Abstract
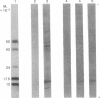
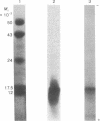
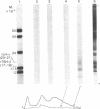
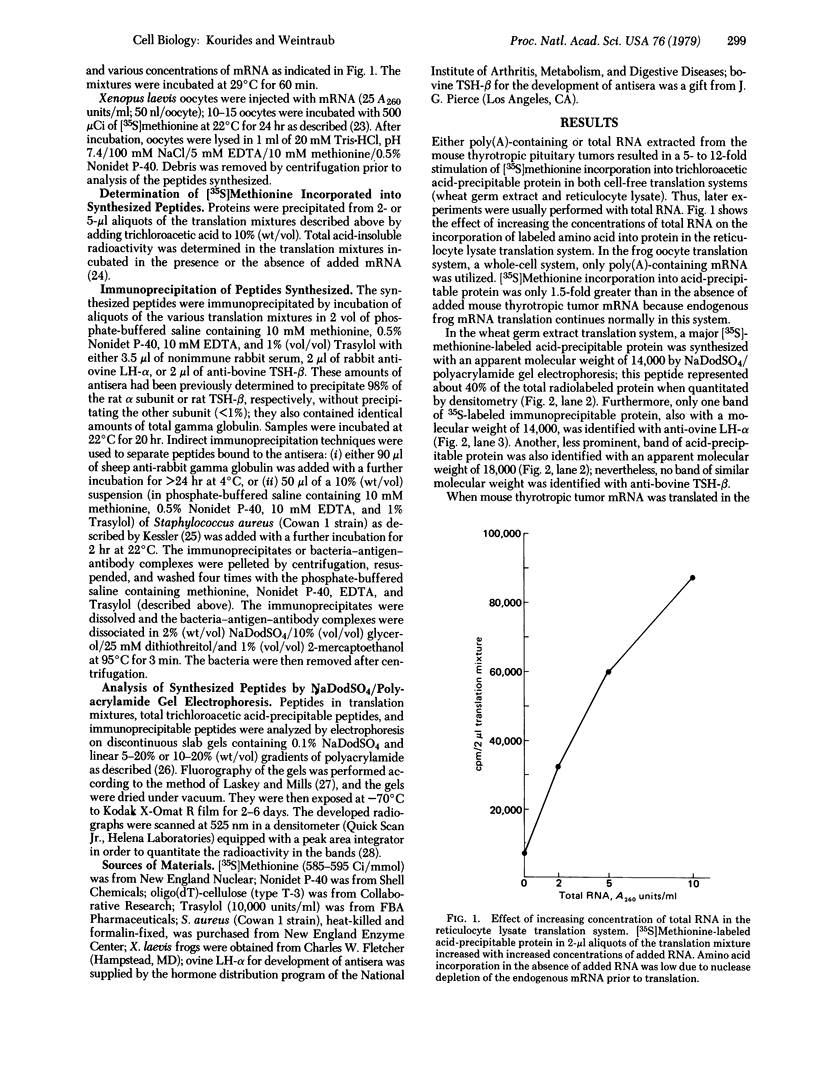
mRNA from mouse thyrotropic pituitary tumors was translated in frog oocytes (a whole-cell system) and in wheat germ extract and reticulocyte lysate (cell-free systems) in the presence of [35S]methionine. Synthesized peptides related to thyrotropin were identified in the three systems by immunoprecipitation with subunit-specific antisera developed against the α subunit of ovine lutropin (luteinizing hormone) and the β subunit of bovine thyrotropin. In wheat germ extract and reticulocyte lysate, a single immunoprecipitable form of the α subunit of thyrotropin was synthesized with an apparent molecular weight of 14,000 by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. In the frog oocyte, three forms of immunoprecipitable α subunit of thyrotropin were synthesized with apparent molecular weights of 20,000, 14,000, and 10,000. The 20,000 form is similar to unlabeled rat pituitary standard α subunit and 35S-labeled mouse tumor α subunit in cell cultures (20,000-21,000); thus, it may represent a precursor-cleaved and glycosylated form. The 14,000 form synthesized in all three systems probably represents the pre-α subunit of thyrotropin; the 10,000 form, synthesized only in the frog oocyte, could be a proteolytically cleaved but unglycosylated form. Because only the α subunit of thyrotropin was identified and no larger molecular weight immunoprecipitable form of either subunit was detected in any of the translation systems, α and β subunits of thyrotropin appear to be translated from separate mRNAs.
Keywords: secretory glycoprotein hormone, mouse thyrotropic pituitary tumor, independent synthesis of thyrotropin subunits
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M., Boime I. mRNA-dependent synthesis of a glycosylated subunit of human chorionic gonadotropin in cell-free extracts derived from ascites tumor cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1768–1772. doi: 10.1073/pnas.75.4.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. R., Gershengorn M. C., Weintraub B. D. Excess production of free alpha subunits by mouse pituitary thyrotropic tumor cells in vitro. Endocrinology. 1978 Feb;102(2):499–508. doi: 10.1210/endo-102-2-499. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M., Munro H. N. Changing ratio of human chorionic gonadotropin subunits synthesized by early and full-term placental polyribosomes. Biochem Biophys Res Commun. 1977 Jul 11;77(1):426–433. doi: 10.1016/s0006-291x(77)80215-5. [DOI] [PubMed] [Google Scholar]
- Furth J., Moy P., Hershman J. M., Ueda G. Thyrotropic tumor syndrome. A multiglandular disease induced by sustained deficiency of thyroid hormones. Arch Pathol. 1973 Oct;96(4):217–226. [PubMed] [Google Scholar]
- Hagen C., McNeilly A. S. Identification of human luteinizing hormone, follicle-stimulating hormone, luteinizing hormone beta-subunit and gonadotrophin alpha-subunit in foetal and adult pituitary glands. J Endocrinol. 1975 Oct;67(1):49–57. doi: 10.1677/joe.0.0670049. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Cavalieri R. L., Yaffe L., Pestka S. Synthesis and glycosylation of the MOPC-46B immunoglobulin in kappa chain in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1977 Dec 7;79(3):625–630. doi: 10.1016/0006-291x(77)91157-3. [DOI] [PubMed] [Google Scholar]
- Kaplan S. L., Grumbach M. M., Aubert M. L. alpha and beta glycoprotein hormone subunits (hLH, hFSH, hCG) in the serum and pituitary of the human fetus. J Clin Endocrinol Metab. 1976 May;42(5):995–998. doi: 10.1210/jcem-42-5-995. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kourides I. A., Re R. N., Weintraub B. D., Ridgway E. C., Maloof F. Metabolic clearance and secretion rates of subunits of human thyrotropin. J Clin Invest. 1977 Mar;59(3):508–516. doi: 10.1172/JCI108666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourides I. A., Ridgway E. C., Weintraub B. D., Bigos S. T., Gershengorn M. C., Maloof F. Thyrotropin-induced hyperthyroidism: use of alpha and beta subunit levels to identify patients with pituitary tumors. J Clin Endocrinol Metab. 1977 Sep;45(3):534–543. doi: 10.1210/jcem-45-3-534. [DOI] [PubMed] [Google Scholar]
- Kourides I. A., Weintraub B. D., Levko M. A., Maloof F. Alpha and beta subunits of human thyrotropin: purification and development of specific radioimmunoassays. Endocrinology. 1974 May;94(5):1411–1421. doi: 10.1210/endo-94-5-1411. [DOI] [PubMed] [Google Scholar]
- Kourides I. A., Weintraub B. D., Ridgway E. C., Maloof F. Pituitary secretion of free alpha and beta subunit of human thyrotropin in patients with thyroid disorders. J Clin Endocrinol Metab. 1975 May;40(5):872–885. doi: 10.1210/jcem-40-5-872. [DOI] [PubMed] [Google Scholar]
- Kourides I. A., Weintraub B. D., Rosen S. W., Ridgway E. C., Kliman B., Maloof F. Secretion of alpha subunit of glycoprotein hormones by pituitary adenomas. J Clin Endocrinol Metab. 1976 Jul;43(1):97–106. doi: 10.1210/jcem-43-1-97. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Landefeld T. D., McWilliams D. R., Boime I. The isolation of mRNA encoding the alpha subunit of human chorionic gonadotropin. Biochem Biophys Res Commun. 1976 Sep 20;72(2):381–390. doi: 10.1016/s0006-291x(76)80054-x. [DOI] [PubMed] [Google Scholar]
- Landefeld T., Boguslawski S., Corash L., Boime I. The cell-free synthesis of the alpha subunit of human chorionic gonadotropin. Endocrinology. 1976 May;98(5):1220–1227. doi: 10.1210/endo-98-5-1220. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis G. M., Ramirez F., Cann A., Marks P. A., Bank A. Translation and stability of human globin mRNA in Xenopus oocytes. J Clin Invest. 1976 Dec;58(6):1419–1427. doi: 10.1172/JCI108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell W. D., Wolfsen A. R. Humoral syndromes associated with cancer. Annu Rev Med. 1978;29:379–406. doi: 10.1146/annurev.me.29.020178.002115. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pierce J. G. Eli Lilly lecture. The subunits of pituitary thyrotropin--their relationship to other glycoprotein hormones. Endocrinology. 1971 Dec;89(6):1331–1344. doi: 10.1210/endo-89-6-1331. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Liao T., Howard S. M., Shome B., Cornell J. S. Studies on the structure of thyrotropin: its relationship to luteinizing hormone. Recent Prog Horm Res. 1971;27:165–212. doi: 10.1016/b978-0-12-571127-2.50029-8. [DOI] [PubMed] [Google Scholar]
- Prentice L. G., Ryan R. J. LH and its subunits in human pituitary, serum and urine. J Clin Endocrinol Metab. 1975 Feb;40(2):303–312. doi: 10.1210/jcem-40-2-303. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. W., Weintraub B. D. Ectopic production of the isolated alpha subunit of the glycoprotein hormones. A quantitative marker in certain cases of cancer. N Engl J Med. 1974 Jun 27;290(26):1441–1447. doi: 10.1056/NEJM197406272902601. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., McKnight G. S., Shapiro D. J., Sullivan D., Palacios R. Hormonal regulation of ovalbumin synthesis in the chick oviduct. Recent Prog Horm Res. 1975;31:175–211. doi: 10.1016/b978-0-12-571131-9.50009-8. [DOI] [PubMed] [Google Scholar]
- Spielman L. L., Bancroft F. C. Pregrowth hormone: evidence for conversion to growth hormone during synthesis on membrane-bound polysomes. Endocrinology. 1977 Sep;101(3):651–658. doi: 10.1210/endo-101-3-651. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Weintraub B. D., Barowsky N. J., Rabson A. S., Rosen S. W. Subunits of human chorionic gonadotropin: unbalanced synthesis and secretion by clonal cell strains derived from a bronchogenic carcinoma. Proc Natl Acad Sci U S A. 1973 May;70(5):1419–1422. doi: 10.1073/pnas.70.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L., Ross G. T., Braunstein G. D., Rayford P. L. Gonadotropins and their subunits: basic and clinical studies. Recent Prog Horm Res. 1976;32:289–331. doi: 10.1016/b978-0-12-571132-6.50019-1. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Rosen S. W. Ectopic production of the isolated beta subunit of human chorionic gonadotropin. J Clin Invest. 1973 Dec;52(12):3135–3142. doi: 10.1172/JCI107513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub B. D., Stannard B. S. Precursor-product relationships in the biosynthesis and secretion of thyrotropin and its subunits by mouse thyrotropic tumor cells. FEBS Lett. 1978 Aug 15;92(2):303–307. doi: 10.1016/0014-5793(78)80775-3. [DOI] [PubMed] [Google Scholar]