Abstract
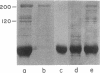
Microtubule protein from HeLa cell extracts was purified by multiple cycles of polymerization and depolymerization in the absence of glycerol or other exogenous polymerization-stimulatory agents. Approximately 4-5% of the extract protein was tubulin, of which more than one-half was competent to participate in polymerization-depolymerization cycles. The purified HeLa microtubule protein preparations contained 95% tubulin after the second cycle of polymerization and depolymerization. Additional protein species bound specifically to and copurified quantitatively with microtubules throughout at least four cycles of polymerization and depolymerization. These microtubule-associated proteins (MAPs) were separated from tubulin by DEAE column chromatography. When added to purified brain or HeLa tubulin, these MAPs stimulated the polymerization of microtubules as assayed by electron microscopy and a quantitative sedimentation assay. The most prominent HeLa MAPs had molecular weights of approximately 210,000 and 120,000.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz S. A., Katagiri J., Binder H. K., Williams R. C., Jr Separation and characterization of microtubule proteins from calf brain. Biochemistry. 1977 Dec 13;16(25):5610–5617. doi: 10.1021/bi00644a035. [DOI] [PubMed] [Google Scholar]
- Borisy G. G. A rapid method for quantitative determination of microtubule protein using DEAE-cellulose filters. Anal Biochem. 1972 Dec;50(2):373–385. doi: 10.1016/0003-2697(72)90046-2. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B., Marcum J. M., Allen C. Microtubule assembly in vitro. Fed Proc. 1974 Feb;33(2):167–174. [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B. Nucleated assembly of microtubules in porcine brain extracts. Science. 1972 Sep 29;177(4055):1196–1197. doi: 10.1126/science.177.4055.1196. [DOI] [PubMed] [Google Scholar]
- Bryan J. B., Nagle B. W., Doenges K. H. Inhibition of tubulin assembly by RNA and other polyanions: evidence for a required protein. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3570–3574. doi: 10.1073/pnas.72.9.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Cleveland D. W., Kirschner M. W. Immunoflourescent staining of cytoplasmic and spindle microtubules in mouse fibroblasts with antibody to tau protein. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2437–2440. doi: 10.1073/pnas.74.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Rosenbaum J. L. Ultrastructural localization of the high molecular weight proteins associated with in vitro-assembled brain microtubules. J Cell Biol. 1975 Apr;65(1):237–241. doi: 10.1083/jcb.65.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Berkowitz A., Kim H., Williams R. C., Jr Binding of glycerol by microtubule protein. Biochem Biophys Res Commun. 1976 Feb 9;68(3):961–968. doi: 10.1016/0006-291x(76)91239-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lockwood A. H. Tubulin assembly protein: immunochemical and immunofluorescent studies on its function and distribution in microtubules and cultured cells. Cell. 1978 Apr;13(4):613–627. doi: 10.1016/0092-8674(78)90212-x. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Vallee R. B., Borisy G. G. Identity and polymerization-stimulatory activity of the nontubulin proteins associated with microtubules. Biochemistry. 1977 Jun 14;16(12):2598–2605. doi: 10.1021/bi00631a004. [DOI] [PubMed] [Google Scholar]
- Nagle B. W., Doenges K. H., Bryan J. Assembly of tubulin from cultured cells and comparison with the neurotubulin model. Cell. 1977 Nov;12(3):573–586. doi: 10.1016/0092-8674(77)90258-6. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Scheele R. B., Borisy G. G. Comparison of the sedimentation properties of microtubule protein oligomers prepared by two different procedures. Biochem Biophys Res Commun. 1976 May 3;70(1):1–7. doi: 10.1016/0006-291x(76)91100-1. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherline P., Schiavone K. Immunofluorescence localization of proteins of high molecular weight along intracellular microtubules. Science. 1977 Dec 9;198(4321):1038–1040. doi: 10.1126/science.337490. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. Removal of the projections from cytoplasmic microtubules in vitro by digestion with trypsin. J Biol Chem. 1977 Jan 10;252(1):377–382. [PubMed] [Google Scholar]
- Weatherbee J. A., Luftig R. B., Weihing R. R. In vitro polymerization of microtubules from HeLa cells. J Cell Biol. 1978 Jul;78(1):47–57. doi: 10.1083/jcb.78.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Wiche G., Cole R. D. Reversible in vitro polymerization of tubulin from a cultured cell line (rat glial cell clone C6). Proc Natl Acad Sci U S A. 1976 Apr;73(4):1227–1231. doi: 10.1073/pnas.73.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Lundblad V. J., Cole R. D. Competence of soluble cell extracts as microtubule assembly systems. Comparison of simian virus 40 transformed and nontransformed mouse 3T3 fibroblasts. J Biol Chem. 1977 Jan 25;252(2):794–796. [PubMed] [Google Scholar]
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970 Dec 8;9(25):4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]