interview) in the months before becoming pregnant with index child (%) | 62.5 | 52.4 | 67.9 | 44.4 | 0.0 | χ2 = 132.15 | <.001* |
| Average # of standard drinks per week during pregnancy, Mean (SD) | 13.4 (14.0) | 13.1 (16.1) | 13.0 (15.0) | 5.6 (5.3) | 0.0 (0.0) | F = 16.43 | <.001* |
| Average # of standard drinks consumed Fri - Sun during pregnancy (all trimesters, drinkers only), Mean (SD) | 15.7 (14.1) | 10.9 (11.7) | 11.9 (13.6) | 5.4 (5.4) | -- | F = 2.84 | .041 |
| Average # of standard drinks consumed Monday – Thursday during pregnancy (all trimesters, drinkers only), Mean (SD) | 2.9 (2.1) | 5.5 (4.0) | 8.5 (7.0) | -- | -- | F = 1.97 | .185 |
| Drinks consumed per drinking day during pregnancy, Mean (SD) | 5.8 (6.6) | 5.6 (6.8) | 4.9 (4.9) | 2.6 (2.7) | 0.0 (0.0) | F = 16.14 | <.001* |
| Binge, 3 drinks per occasion during pregnancy (% Yes) | 78.8 | 74.4 | 80.8 | 70.6 | 0.0 | χ2 = 117.22 | <.001* |
| Binge, 5 drinks per occasion during pregnancy (% Yes) | 59.6 | 53.8 | 61.5 | 41.2 | 0.0 | χ2 = 69.92 | <.001* |
| Timing | |||||||
| 1st Trimester | |||||||
| Drinks consumed per drinking day, 1st trimester, Mean (SD) | 6.8 (6.4) | 6.0 (7.0) | 5.9 (4.5) | 3.8 (3.0) | 0.0 (0.0) | F = 21.71 | <.001* |
| Peak BAC (estimated)** | .175 (.11) | .132 (.09) | .170 (.11) | .110 (.09) | -- | F = 1.77 | .158 |
| 2nd Trimester | |||||||
| Drinks consumed per drinking day, 2nd trimester, Mean (SD) | 6.5 (6.9) | 5.1 (7.2) | 4.9 (5.1) | 2.1 (2.9) | 0.0 (0.0) | F = 15.91 | <.001* |
| Peak BAC (estimated)** | .161 (.10) | .119 (.08) | .167 (.12) | .140 (.09) | -- | F = 1.12 | .346 |
| 3rd Trimester | |||||||
| Drinks consumed per drinking day, 3rd trimester, Mean (SD) | 5.2 (6.9) | 4.3 (7.2) | 3.8 (5.6) | 2.0 (3.0) | 0.0 (0.0) | F = 10.13 | <.001* |
| Peak BAC (estimated)** | .140 (.08) | .108 (.06) | .195 (.12) | .160 (.07) | -- | F = 2.75 | .051 |
| Dunnett’s C Post Hoc Significant Comparisons at p = .05 level. | |
|---|---|
| Maternal Drinking Variables | Significant differences seen between the following groups |
| Total # of standard drinks per week 3 months before pregnancy |
|
| Average # of standard drinks per week during pregnancy |
|
| Drinks consumed per drinking day during pregnancy |
|
| Drinks consumed per drinking day, 1st trimester |
|
| Drinks consumed per drinking day, 2nd trimester |
|
| Drinks consumed per drinking day, 3rd trimester |
|
Significant at the Bonferroni-adjusted value of .006 for Quantity/Frequency, and .008 for Timing.
Blood alcohol concentration (BAC) was estimated via the BACCuS technique (Markham et al., 1993).
3.2 Drinking during pregnancy – quantity and frequency
The average number of standard drinks consumed per week during pregnancy also differed significantly between the FASD groups and controls (F = 16.43, p <.001). Post hoc tests demonstrated differences between the FAS group and both exposed and unexposed controls, as well as between the unexposed controls and PFAS, ARND, and exposed control groups. The average number of standard drinks consumed during pregnancy on weekends (Friday through Sunday) and during the week showed no statistically significant differences between groups. Adding the drinks per weekend to drinks per weekday produces a greater quantity than the simple question, “how many drinks did you have per week,” as the more focused the time period, the higher the reporting of drinking. In Table 1, drinks consumed per drinking day (DDD) during pregnancy is statistically significant overall (F = 16.14, p <.001). In post hoc analyses it also differentiates between each of the diagnostic groups and unexposed controls, FAS and exposed controls, and between exposed and unexposed controls. Finally, bingeing at both 3 drinks (χ2 = 117.22, p <.001) and 5 drinks (χ2 = 69.92, p <.001) per occasion during pregnancy were significantly different across groups. Therefore, most measures of quantity and frequency of alcohol use are useful in to discriminating between FASD diagnostic groups and controls. Drinks per week and DDD are robust measures of combined quantity/frequency.
3.3 Timing of drinking during pregnancy
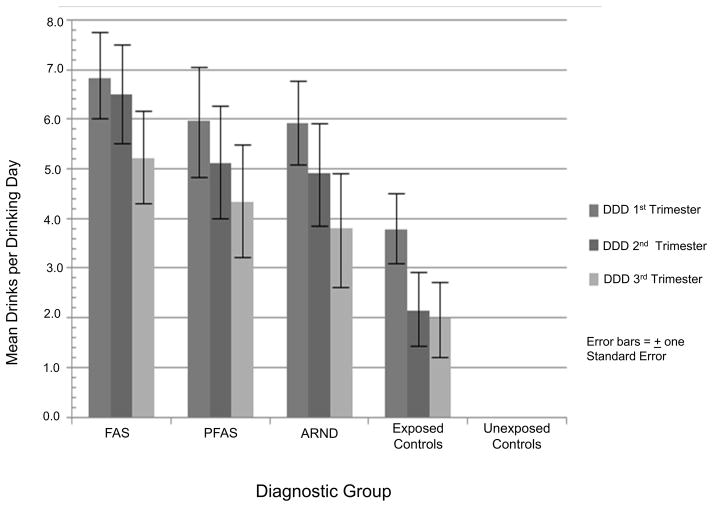
Timing of drinking by trimester (Table 1) demonstrates significant differences for DDD across maternal groups. First trimester means range from 6.8 for mothers of FAS to 3.8 for the mothers of exposed, normal children. On the other hand, estimated peak BAC’s for the drinking in each group do not differ significantly by trimester, indicating that peak BAC is not a good measure of risk for FASD in this population where binge drinking is the normative style and because it does not capture frequency of drinking. Figure 1 illustrates that DDD drop for each group with each trimester; but error bars (noting standard error) indicate much individual variation. Post hoc analyses illustrate significant differences of DDD at all three trimesters between the FAS, PFAS, and ARND groups compared with unexposed controls, and significant differences: for the first trimester between exposed and unexposed controls and for both the second and third trimesters between the FAS group and unexposed controls.
Figure 1.
Mean Drinks per Drinking Day (DDD) by Trimester and Diagnostic Group
In addition to data presented in Table 1, results of a two-way, between-within ANOVA using only the four groups with exposed mothers confirms significant reduction of drinking across trimesters for all drinking groups. The only statistically significant effect was the within-subjects main effect for trimester (F (2, 410) = 31.72, p <.001), partial η2 = .14 with 95% confidence limits between .08 and .19.
Table 2 presents data on a simple frequency variable: how many days per week did you drink? Overall, mothers of children with FAS and ARND in this ZA population reported more drinking days per week during pregnancy and in the first trimester than did the others. There was no significant difference in current frequency of drinking across groups at interview or in 2nd and 3rd trimester.
Table 2.
Frequency of Maternal Drinking Behavior During Pregnancy and by Trimester by Diagnostic Group within FASD: Drinking Days per Week (Drinkers Only)
| Maternal Drinking Variables | Mothers of Children with FAS (n = 63) | Mothers of Children with PFAS (n = 48) | Mothers of Children with ARND (n = 32) | Mothers of Exposed Control Children (n = 26) | Mothers of Unexposed Control Children (n = 81) | F | p |
|---|---|---|---|---|---|---|---|
| Quantity/Frequency | |||||||
| Number of drinking days per week, Current (at interview) Mean (SD) | 2.25 (1.1) | 1.80 (0.9) | 2.50 (1.1) | 2.20 (0.9) | 1.59 (0.9) | 2.45 | .052 |
| Number of drinking days per week, During Pregnancy Mean (SD) | 2.27 (1.2) | 1.75 (1.1) | 1.88 (1.1) | 1.02 (0.6) | -- | 5.29 | .002* |
| Number of drinking days per week, 1st Trimester Mean (SD) | 2.66 (1.1) | 2.22 (0.9) | 2.31 (0.9) | 1.67 (0.5) | -- | 4.26 | .007* |
| Number of drinking days per week, 2nd Trimester Mean (SD) | 2.66 (1.2) | 2.22 (0.9) | 2.36 (1.1) | 1.63 (0.5) | -- | 2.47 | .067 |
| Number of drinking days per week, 3rd Trimester Mean (SD) | 2.56 (1.1) | 2.24 (1.0) | 2.69 (0.9) | 1.57 (0.5) | -- | 2.33 | .082 |
Significant at Bonferroni-adjusted value of .01
3.4 Correlations with child physical traits and cognitive/behavioral outcomes
Table 3 presents bivariate correlations of maternal drinking measures with four child physical traits characteristic of children with an FASD and total dysmorphology score of anomalies common to FASD. Most quantity/frequency drinking measures (30 of 40) are significantly associated with child physical indicators of FASD at the alpha level of <.05. Similarly, 16 of 30 measures of timing are significantly associated at alpha <.05. With Bonferroni adjustment of alpha level, many correlations of particular drinking measures remain statistically significant, indicating a more robust association: total dysmorphology score, vermillion score, and palpebral fissure length are significant in 6 of 8 correlations; head circumference in 5 of 8 correlations; and philtrum with 2 of 8. Women who report more drinking during pregnancy than their current drinking, and those reporting bingeing correlate significantly with most FASD physical features. Drinks per week 3 months prior to pregnancy and mean drinks per week, DDD, and bingeing 5 or more drinks during pregnancy all correlate significantly with 4 of 5 physical measures. Head circumference, palpebral fissure length, vermillion score, and total dysmorphology score are all linked to DDD during pregnancy. Estimated peak BAC is again not statistically significant using the adjusted alpha level, probably because it is not a measure that combines quantity and frequency concepts.
Table 3.
Bivariate Correlations of Maternal Drinking Measures with Major Child Physical Variables and Characteristics of FASD
| Maternal Drinking Variables | Child Physical Trait Measures | ||||
|---|---|---|---|---|---|
| Head Circumference (n = 250) | Palpebral Fissure Length (PFL) (n =250) | Vermilion Score (1–5) (n =250) | Philtrum Score (1–5) (n=250) | Total Dysmorphology Score (n=250) | |
| Quantity/Frequency | |||||
| Total # of standard drinks per week 3 months before pregnancy | −.245* p<.001 |
−.287* p<.001 |
.310* p<.001 |
.085 p=.196 |
.360* p<.001 |
| Percent drinking more (than currently) in the months before becoming pregnant with index child? | −.388* p<.001 |
−.362* p<.001 |
.306* p<.001 |
.266* p<.001 |
.468* p<.001 |
| Average # of standard drinks per week during pregnancy | −.226* p=.001 |
−.284* p<.001 |
.298* p<.001 |
.113 p=.102 |
.389* p<.001 |
| Average # of standard drinks consumed Fri - Sun during pregnancy (all trimesters, drinkers only) | −.180 p=.049 |
−.125 p=.174 |
.202 p=.027 |
−.097 p=.289 |
.221 p=.015 |
| Average # of standard drinks consumed Monday – Thursday during pregnancy (all trimesters, drinkers only) | .066 p=.823 |
.462 p=.097 |
−.067 p=.821 |
−.119 p=.687 |
−.547 p=.043 |
| Drinks consumed per drinking day (DDD) during pregnancy | −.262* p<.001 |
−.290* p<.001 |
.307* p<.001 |
.132 p=.055 |
.401* p<.001 |
| Binge, 3 drinks per occasion during pregnancy (% Yes) | −.358* p<.001 |
−.338* p<.001 |
.352* p<.001 |
.285* p<.001 |
.527* p<.001 |
| Binge, 5 drinks per occasion during pregnancy (% Yes) | −.293* p<.001 |
−.287* p<.001 |
.324* p<.001 |
.150 p=.029 |
.422* p<.001 |
| Timing | |||||
| 1st Trimester | |||||
| Drinks consumed per drinking day (DDD), 1st trimester | −.253* p<.001 |
−.300* p<.001 |
.308* p<.001 |
.166 p=.014 |
.435* p<.001 |
| Peak BAC (estimated)** | −.129 p=.200 |
−.055 p=.585 |
.112 p=.268 |
.041 p=.684 |
.243 p=.015 |
| 2nd Trimester | |||||
| Drinks consumed per drinking day (DDD), 2nd trimester | −.226* p=.001 |
−.299* p<.001 |
.271* p<.001 |
.144 p=.037 |
.413* p<.001 |
| Peak BAC (estimated)** | −.097 p=.325 |
−.092 p=.353 |
−.023 p=.813 |
.044 p=.656 |
.233* p=.017 |
| 3rd Trimester | |||||
| Drinks consumed per drinking day (DDD), 3rd trimester | −.183 p=.008 |
−.228* p=.001 |
.259* p=.000 |
.081 p=.244 |
.346* p=.000 |
| Peak BAC (estimated)** | −.069 p=.483 |
.014 p=.883 |
.016 p=.874 |
.005 p=.960 |
.176 p=.070 |
| Addendum. Bivariate Correlations of Maternal Drinking (DDD) Timing Measures by Trimester with Child Physical Variables, Controlling for First Trimester Drinking | |||||
|---|---|---|---|---|---|
| Maternal Drinking Variables*** | Child Physical Trait Measures | ||||
| Head Circumference (n = 250) | Palpebral Fissure Length (PFL) (n =250) | Vermilion Score (1–5) (n =250) | Philtrum Score (1–5) (n=250) | Total Dysmorphology Score (n=250) | |
| Timing | |||||
| 2nd Trimester | |||||
| Drinks consumed per drinking day, 2nd trimester | −.028 p=.787 |
−.126 p=.216 |
−.018 p=.860 |
.006 p=.956 |
.028 p=.783 |
| 3rd Trimester | |||||
| Drinks consumed per drinking day, 3rd trimester | .069 p=.500 |
−.003 p=.978 |
.040 p=.698 |
−.078 p=.445 |
−.078 p=.443 |
Bonferroni adjusted significance level for multiple comparisons is p = .0013 for Quantity/Frequency variables and .0017 for Timing variables.
Blood alcohol concentration (BAC) was estimated via the BACCuS technique (Markham et al., 1993).
Bonferroni adjusted significance level for multiple comparisons in the addendum is p = .005. No variables produced significant correlations for the 2nd and 3rd trimesters after 1st trimester drinking was controlled.
The addendum at the bottom of Table 3, repeats the correlations of physical features and DDD for 2nd and 3rd trimesters, while controlling for 1st trimester drinking. With this control, statistical significance is not attained for any of these physical features. Most facial dysmorphia and many of the minor anomalies of FASD are developed primarily in the 1st trimester, which may account for the lack of significance in the addendum comparison.
For behavioral outcomes, fewer measures were highly correlated with child cognitive and behavioral performance (see Table 4). Generally, the greater the maternal drinking, the greater the child’s problems with non-verbal IQ, problem behavior, and attention. The most discriminating drinking variables were DDD and bingeing of 3 and 5 drinks per occasion, which at the adjusted alpha level of p =.0014, are significantly associated with 3 or more of the 5 tests (non-verbal IQ, problem behavior, attention problems, and working memory). Similarly, drinks per week consumed 3 months prior to pregnancy and mean drinks per week during pregnancy are each significantly correlated with 2 of the 5 tests (problem behavior and attention problems, and attention problems and verbal IQ; respectively).
Table 4.
Bivariate Correlations of Maternal Drinking Measures with Child Cognition and Behavioral Tests
| Maternal Variable | Raven Percentile (Non-Verbal Intelligence) (n = 250) | TROG Percentile (Verbal Intelligence) (n =250) | Digit Span Scaled Score (Working Memory) (n =250) | Achenbach Teacher Report Form for Problem Behavior (n=250) | Attention Problems Score (n=250) |
|---|---|---|---|---|---|
| Quantity/Frequency | |||||
| Total # of standard drinks per week 3 months before pregnancy | −.195 p= .003 |
−.174 p=.008 |
−.147 p=.027 |
.211* p=.001 |
.234* p<.001 |
| Percent drinking more (than currently) in the months before becoming pregnant with index child? | −.184 p=.008 |
−.042 p=.542 |
−.122 p=.080 |
.209 p=.002 |
.207 p=.003 |
| Average # of standard drinks per week during pregnancy | −.231* p=.001 |
−.151 p=.029 |
−.174 p=.012 |
.203 p=.003 |
.247* p<.001 |
| Average # of standard drinks consumed Fri - Sun during pregnancy (all trimesters, drinkers only) | −.122 p=.186 |
−.091 p=.321 |
−.209 p=.022 |
.153 p=.094 |
.196 p=.032 |
| Drinks consumed per drinking day (DDD) during pregnancy | −.247* p<.001 |
−.181 p=.009 |
−.216 p=.002 |
.236* p=.001 |
.252* p<.001 |
| Binge, 3 drinks per occasion during pregnancy (% Yes) | −.304* p<.001 |
−.203 p=.003 |
−.197 p=.004 |
.257* p<.001 |
.270* p<.001 |
| Binge, 5 drinks per occasion during pregnancy (% Yes) | −.279* p<.001 |
−.166 p=.017 |
−.232* p=.001 |
.255* p<.001 |
.272* p<.001 |
| Timing | |||||
| 1st Trimester | |||||
| Drinks consumed per drinking day, 1st trimester | −.241* p<.001 |
−.160 p=.019 |
−.192 p=.005 |
.214 p=.002 |
.250* p<.001 |
| Peak BAC (estimated)** | −.196 p=.053 |
−.132 p=.195 |
−.286 p=.004 |
.170 p=.094 |
.134 p=.188 |
| 2nd Trimester | |||||
| Drinks consumed per drinking day, 2nd trimester | −.235* p=.001 |
−.130 p=.061 |
−.182 p=.008 |
.200 p=.004 |
.239 p=.001 |
| Peak BAC (estimated)** | −.105 p=.292 |
−.108 p=.278 |
−.226 p=.023 |
.253* p=.010 |
.215 p=.030 |
| 3rd Trimester | |||||
| Drinks consumed per drinking day, 3rd trimester | −.187 p=.007 |
−.101 p=.148 |
−.138 p=.047 |
.177 p=.010 |
.221* p=.001 |
| Peak BAC (estimated)** | −.069 p=.482 |
−.079 p=.423 |
−.163 p=.096 |
.287 p=.003 |
.257 p=.008 |
Bonferroni adjusted significance level for multiple comparisons is p = .0014 for Quantity/Frequency variables and .0017 for Timing variables.
Blood alcohol concentration (BAC) was estimated via the BACCuS technique (Markham et al., 1993).
For timing, DDD during the pregnancy is a better measure (as indicated by stringent alpha levels) than are estimated peak BAC measures when predicting child performance on non-verbal intelligence in the first two trimesters and attention in the first and third trimesters.
3.5 Predicting FASD from drinking behavior over pregnancy trimesters
Based on these results, binary logistic regression analyses were utilized to predict FASD from maternal drinking (DDD) by trimester (Table 5). Seven analyses were conducted, and three were statistically significant at the Bonferroni-adjusted level (α= .007). The odds of giving birth to an FASD child are 12 times greater for mothers who reported drinking only during the first trimester compared to those who reported no drinking, although the 95% confidence interval is quite large (4.13 to 35.8). Nagelkerke R2 = .27 suggests that a little over a quarter of the variability in FASD is predictable from first trimester drinking. The odds of giving birth to an FASD child increase to about 61 times greater than for non-drinking mothers (95% CI, 13 to 291) for mothers who drink during the first and second trimesters only. The odds of giving birth to an FASD child further increase to about 65 times greater (95% CI, 23 to 180, Nagelkerke R2 = .65) for mothers who reported drinking during all trimesters.
Table 5.
Comparisons of Binge Logistic Regression-Generated Odds of FASD Births for Mothers’ Drinking Behavior over Pregnancy Trimesters
| Drinking Behavior | n1/n2 | Wald (1 df) | p | Odds Ratio | 95% CI for Odds Ratio | Nagelkerke R2 | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| First trimester only vs. no drinking | 21/92 | 20.53 | <.001* | 12.15 | 4.13 | 35.8 | .27 |
| First and second trimesters only vs. no drinking | 22/92 | 26.37 | <.001* | 60.76 | 12.68 | 291.36 | .50 |
| All trimesters vs. no drinking | 70/92 | 64.01 | <.001* | 64.81 | 23.33 | 180.11 | .65 |
| Third trimester only vs. no drinking | 2/92 | 1.56 | .212 | 6.08 | 0.36 | 103.31 | .28 |
| First and second trimesters vs. first trimester only | 22/21 | 3.39 | .066 | 5.00 | 0.91 | 27.74 | .14 |
| All trimesters vs. first trimester only | 70/21 | 7.07 | .008 | 5.33 | 1.55 | 18.33 | .13 |
| All trimesters vs. first and second trimesters only | 70/22 | 0.01 | .940 | 1.07 | 0.20 | 5.71 | .00 |
p < .007
Note: There were no Drinking Behavior cases for which there were both second and third trimester data, and no corresponding first trimester data; therefore, this particular combination of drinking behavior is not represented on the table above.
Although not quite statistically significant by the Bonferroni criterion, continuing to drink during all trimesters increases the odds of a child with FASD by about 5 times compared with drinking only during the first trimester, (95% CI, 2 to 18, Nagelkerke R2 = .65).
4. DISCUSSION
4.1 QFT measures of prenatal alcohol consumption characterize differential drinking leading to the FASD continuum
From this sample and the variables examined, a number of conclusions follow. First, several measures of QFT distinguish between drinking patterns of mothers of children with diagnoses within FASD and unexposed controls; and a few even distinguish between FAS and exposed controls and between exposed and unexposed controls. Few summary alcohol use measures have been identified before that have made these distinctions between drinking patterns that lead to the birth of children with and without an FASD. Second, even though they do not drink the most alcohol 3 months prior to pregnancy, the mothers of FAS and PFAS children in this population drink consistently in a binge fashion (primarily on weekends) throughout the entire pregnancy and taper off the least in later pregnancy. Third, on average, mothers of children with ARND drink the most per week 3 months prior to pregnancy, and drink almost as much as the mothers of children with PFAS in the first trimester. But overall, mothers of children with ARND cut down more in the middle and later stages of pregnancy, yet when they do drink, they binge heavily and report spacing out consumption throughout various days of the week. Fourth, mothers of the normal/control children who drank during pregnancy drink the least of any of the drinking mothers (about 50% less than the mothers of FASD children) and did not drink at all during the weekdays. Mothers of normal controls report cutting down on the drinking greatly in the second and third trimesters, even though when they drink they binge drink. Fifth, with only a few exceptions, average drinking during pregnancy for the mothers of FASD children form a spectrum from the heaviest sustained bingeing practiced by the mothers of the FAS children to the least drinking and fewest binges among mothers of the children without FASD.
4.2 Variation in maternal drinking levels and patterns found both between and within the diagnostic groups
Therefore, individual variation in quantity, frequency, and timing of drinking exists between the groups of mothers in ways that one would generally expect given the severity of FASD characteristics in the sample. Higher average quantities consumed more frequently throughout pregnancy produce the predicted variation in diagnoses across the continuum. But a sixth and final point can be made from these data. Variation in drinking QFT is also striking within each group as evidenced by the large standard deviations for most every variable (e.g. DDD) and is illustrated by the error bars in Figure 1. Drinking quantities of individual woman are frequently similar across FASD diagnostic groups, indicating that there are many mothers of children with ARND who drank as much or more when they drank, especially in the 1st trimester, as did some mothers of children with FAS and PFAS. And there are mothers of children with FAS or PFAS who drank less, especially in the first trimester, than some of the mothers who have children with ARND or are children who are physically and behaviorally normal. Therefore, there is individual variation among mothers in quantity, but especially in frequency and timing.
While much of the variation in child outcomes is due to major drinking variation by QFT, other maternal risk co-factors such as maternal age, gravidity, maternal body mass index, nutrition, and socioeconomic status also play a significant and meaningful role. But including these other maternal risk factors in these analyses is beyond the scope of this paper and have been analyzed elsewhere (May and Gossage, 2011; May et al., 2007, 2008a, 2013b). It is interesting, however, that the drinking data used in the regression model explain 60 to 65% of the variance in diagnosis, which is similar to the variance in child outcomes explained in complex structural equation models of child dysmorphology and neurobehavior (62 to 55%) that incorporate multiple measures of risk: socioeconomic status, childbearing history, and maternal physical variables (May et al., 2011, 2013b). Furthermore, genetic (Khaole et al., 2004) and epigenetic variables also influence outcome. They are also beyond the scope of these epidemiologic sample data and of this paper. In light of these findings, it seems impossible to easily determine that there is a specific threshold level of drinking that might be safe for any individual mother. Even by drinking exclusively in the first trimester a woman in this sample increases the likelihood of a child with an FASD twelve fold. Therefore, these data clearly reinforce that there is no safe level of alcohol consumption that can be recommended for any woman at any time of pregnancy, especially in the first trimester when many do not yet know that they are pregnant.
4.3 The effect of prenatal drinking on child physical features, cognitive skills, and behavior
Maternal drinking variables in this study, especially the binge measures, correlate significantly with increased dysmorphology, decreased head circumference, short palpebral fissures, and indistinct vermillion boarder. These simple physical measures/traits can provide a clinician with clues of prenatal alcohol exposure, at least in the first trimester (Hoyme et al., 2005; May et al., 2011). Many of the alcohol consumption measures that combine quantity and frequency (Q/F) concepts, and therefore characterize binges, are also substantially associated with many measures of cognitive/behavioral performance. But only non-verbal intelligence correlates significantly at the most strict significance levels. Furthermore, when adding timing of drinking during the pregnancy, the drinks per drinking day (DDD) measure is associated with poor performance on cognitive and behavioral scales in all virtually trimesters. Non-verbal IQ and attention are significantly correlated in 2 of the 3 trimesters. As has been demonstrated with other analyses and samples, prenatal alcohol use is more directly correlated with physical effects on children (May et al., 2011) than its effect on neurobehavior. The effect of prenatal drinking on neurobehavior is filtered through multiple environmental conditions in the child’s formative years such as: maternal education, maternal health, and cognitive stimulation which are often mediated by socioeconomic conditions (May et al., 2013b). With good postnatal conditions the brain development of a child with FASD can improve in areas such as verbal acuity and ability to focus attention as is demonstrated in this paper.
4.4 Assessing global risk by trimester – regression analysis findings
In this sample, drinking throughout all trimesters increases the odds of having a child with an FASD diagnosis by 65 times over that of non-drinking mothers, while first trimester drinking alone increases the odds 12 times. But intervention after 1st trimester drinking is promising. When a mother ceases drinking after the first trimester it appears to decrease the odds of an FASD child by 5 times. This finding reinforces studies of intervention where case management and other techniques have been proven effective in preventing FASD when introduced early or at least in the second trimester (Grant et al., 2013; May et al., 2008b, in press).
4.5 Limitations of this study
There are limitations to this study. First, the data utilized are from one unique, population where rates of FASD are the highest in the world, and alcohol is the exclusive drug of choice. Social, economic and cultural conditions associated with FASD in this population are severe and prominent. Binge drinking on weekends is normative for up to 40% of the women in this population, while drinking moderately and during the weekday is rare. This means that patterns of association, and possibly etiology, are robust, lend themselves well to discovery, and are more easily determined than in other populations where maternal drinking exists with lower prevalence, is obscured or hidden, and is likely cofounded by other drugs of abuse. While these conditions allow us to isolate the study variable, alcohol, its applicability to other populations may be questioned. Second, ZA mothers of this mixed race population are rather unique regarding their physical, nutritional, socioeconomic status, and historically unique relationship to alcohol. But, multiple studies of FASD in ZA have demonstrated that the sample mothers are extraordinarily forthcoming and reliable in reporting alcohol use. We think that these conditions make the link between detailed alcohol use information and child diagnosis more accurate and valid, but the study can be criticized for relying on self-reported information. Third, the outcome variables utilized in this study are diagnostic variables of both child physical traits and cognitive/behavioral performance drawn from clinical exams. While these are measured by highly experienced professionals from multiple disciplines, and few human studies of FASD have the benefit of multiple measures of alcohol use to pair with these specific outcome measures, some have questioned the accuracy of specific diagnostic categories within the FASD continuum. Forth, while it would be desirable to collect biological samples for analyzing biomarkers of alcohol and drug use, genetic risk factors, and epigenetic influences, we did not. Therefore, more detailed studies of the molecular influence of prenatal alcohol exposure in humans await further study.
4.6 Conclusions
The specific quantity, frequency, and timing of prenatal exposure to alcohol that will produce a child with a specific diagnosis within the FASD continuum varies greatly by individual mothers, and furthermore the maternal drinking variance is demonstrated in this study to exist both between and within FASD diagnostic categories. Binge drinking of at least two days a week during all trimesters in this population may produce FAS or PFAS, while mothers of children with ARND and exposed children without an FASD are most likely to reduce their average and peak alcohol consumption in the later trimesters. Overall, however, regular drinking in a binge fashion is the common pattern for all mothers of children with any of the FASD diagnoses.
Acknowledgments
Role of the funding source
This project was funded by the National Institute on Alcohol Abuse, and Alcoholism (NIAAA) Grants RO1 AA09440, R01 AA11685, and RO1/UO1 AA01115134.
We thank Faye Calhoun, D.P.A., Kenneth Warren, Ph.D., T-K Li, M.D., and Marcia Scott, Ph.D. of NIAAA who have provided intellectual guidance and support in the South African studies of FASD since 1996. Teresa Alexander and Simone Europa played a vital role in the data collection process. Our deepest thanks are extended to Mayor Herman Bailey, education officials and teachers, and many others in the study community who have hosted and assisted in the research process over the years. Protocols and consent forms were approved by: The University of New Mexico (UNM) 09-97-90-9805, 01-93-86-9808R; UNM School of Medicine, HRRC # 96-209, and 06-199;The University of Cape Town, #101/2004U and Stellenbosch University, Faculty of Health Sciences, # N06/07/129. Active consent for children to participate in all phases of this study was obtained from parents or other legal guardians. Mothers interviewed for maternal risk factors also provided a separate consent for the interview process.
Footnotes
Conflict of interests
None of the authors have any conflicts of interest to declare.
Contributors
Philip May was the principle investigator of the NIH grant that funded this research and he, along with assistance from Julie Hasken, was the major writer and final editor of all drafts. Jason Blankenship, Barbara Tabachnick, Wendy Kalberg, and David Buckley all played major roles in data analysis and preparation of text for the methods and results sections. Anna-Susan Marais, Jan Gossage, Belinda Joubert, Marise Cloete, Ronel Barnard, Marlene De Vries, Charles Parry, and Soraya Seedat all collected and managed all data in the field and in program offices in both South Africa and the USA. Luther Robinson, Melanie Manning, and Gene Hoyme generated all dysmorphology data and made final diagnoses of the children in the field. Colleen Adnams directed and supervised all of the cognitive and behavioral testing and initial analyses used in the diagnostic process. Soraya Seedat and Charles Parry are the South African co-investigators who oversaw all study activities in South Africa both in the field and Stellenbosch University. Each author read, edited, contributed to, and approved various drafts of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Fetal Alcohol Abuse Syndrome. Plenum Press; New York: 1998. [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:445–465. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- Alvik A, Haldorsen T, Groholt B, Lindeman R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006a;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Alvik A, Haldorsen T, Lindeman R. Alcohol consumption, smoking and breastfeeding in the first six months after delivery. Acta Pediatr. 2006b;95:686–693. doi: 10.1080/08035250600649266. [DOI] [PubMed] [Google Scholar]
- Alvik A, Heyerdahl S, Haldorsen T, Lindeman R. Alcohol use before and during pregnancy: a population-based study. Acta Obstet Gynecol Scand. 2006c;85:1292–1298. doi: 10.1080/00016340600589958. [DOI] [PubMed] [Google Scholar]
- Bailey BN, Sood BG, Sokol RJ, Ager J, Hanise J, Hannigan JH, Covington C, Delaney-Black V. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005;27:181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Test of the Reception of Grammar (TROG) 2. University of Manchester; Manchester: 1989. [Google Scholar]
- Bonati M, Fellin G. Changes in smoking and drinking behavior before and during pregnancy in Italian mothers: implications for public health intervention. Int J Epidemiol. 1991;20:927–932. doi: 10.1093/ije/20.4.927. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. Alcohol consumption among pregnant and child-bearing aged women: United States, 1991 and 1995. MMWR. 1997;46:346–350. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults-United States, 2004. MMWR. 2005;54:1121–1124. [PubMed] [Google Scholar]
- Colvin L, Payne J, Parsons D, Kurinczuk JJ, Bower C. Alcohol consumption during pregnancy in nonindigenous West Australian women. Alcohol Clin Exp Res. 2007;31:276–284. doi: 10.1111/j.1530-0277.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- Croxford J, Viljoen D. Alcohol consumption by pregnant women in the Western Cape. S Afr Med J. 1999;89:962–965. [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D. Five-year reliability of self-reported alcohol consumption. J Stud Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- Day NL, Zuo Y, Richardson GA, Goldschmidt L, Larkby CA, Cornelius MD. Prenatal alcohol use and offspring size at 10 years of age. Alcohol Clin Exp Res. 1999;23:863–869. [PubMed] [Google Scholar]
- Dean AG, Dean JA, Coulambier D, Brendel KA, Smith DC, Burton AH, Dickers RC, Sullivan K, Faglen RF, Arnir RG. Epi Info, Version 6: A Word Processing Data Base, and Statistical Program for Epidemiology in Microcomputers. Centers for Disease Control and Prevention; Atlanta, Georgia: 1994. [Google Scholar]
- Floyd RL, Decouflé P, Hungerford DW. Alcohol use prior to pregnancy recognition. Am J Prev Med. 1999;17:101–107. doi: 10.1016/s0749-3797(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Gomberg ESL. Women and alcohol: use and abuse. Nerv Ment Disease. 1993;181:211–219. doi: 10.1097/00005053-199304000-00001. [DOI] [PubMed] [Google Scholar]
- Grant TM, Brown NN, Dubovsky D, Sparrow J, Ries R. The impact of prenatal alcohol exposure on addiction treatment. J Addict Med. 2013;7:87–95. doi: 10.1097/ADM.0b013e31828b47a8. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwalk MK, Delaney Black V. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44:583–594. doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. An alternative to standard drinks as a measure of alcohol consumption. J Subst Abuse. 2000;12:67–78. doi: 10.1016/s0899-3289(00)00042-0. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. Pre-pregnancy drinking: how drink size affects risk assessment. Addiction. 2001;96:1199–1209. doi: 10.1046/j.1360-0443.2001.968119912.x. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Kerr WC. Accuracy of photographs to capture respondent-defined drink size. J Stud Alcohol Drugs. 2008;69:605–610. doi: 10.15288/jsad.2008.69.605. [DOI] [PubMed] [Google Scholar]
- Khaole NCO, Ramchandani VA, Viljoen DL, Li TK. A pilot study of alcohol exposure and pharmacokinetics in women with or without children with fetal alcohol syndrome. Alcohol Alcohol. 2004;39:503–508. doi: 10.1093/alcalc/agh089. [DOI] [PubMed] [Google Scholar]
- King AC. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am J Public Health. 1994;84:294–296. doi: 10.2105/ajph.84.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjanson AF, Wilsnack SC, Zvartau E, Tsoy M, Nivikov B. Alcohol use in pregnant and non-pregnant Russian women. Alcohol Clin Exp Res. 2007;31:299–307. doi: 10.1111/j.1530-0277.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- Kvigne VL, Leonardson GR, Borzelleca J, Brock E, Neff-Smith M, Welty TK. Characteristics of mothers who have children with fetal alcohol syndrome or some characteristics of fetal alcohol syndrome. J Am Bd Fam Pract. 2003;16:296–303. doi: 10.3122/jabfm.16.4.296. [DOI] [PubMed] [Google Scholar]
- Markham MR, Miller WR, Arciniega L. BACCuS 2.01: computer software for quantifying alcohol consumption (a blood alcohol concentration calculating system) Behav Res Method Instr Comp. 1993;25:420–421. [Google Scholar]
- May PA. A multiple-level, comprehensive approach to the prevention of fetal alcohol syndrome (FAS) and other alcohol-related birth defects (ARBD) Int J Addict. 1995;30:1549–1602. doi: 10.3109/10826089509104417. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais AS, Hendricks LS, Croxford JA, Viljoen DL. Maternal risk factors for fetal alcohol syndrome in the Western Cape province of South Africa: a population-based study. Am J Public Health. 2005;95:1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Gossage JP, Kalberg WO, Hoyme HE, Robinson LK, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M. Epidemiology of FASD in a province in Italy: prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res. 2006;30:1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellevato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Hendricks L, Snell C, Tabachnick BG, Stellavate C, Buckley DG, Brooke L, Viljoen DL. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. 2008a;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- May PA, Miller JH, Goodhart KA, Maestas OR, Buckley D, Trujillo PM, Gossage JP. Enhanced case management to prevent fetal alcohol spectrum disorders in Northern Plains communities. Matern Child Health J. 2008b;12:747–59. doi: 10.1007/s10995-007-0304-2. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley DG, Manning M, Hoyme HE. The prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on in-school studies. Devel Dis Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders not as simple as it might seem. Alcohol Res Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais AS, Robinson LK, Manning M, Buckley D, Hoyme HE. Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend. 2011;119:18–27. doi: 10.1016/j.drugalcdep.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013a;37:818–30. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick B, Gossage JP, Kalberg WO, Marais AS, Robinson LK, Manning M, Blankenship J, Buckley D, Hoyme HE, Adnams C. Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrums disorders. J Dev Behav Pediatr. 2013b;34:314–325. doi: 10.1097/DBP.0b013e3182905587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Marais AS, Gossage JP, Barnard R, Joubert B, Cloete M, Hendricks N, Roux S, Blom A, Steenekamp J, Alexander T, Andreas R, Human S, Snell C, Seedat S, Parry CD, Kalberg WO, Buckley D, Blankenship J. Case management reduces drinking during pregnancy among high-risk women. Int J Alcohol Drugs Res. doi: 10.7895/ijadr.v2i3.79. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Kennedy D, Wasson C, Gladstone J, Fried S, Koren G. Binge alcohol consumption by non-alcohol – dependent women during pregnancy affects child behavior, but not general intellectual function; a prospective controlled study. Arch Womens Ment Health. 2004;7:173–181. doi: 10.1007/s00737-004-0055-7. [DOI] [PubMed] [Google Scholar]
- Petković G, Barišić I. FAS prevalence in a sample of urban schoolchildren in Croatia. Reprod Toxicol. 2010;29:237–272. doi: 10.1016/j.reprotox.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Primatesta P, DelCorno G, Bonazzi MC, Waters WE. Alcohol and pregnancy: an international comparison. J Pub Health Med. 1993;15:69–76. doi: 10.1093/oxfordjournals.pubmed.a042822. [DOI] [PubMed] [Google Scholar]
- Raven J. Research supplement No. 1: 1979 British Standardisation of the standard progressive matrices and mill hill vocabulary scales, together with comparative data from earlier studies in the UK, US, Canada, Germany and Ireland. Harcourt Assessment; San Antonio, TX: 1981. Manual for Raven’s Progressive and Vocabulary Scales. [Google Scholar]
- Sayal K, Heron J, Golding J, Alati R, Smith GD, Gray R, Emond A. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009:e289–e296. doi: 10.1542/peds.2008-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerbø Å, Kesmodel U, Wimberley T, Støvring H, Bertrand J, Landrø N, Mortensen E. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG. 2012;119:1201–1210. doi: 10.1111/j.1471-0528.2012.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrwal S, Annis H, Ayala-Velasquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M. Cross-cultural evaluation of two drinking assessment instruments: Alcohol timeline follow back and inventory of drinking situations. Subst Use Misuse. 2001;36:313–331. doi: 10.1081/ja-100102628. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC, editors. Fetal Alcohol Syndrome Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press; Washington, DC: 1996. [Google Scholar]
- SPSS, Inc. SPSS for Windows [Computer Software] SPSS Inc; Chicago: 2010. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6. Pearson; Boston: 2013. [Google Scholar]
- Underbjerg M, Kesmodel U, Landrø N, Bakketeig L, Grove J, Wimberley T, Kilburn T, Sværke C, Thorsen P, Mortensen E. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on selective and sustained attention in 5-year-old children. BJOG. 2012;119:1211–1221. doi: 10.1111/j.1471-0528.2012.03396.x. [DOI] [PubMed] [Google Scholar]
- Urban M, Chersich MF, Fourie LA, Chetty C, Olivier L, Viljoen D. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. S Afr Med J. 2008;98:877–882. [PubMed] [Google Scholar]
- Viljoen D, Croxford J, Gossage JP, Kodituwakku PW, May PA. Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: a case control study. J Stud Alcohol. 2002;63:6–17. [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Brooke L, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell CL, Khaole N, Kodituwakku P, Asante KO, Findlay R, Quinton BA, Marais AS, Kalberg WO, May PA. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson EJ, Murray-Lyon IM. Drinking and smoking patterns amongst women attending an antenatal clinic — II during pregnancy. Alcohol Alcohol. 1989;24:163–173. doi: 10.1093/oxfordjournals.alcalc.a044880. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]