Abstract
Background
Stroke is the first cause of morbidity all around the world. Entrapment neuropathies are a known complication of stroke. The objective of this study is to assess the frequency of subclinical carpal tunnel syndrome in the healthy and paretic hands of stroke patients.
Methods
The authors performed nerve conduction study in the first three days after admission in 39 stroke patients without subclinical carpal tunnel syndrome and 30 days after admission. Electrophysiological studies were done in both paretic and non-paretic hands. Both ulnar and median nerves were studied.
Results
After one month we found subclinical carpal tunnel syndrome in 16 paretic hands and 13 healthy hands. We did not find any difference in the frequency of carpal tunnel syndrome in two sides.
Conclusion
The authors suggest that simultaneous different mechanisms may act in inducing carpal tunnel syndrome in both hands of hemiparetic patients.
Keywords: Stroke, Carpal Tunnel Syndrome, Paretic Hand
Introduction
Carpal tunnel syndrome (CTS) is the most frequent type of compressive neuropathies of the humankind. It results from entrapment of the median nerve at the level of carpal tunnel.1 The majority of cases are idiopathic and present in women, however, there are other important and independent risk factors such as obesity, square wrists,2, 3 rheumatoid arthritis, diabetes, wrist fracture and hypothyroidism.4 The diagnosis of CTS is based on a collection of clinical symptoms and signs including pain and numbness in the territory of median nerve in the hand and related electrophysiological findings.5, 6 CTS may only be a demyelinative process or with axonal injury due to chronic nerve compression in the canal. Asymptomatic or subclinical CTS is common and especially frequent in diabetic patients and Down syndrome.7, 8 The clinical significance of asymptomatic CTS is its ability to change into the symptomatic type due to the vulnerability of the median nerve. It may be found in patients without clinically prominent symptoms of CTS.
Stroke after heart disease is the third most prevalent cause of death worldwide and the first important cause of morbidity all around the world. It has been shown that the age at first manifestation in Iran is less than developed countries and its complication should be higher in our population.9 Stroke patients are vulnerable to compression neuropathy due to inability to move or using crutches during rehabilitation.10, 11 It is usually missed to assess the status of the peripheral nerves in such patients due to motor disability especially in the first month after stroke. Electrophysiologic study is useful for confirming the presence of entrapment neuropathies which are occurred in the fibrous tunnels of the limbs. Increased pressure in the carpal tunnel is the main described mechanism of median nerve compression in carpal tunnels of the paretic hands. However, in stroke patients the use of different types of crutches for rehabilitation may cause more pressure in the healthy limb and may be an important factor of CTS presentation in these patients.12.
Materials and Methods
Patient Selection
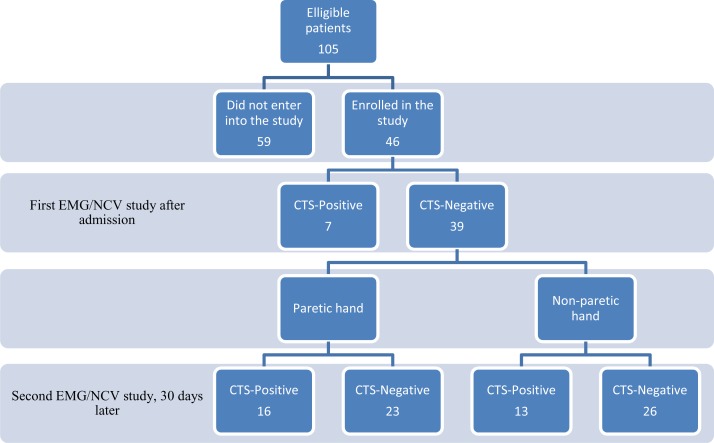
After obtaining the University Ethics Committee approval, we conducted an 11-month cross-sectional study from May 2011 to April 2012. The authors studied a local population of stroke patients admitted to our central university hospital in southeastern province of Iran. One hundred and five patients with non-hemorrhagic stroke were eligible to enter into the study. However, only 46 patients enrolled in the study. 59 patients did not continue the study due to different reasons such as inability to be visited at the right time (30 days later) or death. Motor power of the paretic hand of all patients was ≤ 2/5 based on Medical Research Council (MRC) scale.13 The diagnosis was confirmed by clinical examination and imaging studies (Fig. 1). The researcher at first explained the nature of the study and after obtaining informed consent, the examiner filled out a questionnaire about demographic characters of the participants such as age, gender, occupation and any associated disorder like hypertension, hypercholesterolemia, diabetes, trauma and fractured wrists.
Figure 1.
Profile of the study
EMG/NCV: Electromyography and nerve conduction studies
CTS: Carpal tunnel syndrome
Electrophysiological study
The electrophysiologic studies were performed in the first three days after admission of patients to the neurology department and 30 days later for evaluation of the presence of CTS. Median and ulnar nerves of both hands were evaluated in an air-conditioned room at 23-25° C. The diagnosis of electrophysiological CTS was based on previously validated electrodiagnostic criteria according to the American Academy of Neurology (AAN)14 consisting evidence of slowing of distal median nerve conduction. Only one of the researchers (AM) did all electrodiagnostic studies with a Medtronic-Keypoint® 4 apparatus (Skovlunde, Denmark) on subjects lying on a bed in a quiet and warm room. All studies were done in the same room and in similar temperature using a surface temperature recorder. All nerve stimulations were delivered with a constant current standard bipolar surface stimulator (cathode distal). The sweep speed were set at 2 ms/division and the recording of the median nerve compound muscle action potential (CMAP) were performed using standard bar electrode supplied by the equipment, which was placed on the thenar muscle at a distance of 8 cm from the stimulator. CMAP for median nerve had been measured from the baseline to the negative peak. Interelectrode distance (between active and reference electrodes) was 4 cm. Sensory nerve action potentials (SNAPs) were obtained antidromically, using ring electrode after stimulation at the wrist and registered at second digit for the median nerve and at fifth digit in the same way for ulnar nerve. Active and reference electrodes were placed on proximal and distal interphalangeal joints, respectively, with interelectrode distance of 2 cm. Supramaximal stimulation was used for motor conduction studies, while up to 50 mA stimulation intensity was delivered for sensory nerves. Obtained sensory responses were averaged. Maximum antidromic sensory conduction velocity (SCV) and maximum sensory action potential amplitude (SAP) were determined. The mean values of distal latencies, conduction velocities and amplitudes were calculated for motor and sensory branches of median nerve. Normal reference values for age and sex were based on a previous local population study of healthy subjects in this region. Distal and peak latencies for sensory branches of median nerve in CTS and non-CTS patients were measured. Electromyographic study was carried out in all individuals. Electrophysiologic diagnostic criteria of CTS include presence of at least two of the followings: Prolonged distal latency of motor fibers of median nerve ≥ 4 ms; prolonged median nerve digit 2 sensory onset latency ≥ 2.5 ms; prolongation of onset latency of the median SNAP of digit two relative to the ulnar SNAP of digit five ≥ 0.5 ms.15 Normal median conduction velocity along the forearm was mandatory (# 50 m/s). Those parameters were used as the reference values for the diagnosis of CTS in our electromyography laboratory. Our patients were divided into three groups on the basis of electrophysiological severity:16 (i) Mild: Prolonged sensory distal latency ± SNAP amplitude reduction; (ii) Moderate: Prolongation of both median motor and sensory distal latencies; (iii) Severe: Electrodiagnostic criteria of moderate type of CTS, with either an absence of SNAP, or low amplitude or absent thenar CMAP, or findings compatible with axonal injury in electromyography.
Statistical analysis
The authors made a standardized report sheet in which data were collected step-by-step as described earlier. After an evaluation of the assumption of normal distribution using SPSS software for Windows, version 17 (SPSS Inc., Chicago, IL, USA), arithmetic mean values and standard deviations (SD) of the data were calculated. Then we carried out paired t-test to compare electrophysiologic parameters of the first and second assessments. Chi-square test was used for comparing the paretic and non-paretic hands against the presence or absence of CTS. A two-sided significance level of 0.05 was used.
Results
Among 46 studied patients, 7 subjects had asymptomatic CTS in the first electrophysiological study; therefore, we excluded them. The remaining 39 patients (18 females), aged between 35 and 89 years (mean: 64.1 ± 7.2 years). All patients had ischemic cerebrovascular disease. Twenty-four patients (61.5%) had hypertension and 30 cases (76.9%) had diabetes. Ten patients had BMI of more than 30 and the remainder 29 cases (74.3%) had BMI between 25 and 30. Mean values and standard deviations for laboratory results are shown in Table 1.
Table 1.
Demographic characteristics of 39 stroke patients
| Sex (M/F) | 21/18 |
| Hypertension (%) | 24 (61.5%) |
| Diabetes (%) | 30 (76.9%) |
| Mean ± SD | |
| Age (year) | 64.1 ± 7.2 |
| Body mass index (kg/m2) | 28.1 ± 7.2 |
| Fasting blood sugar (mg/dl) | 165.1 ± 15.2 |
| Triglyceride (mg/dl) | 195.5 ± 65.2 |
| Cholesterol (mg/dl) | 210.2 ± 45.1 |
| HDL-Cholesterol (mg/dl) | 49.1 ± 9.1 |
| LDL-Cholesterol (mg/dl) | 133.1 ± 35.2 |
After performing the second electrophysiological study, it was revealed that 16 paretic hands and 13 non-paretic hands were affected with CTS. Chi-square analysis revealed that there was not any significant association between limb paresis and CTS. In terms of nerve conduction studies in the paretic hands, paired t-test revealed that the only significant relationship between two assessments were in distal motor latency of the motor branches (P = 0.039) and conduction velocity of the sensory branches (P = 0.031) of median nerve (Table 2). Same analysis for the non-paretic hand showed that significant findings were between distal latency of motor branches (P = 0.041) and peak latency of the sensory branches (P = 0.031) of the related median nerves (Table 3). Concerning the presence of subclinical CTS, we did not find any difference between two sides after one month.
Table 2.
Parameters of nerve conduction studies of median nerve in the paretic hand of 39 stroke patients
| First study | Second study | P | |
|---|---|---|---|
| Distal latency-M (m/sec) | 3.4 ± 6.6 | 3.8 ± 0.5 | 0.039 |
| Amplitude-M (mV) | 6.2 ± 1.7 | 6.1 ± 1.2 | 0.170 |
| Conduction velocity-M (m/sec) | 65.2 ± 7.2 | 62.3 ± 6.7 | 0.051 |
| Onset latency-S (m/sec) | 2.8 ± 0.3 | 3.0 ± 0.1 | 0.045 |
| Amplitude-S (mV) | 15.7 ± 7.1 | 12.1 ± 6.1 | 0.055 |
| Conduction velocity-S (m/sec) | 59.1 ± 6.5 | 51.7 ± 4.2 | 0.031 |
M: Motor fibers; S: Sensory fibers
Table 3.
Parameters of nerve conduction studies of median nerve in the non-paretic hand of 39 stroke patients
| First study | Second study | P | |
|---|---|---|---|
| Distal latency-M (m/sec) | 3.3 ± 0.5 | 3.9 ± 0.6 | 0.041 |
| Amplitude-M (mV) | 7.2 ± 1.9 | 7.1 ± 1.3 | 0.210 |
| Conduction velocity-M (m/sec) | 64.2 ± 6.1 | 63.1 ± 3.9 | 0.181 |
| Onset latency-S (m/sec) | 2.7 ± 0.2 | 3.2 ± 0.2 | 0.031 |
| Amplitude-S (mV) | 19.2 ± 6.1 | 15.2 ± 4.3 | 0.150 |
| Conduction velocity-S (m/sec) | 62.1 ± 6.1 | 54.1 ± 5.3 | 0.052 |
M: Motor fibers; S: Sensory fibers
Discussion
According to this study, distal latency for motor branches of both healthy and paretic hands has been increased after stroke. Onset latency and conduction velocity of sensory branches of median nerve in the paretic hand were altered and revealed statistically significant changes between two electrodiagnostic studies, immediately after admission and one month later. In the non-paretic hand, onset latency of sensory fibers showed significant change, however, conduction velocity did not show any abnormality between two studies. Distal latencies and conduction velocities of ulnar nerves in both sides did not reveal any significant difference. It seems that stroke may be an independent risk factor CTS after one month. Increased pressure in the carpal tunnel due to edema of the upper extremity is probably the main reason for inducing CTS in the paretic hand. On the other hand, overuse of the other hand especially for rehabilitation is an important risk factor for increased pressure in the same tunnel of the non-paretic hand.
There are three main leading theories of causation of entrapment neuropathies. First theory is the repeated compression that may cause edema in the sub-endoneurial space and synovium along with ischemia.17 Schneider and Dellon18 proposed a model in Rhesus monkey in which A-beta wave amplitude was decreased after intentional increase in carpal tunnel pressure. Moreover, localized mechanical pressure of the surrounding structures such as flexor retinaculum and Palmaris longus tendon during normal movements or changes in position of the limbs may cause local nerve damage.19, 20 Finally, the third mechanism which is not relevant here is tethering of the nerve due to scar tissues.21
Sato and Kaji22 reported a higher rate of CTS in the non-paretic hand of stroke patients. Frequent and repetitive use of hand or wrist is a common cause of CTS. Therefore, they proposed that a higher rate of use in the non-paretic hand when the other hand of the patient is paretic or plegic is the main mechanism of inducing CTS. Increased pressure in the carpal tunnel may be due to flexor tenosynovitis. However, they confirmed that the distal latency of the median nerve in the paretic hand also increased and atrophy of thenar muscles in the involved hand may have been overlooked. They have shown that sensory fibers are involved more than motor branches.
In another study Kabayel and his colleagues10 reported that in severe hemiparetic patients, the paretic hand is involved more than the other hand. They proposed that inflammation and edema are the most important risk factors that cause median nerve compression. The pathologic process has been described earlier in the sciatic nerve models. Proliferation of fibrous tissue, demyelination and axonal loss may be seen after the first week and finally marked fibrosis was seen after 28 days.23
Moreover, simultaneous mechanisms may act in both healthy and paretic limbs. Inflammation and edema is the major factor in the paretic limb, while sliding, tension and increased pressure in the carpal tunnel may play an important role in inducing CTS in the healthy hand. In conclusion, we did not find any difference between two sides and it seems that both mechanisms may act in two limbs. Longer duration of follow-up may help in distinguishing the underlying roles of different pathophysiologic mechanisms in human beings.
Acknowledgements
This paper was based on research grant (code: 88-1921) approved and funded by the Dean for Research Affairs at the Zahedan University of Medical Sciences (Iran). The authors do not have any conflict of interest.
References
- 1.Boz C, Ozmenoglu M, Altunayoglu V, et al. Individual risk factors for carpal tunnel syndrome: an evaluation of body mass index, wrist index and hand anthropometric measurements. Clin Neurol Neurosurg. 2004;106(4):294–9. doi: 10.1016/j.clineuro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Moghtaderi A, Izadi S, Sharafadinzadeh N. An evaluation of gender, body mass index, wrist circumference and wrist ratio as independent risk factors for carpal tunnel syndrome. Acta Neurol Scand. 2005;112(6):375–9. doi: 10.1111/j.1600-0404.2005.00528.x. [DOI] [PubMed] [Google Scholar]
- 3.Nordstrom DL, Vierkant RA, DeStefano F, et al. Risk factors for carpal tunnel syndrome in a general population. Occup Environ Med. 1997;54(10):734–40. doi: 10.1136/oem.54.10.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan PA, Meadows KD, Istvan JA. Predictors of carpal tunnel syndrome: an 11-year study of industrial workers. J Hand Surg Am. 2002;27(4):644–51. doi: 10.1053/jhsu.2002.34003. [DOI] [PubMed] [Google Scholar]
- 5.Uchiyama S, Itsubo T, Nakamura K, et al. Current concepts of carpal tunnel syndrome: pathophysiology, treatment, and evaluation. J Orthop Sci. 2010;15(1):1–13. doi: 10.1007/s00776-009-1416-x. [DOI] [PubMed] [Google Scholar]
- 6.Roh YH, Chung MS, Baek GH, et al. Incidence of clinically diagnosed and surgically treated carpal tunnel syndrome in Korea. J Hand Surg Am. 2010;35(9):1410–7. doi: 10.1016/j.jhsa.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Kim WK, Kwon SH, Lee SH, et al. Asymptomatic electrophysiologic carpal tunnel syndrome in diabetics: entrapment or polyneuropathy. Yonsei Med J. 2000;41(1):123–7. doi: 10.3349/ymj.2000.41.1.123. [DOI] [PubMed] [Google Scholar]
- 8.Christensen JE, Peter PJ, Nielsen VK, et al. Prevalence of carpal tunnel syndrome among individuals with Down syndrome. Am J Ment Retard. 1998;102(6):547–51. doi: 10.1352/0895-8017(1998)102<0547:poctsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Azarpazhooh MR, Etemadi MM, Donnan GA, et al. Excessive incidence of stroke in Iran: evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 2010;41(1):e3–e10. doi: 10.1161/STROKEAHA.109.559708. [DOI] [PubMed] [Google Scholar]
- 10.Kabayel L, Balci K, Turgut N, et al. Development of entrapment neuropathies in acute stroke patients. Acta Neurol Scand. 2009;120(1):53–8. doi: 10.1111/j.1600-0404.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 11.Katirji MB, Wilbourn AJ. Common peroneal mononeuropathy: a clinical and electrophysiologic study of 116 lesions. Neurology. 1988;38(11):1723–8. doi: 10.1212/wnl.38.11.1723. [DOI] [PubMed] [Google Scholar]
- 12.Mondelli M, Padua L, Reale F. Carpal tunnel syndrome in elderly patients: results of surgical decompression. J Peripher Nerv Syst. 2004;9(3):168–76. doi: 10.1111/j.1085-9489.2004.09309.x. [DOI] [PubMed] [Google Scholar]
- 13.London Medical Research Council. London, UK: London Her Majesty's Stationery Office; 1976. Aids to the Examination of the Peripheral Nervous System - Medical Research Council Memorandum No. 45. [Google Scholar]
- 14.Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25(6):918–22. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]
- 15.Jablecki CK, Andary MT, Floeter MK, et al. Withdrawn: Second AAEM literature review of the usefulness of nerve conduction studies and needle electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve. 2002 doi: 10.1002/mus.10215. [DOI] [PubMed] [Google Scholar]
- 16.Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1997;20(12):1477–86. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Nakamichi K, Tachibana S. Restricted motion of the median nerve in carpal tunnel syndrome. J Hand Surg Br. 1995;20(4):460–4. doi: 10.1016/s0266-7681(05)80153-6. [DOI] [PubMed] [Google Scholar]
- 18.Schneider RJ, Dellon AL. Median nerve evoked potential changes in an acute carpal tunnel syndrome model in Macaca mulatta. Electroencephalogr Clin Neurophysiol. 1983;56(2):224–31. doi: 10.1016/0013-4694(83)90076-7. [DOI] [PubMed] [Google Scholar]
- 19.Keese GR, Wongworawat MD, Frykman G. The clinical significance of the palmaris longus tendon in the pathophysiology of carpal tunnel syndrome. J Hand Surg Br. 2006;31(6):657–60. doi: 10.1016/j.jhsb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Valls-Sole J, Alvarez R, Nunez M. Limited longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. Muscle Nerve. 1995;18(7):761–7. doi: 10.1002/mus.880180713. [DOI] [PubMed] [Google Scholar]
- 21.Steyers CM. Recurrent carpal tunnel syndrome. Hand Clin. 2002;18(2):339–45. doi: 10.1016/s0749-0712(01)00005-1. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y, Kaji M, Tsuru T, et al. Carpal tunnel syndrome involving unaffected limbs of stroke patients. Stroke. 1999;30(2):414–8. doi: 10.1161/01.str.30.2.414. [DOI] [PubMed] [Google Scholar]
- 23.Dyck PJ, Lais AC, Giannini C, et al. Structural alterations of nerve during cuff compression. Proc Natl Acad Sci U S A. 1990;87(24):9828–32. doi: 10.1073/pnas.87.24.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]