Abstract
Background
There is a known inverse association between solar radiation and the prevalence of multiple sclerosis (MS). Some studies have investigated the link between vitamin D and MS. The aim of this study was to investigate the possible association between serum 25(OH) vitamin D3 concentration and the severity of disease in Iranian patients with MS.
Methods
Patients with relapsing–remitting MS underwent neurological examination, including measurement of Expanded Disability Status Scale (EDSS) score, and were categorized by disease severity into mild (0 ≤ EDSS ≤3), moderate (3.5 ≤ EDSS ≤5.5) and severe (6 ≤ EDSS). Serum concentrations of 25(OH) vitamin D3, calcium, phosphorus, magnesium and parathyroid hormone were also measured.
Results
A total of 78 (73.1% female) patients with MS were evaluated. The mean (± standard deviation) of age was 33.9 ± 9.2 years. The mean (± standard error) serum concentrations of 25(OH) vitamin D3 were 36.6 ± 5.1 mg/dL, 50.1 ± 12.6 mg/dL and 19.8 ± 6.5 mg/dL in patients with mild, moderate and severe disease, respectively. There was a statistically significant inverse correlation between 25(OH) vitamin D3 concentration and EDSS score (P = 0.016, r= –0.273 by Spearman rank correlation test), which was observed in women only (P = 0.021, r = –0.305). Receiver operating characteristic curve analysis suggested that a serum 25(OH) vitamin D3 concentration cutoff of 16.5 mg/dL could differentiate patients with mild/moderate MS from severe disease with 74.6% accuracy.
Conclusion
Our findings further support the association between vitamin D and disease severity in MS.
Keywords: Multiple Sclerosis, 25(OH) Vitamin D3, Expanded Disability Status Scale, Severity
Introduction
The prevalence of multiple sclerosis (MS) is increasing in many developing countries, and this has drawn research attention towards various factors that might affect the incidence or severity of this disease. Indeed, one of the most striking epidemiological features of MS is a gradient of increasing prevalence with geographic latitude (i.e. greater prevalence with increasing distance from the Equator).1 This gradient has been observed to persist in some regions even after adjusting for confounding factors such as migration patterns.2–4 This inverse association between the intensity of solar radiation and the prevalence of MS was first observed in 1960.5 In latitudes greater than 45o, during the winter months, even prolonged sunlight exposure is inadequate to support vitamin D synthesis. During these periods, general populations in these regions are at risk of developing vitamin D deficiency.6, 7 Therefore, vitamin D represents one potential link to explain the geographic gradient of MS prevalence.
A study suggested that an individual's vitamin D status may influence susceptibility to certain immune-mediated diseases.8 Some studies about the relation between vitamin D and MS have revealed that vitamin D deficiency often coexists with established MS,9, 10 and that oral supplementation may be associated with a lower risk of the disease.11–13
Iran is located nearer to the equator than countries with a traditionally higher prevalence of MS. Nevertheless, the incidence of MS in Iran has recently increased. Some clinicians believe that despite high solar radiation, Iranians do not generally receive adequate and effective radiation as a result of certain ethnic and cultural factors, and consequently suffer from low vitamin D serum levels.14 This study was therefore performed to further investigate the association between serum 25(OH) vitamin D3 concentration and disease severity in Iranian patients with MS.
Materials and Methods
Patients
This cross-sectional study was conducted at Jondishapour Clinic in Tehran, Iran, between March and September 2007. Eligible patients had relapsing–remitting MS (RRMS), as determined by the McDonald criteria (revision 2005).15 Reasons for exclusion were progressive MS (primary or secondary), use of digitalis or vitamin D supplementation, any condition predisposing to hypercalcemia, nephrolithiasis or renal insufficiency, pregnancy or unwillingness to use contraception and unwillingness to restrict dietary calcium.
The study was approved by the ethics committee of Shaheed Beheshti University of Medical Sciences, Tehran, Iran, and was conducted in accordance with the Declaration of Helsinki. The protocol of the treatment was explained for the patients and written informed consent (approved by the appropriate Institutional Board Review) was obtained from each patient prior to enrollment.
Clinical assessments
Neurological, clinical and magnetic resonance imaging (MRI) assessments were conducted at the time of patient enrollment. All clinical assessments were performed by a neurologist. At enrollment, patients’ neurological impairment was assessed using the Expanded Disability Status Scale (EDSS).16 Assessment of EDSS score was performed at almost the same time of day for all patients, and when patients were not in relapse or not receiving steroids. The EDSS score is based on the neurological examination and the patient's ability to walk and ranges from 0 (indicating no neurological abnormality) to 10 (death caused by MS). Patients were categorized by the severity of their disease: mild (0 ≤ EDSS ≤3), moderate (3.5 ≤ EDSS ≤ 5.5) and severe (6 ≤ EDSS).
Laboratory measurements
Serum concentrations of 25(OH) vitamin D3, calcium, phosphorus, magnesium and parathyroid hormone (PTH) were measured during the summer months. Serum 25(OH) vitamin D3 levels were measured using a semi-automated solid-phase extraction reverse-phase high-performance liquid chromatography assay. The vitamin D was evaluated in the same laboratory.
Statistical analysis
The data were analysed using SPSS version 15 software for Windows (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was performed to evaluate normal distribution of the quantitative variables. To test the differences of continuous variables in study groups based on disease severity, the Mann–Whitney U test and independent t test were used. The Kruskal–Wallis test was used to compare the differences between various levels of disease severity. Associations between quantitative variables were investigated using the Spearman correlation test. Receiver operating characteristic (ROC) curve analysis was also performed to assess the predictability of disease severity with serum vitamin D3 level, and the area under curve (AUC) and appropriate cutoff point were determined. A 5% probability of a type I error (two-tailed) and a power of 80% were considered in the analysis. All reported P-values are two-tailed.
Results
A total of 78 patients with MS were evaluated. Almost three-quarters (73.1%) were women. The mean age was 33.9 ± 9.2 years and the mean disease duration was 6.41 ± 5.2 years. The mean EDSS score was 3.26 ± 2.2 [mean ± standard deviation (SD)]. Disease severity was mild in 47 patients (60.3%), moderate in 12 (15.4%) and severe in 19 (24.4%).
Serum concentrations of 25(OH) vitamin D3, calcium, phosphorus, magnesium and PTH were measured in all patients. The mean values of 25(OH) vitamin D3 were 36.6 ± 5.1 mg/dL, 50.1 ± 12.6 mg/dL and 19.8 ± 6.5 mg/dL in patients with mild, moderate and severe disease, respectively [mean ± standard error (SE)]. A statistically significant inverse correlation was found between 25(OH) vitamin D3 level and EDSS score (P = 0.016, r = –0.273). A statistically significant inverse correlation was also seen between serum calcium concentration and EDSS score (P = 0.045, r = –0.244).
Further analysis of the inverse correlation between serum 25(OH) vitamin D3 concentration and disease severity showed that this correlation was only observed in women (P = 0.021, r = –0.305). On the other hand, there were no significant differences between women and men in either overall mean (± SE) serum 25(OH) vitamin D3 concentration (34.6 ± 5.1 mg/dL vs. 34.5 ± 6.2 mg/dL, P = 0.988) or EDSS score (3.07 ± 0.2 vs. 3.79 ± 0.5, P = 0.205).
When patients were categorized as having mild/moderate disease (n = 59) or severe disease (n = 19), mean serum levels of calcium, phosphorus, magnesium and PTH were not significantly different between these two patient groups (P > 0.05), whereas the mean (± SE) serum 25(OH) vitamin D3 concentrations were significantly (P = 0.001) lower in patients with severe MS (19.8 ± 28.6 mg/dL) than in those with mild or moderate MS (39.3 ± 37.2 mg/dL; Table 1). Although patients with severe MS were significantly older than those with mild/moderate disease (P = 0.003), there was no significant correlation between age and serum 25(OH) vitamin D3 concentration (P = 0.528).
Table 1.
Demographic and clinical characteristics of patients with multiple sclerosis by disease severity
| Disease severity | P-value | ||
|---|---|---|---|
|
| |||
| Mild to moderate (n = 59) | Severe (n = 19) | ||
| Age (years) | 32.1 ± 8.9 | 39.3 ± 7.8 | 0.003 |
| Women | 46 (77.9%) | 11 (57.9%) | 0.086 |
| Duration of disease (years) | 4.73 ± 3.77 | 11.6 ± 5.7 | <0.001 |
| Expanded Disability Status Scale | 2.22 ± 1.3 | 6.5 ± 0.3 | <0.001 |
| Serum phosphorus (mg/dL) | 3.72 ± 0.5 | 3.67 ± 0.7 | 0.770 |
| Serum calcium (mg/dL) | 9.48 ± 0.5 | 8.57 ± 2.0 | 0.059 |
| Serum magnesium (mg/dL) | 2.18 ± 0.3 | 2.21 ± 0.3 | 0.573 |
| Serum parathyroid (mg/dL) | 62.8 ± 37.5 | 43.1 ± 18.7 | 0.064 |
| Serum vitamin D3 (mg/dL) | 39.3 ± 37.2 | 19.8 ± 28.6 | 0.001 |
Data are are presented as mean ± standard deviation unless indicated otherwise.
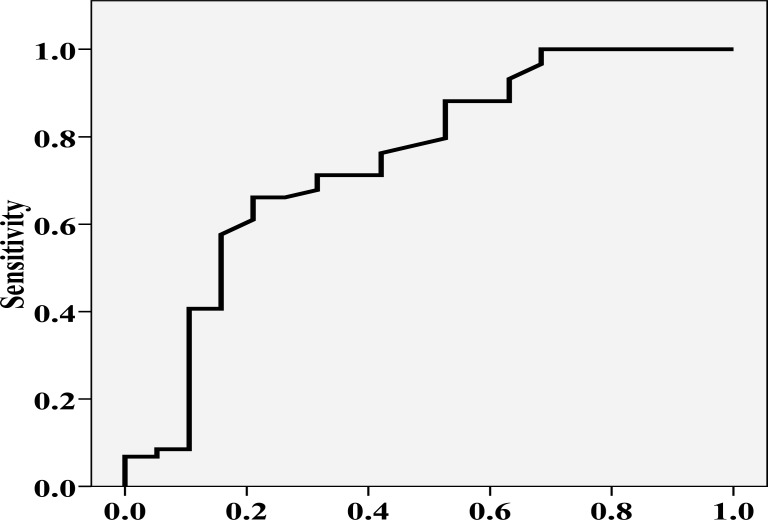
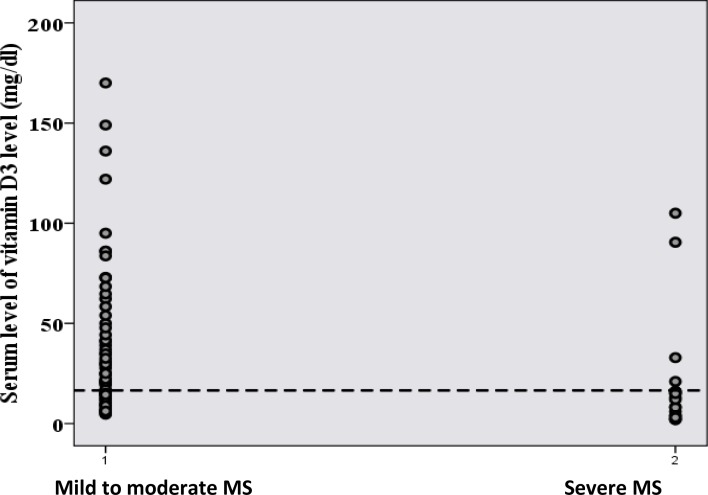
ROC curve analysis showed that with an AUC of 0.746, serum 25(OH) vitamin D3 distinguished patients with mild/moderate MS from those with severe disease (P = 0.001, Figure 1). The cutoff of 16.5 mg/dL for serum 25 (OH) vitamin D3 concentrations could potentially differentiate patients with mild/moderate MS from those with severe disease, with a sensitivity of 66.1% and specificity of 78.9% (Figure 2).
Figure 1.
Receiver operating characteristic curve analysis of serum 25(OH) vitamin D3 concentration to distinguish patients with mild/moderate multiple sclerosis from those with severe disease (area under the curve = 0.746, P = 0.001)
Figure 2.
Plot of serum vitamin D3 concentration versus individuals’ severity of disease. The dotted line shows the cutpoint of 16.55 mg/dL to differentiate patients with mild/moderate multiple sclerosis from those with severe disease.
Discussion
We evaluated the association between serum 25(OH) vitamin D3 concentrations and disease severity in Iranian patients with MS, and found a statistically significant inverse correlation between 25(OH) vitamin D3 level and EDSS score. Sex also appeared to be an important factor, as a statistically significant inverse relation was only found in female patients. This correlation was not affected by patients’ age. Our findings support the possible importance of vitamin D status in patients with MS and its association with disease severity.
The role of vitamin D in MS has been assessed over the last two decades. One of the first animal studies showed that vitamin D deficiency resulted in increased susceptibility to experimental allergic encephalomyelitis (EAE, an animal model of MS).17 Some of the most direct evidence comes from a large prospective epidemiological study, which demonstrated that the intake of vitamin D from multivitamin supplements led to a 40% reduction in the risk of MS among female nurses in the USA.13 Other studies reported no difference in serum 25(OH) vitamin D3 levels between patients with MS at diagnosis and controls, when samples were obtained during the winter months, but found lower serum 25(OH) vitamin D3 concentrations in patients with MS during June to September.18 In addition, patients had lower vitamin D levels during MS relapses than in remission, which suggests that vitamin D could be involved in the regulation of clinical disease activity.18 An inverse correlation between brain MRI activity in patients with MS and serum 25(OH) vitamin D3 levels in the general population in southern Germany was demonstrated in another study.19 Further, 25(OH) vitamin D3 levels were found to be lower in patients with progressive forms of MS compared with RRMS, and low levels were also associated with an increase in clinical MS severity, as measured by EDSS score and the occurrence of relapses.20
The effects of patient sex on the correlation between serum vitamin D concentration and MS severity have also been demonstrated in other studies. In EAE, dietary vitamin D delayed the onset and severity of the disease in female but not male mice.21 In a study on women with and without MS, every 10 nmol/L increase in serum 25(OH) vitamin D3 concentration reduced an individual's risk of a diagnosis of MS by 19%. In the same study, a negative correlation was also found between EDSS score and serum 25(OH) vitamin D3 levelin women (P = 0.020, r = –0.29).22 In contrast, a recent study reported no differences in serum 25(OH) vitamin D levels between patients with MS and controls, but demonstrated higher levels in female patients with MS than in male patients.23 These results may provide some clues into the pathogenesis of the sex difference in the risk and clinical manifestations of MS, and in the nature of the environmental factors involved in MS.
The underlying mechanism for the association between serum 25(OH) vitamin D3 concentration and MS severity is currently unknown. In vitro, 1,25(OH)2 vitamin D3, which is produced mainly by hydroxylation of 25(OH) vitamin D3 in the kidney,24 is a potent immune modulator that inhibits pro-inflammatory cells and promotes anti-inflammatory cells and cytokines.25 Patients with MS showed changes in their cytokine profiles following dietary supplementation with vitamin D (1000 IU/day) plus calcium (800 mg/day).26 However, more studies are needed to clarify the mechanism of the apparent modulating effect of vitamin D on MS pathology.
The current study had important limitations, including its cross-sectional design, small sample size, and uneven group sizes when patients were categorized by disease severity. Nevertheless, the results from this study have allowed us to propose a cutoff of 16.55 mg/dLfor serum 25 (OH) vitamin D3 concentrations in order to differentiate patients with mild-to-moderate from those with severe MS. To the best of our knowledge, our study is the first to propose such a cutoff, which should also inform future research into the optimal serum level of vitamin D that is needed for the modification of immune responses. Our findings may also be important for understanding the increasing incidence of MS in Iran, where recent reports have shown prevalence of vitamin D deficiency as high as 86% in women, 53.6% in girls and 75% in newborns during winter in some regions of Iran.27, 28
In conclusion, our findings suggest that vitamin D could be involved in the regulation of clinical disease activity in MS, based on its inverse correlation with disease severity as measured using the EDSS score. Pilot studies have already been performed to evaluate the effects of vitamin D supplementation on cytokine levels and safety and tolerability in patients with MS.26, 29 We believe that trials of vitamin D supplements, both as a preventive agent for individuals at risk and as a therapeutic agent in female patients with MS, should be considered. In addition, other studies designed to evaluate the possible mechanisms of the modulatory role of vitamin D in MS are warranted.
References
- 1.Ebers GC, Sadovnick AD. The geographic distribution of multiple sclerosis: a review. Neuroepidemiology. 1993;12(1):1–5. doi: 10.1159/000110293. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzke JF. Geography in multiple sclerosis. J Neurol. 1977;215(1):1–26. doi: 10.1007/BF00312546. [DOI] [PubMed] [Google Scholar]
- 3.Compston A. Distribution of multiple sclerosis. In: Compston A, Ebers GC, Lassmann H, editors. McAlpine's multiple sclerosis. London: Churchill Livingstone Elsevier; 1998. pp. 63–100. [Google Scholar]
- 4.Hammond SR, McLeod JG, Millingen KS, et al. The epidemiology of multiple sclerosis in three Australian cities: Perth, Newcastle and Hobart. Brain. 1988;111(Pt 1):1–25. doi: 10.1093/brain/111.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Acheson ED, Bachrach CA, Wright FM. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand Suppl. 1960;35(147):132–47. doi: 10.1111/j.1600-0447.1960.tb08674.x. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 7.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 8.Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 9.Hayes CE, Cantorna MT, DeLuca HF. Vitamin D and multiple sclerosis. Proc Soc Exp Biol Med. 1997;216(1):21–7. doi: 10.3181/00379727-216-44153a. [DOI] [PubMed] [Google Scholar]
- 10.Hayes CE. Vitamin D: a natural inhibitor of multiple sclerosis. Proc Nutr Soc. 2000;59(4):531–5. doi: 10.1017/s0029665100000768. [DOI] [PubMed] [Google Scholar]
- 11.Nieves J, Cosman F, Herbert J, et al. High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis. Neurology. 1994;44(9):1687–92. doi: 10.1212/wnl.44.9.1687. [DOI] [PubMed] [Google Scholar]
- 12.Cosman F, Nieves J, Komar L, et al. Fracture history and bone loss in patients with MS. Neurology. 1998;51(4):1161–5. doi: 10.1212/wnl.51.4.1161. [DOI] [PubMed] [Google Scholar]
- 13.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 14.Bassir M, Laborie S, Lapillonne A, et al. Vitamin D deficiency in Iranian mothers and their neonates: a pilot study. Acta Paediatr. 2001;90(5):577–9. [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 17.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93(15):7861–4. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soilu-Hanninen M, Airas L, Mononen I, et al. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler. 2005;11(3):266–71. doi: 10.1191/1352458505ms1157oa. [DOI] [PubMed] [Google Scholar]
- 19.Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;48(2):271–2. [PubMed] [Google Scholar]
- 20.Smolders J, Menheere P, Kessels A, et al. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14(9):1220–4. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 21.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175(6):4119–26. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 22.Kragt J, van Amerongen B, Killestein J, et al. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler. 2009;15(1):9–15. doi: 10.1177/1352458508095920. [DOI] [PubMed] [Google Scholar]
- 23.Barnes MS, Bonham MP, Robson PJ, et al. Assessment of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D3 concentrations in male and female multiple sclerosis patients and control volunteers. Mult Scler. 2007;13(5):670–2. doi: 10.1177/1352458506072666. [DOI] [PubMed] [Google Scholar]
- 24.Jongen MJ, van der Vijgh WJ, Lips P, et al. Measurement of vitamin D metabolites in anephric subjects. Nephron. 1984;36(4):230–4. doi: 10.1159/000183159. [DOI] [PubMed] [Google Scholar]
- 25.Smolders J, Damoiseaux J, Menheere P, et al. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol. 2008;194(1-2):7–17. doi: 10.1016/j.jneuroim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Mahon BD, Gordon SA, Cruz J, et al. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134(1-2):128–32. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 27.Kazemi A, Sharifi F, Jafari N, et al. High prevalence of vitamin D deficiency among pregnant women and their newborns in an Iranian population. J Womens Health (Larchmt) 2009;18(6):835–9. doi: 10.1089/jwh.2008.0954. [DOI] [PubMed] [Google Scholar]
- 28.Rabbani A, Alavian SM, Motlagh ME, et al. Vitamin D insufficiency among children and adolescents living in Tehran, Iran. J Trop Pediatr. 2009;55(3):189–91. doi: 10.1093/tropej/fmn078. [DOI] [PubMed] [Google Scholar]
- 29.Wingerchuk DM, Lesaux J, Rice GP, et al. A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76(9):1294–6. doi: 10.1136/jnnp.2004.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]