Abstract
Background
The existence of a pathophysiological link between headaches and muscle activity pattern is still being debated. The purpose of this study was to investigate the effect of pain on the timing pattern of the masseter muscle in patients with tension-type headache (TTH) and migraine without aura (MOA).
Methods
57 women (22 controls, 19 MOA and 16 TTH) participated in the study. The electromyographic (EMG) activity of masseter during the open-close-clench cycle (OCC) was recorded in the interictal and ictal stages.
Results
In the interictal stage, the results showed no significant difference in EMG activity between patients and control groups. However, masseter muscles in subjects with TTH (both sides) and in MOA patients (left side) activated significantly earlier than the control in the ictal stage. The duration of left masseter was also significantly greater in the TTH than in the control group (P < 0.05).
Conclusion
The findings of this study showed that activity pattern of masticatory muscles in headaches patients were affected by existence of pain. Furthermore, this study confirmed that temporal variables of EMG such as onset and duration rather than amplitude could be more reliable to identify altered activity pattern of muscles.
Keywords: Tension-type Headache, Migraine Without Aura, Electromyography, Masseter, Pain
Introduction
Several studies have shown the effect of pain on motor activity pattern of muscles during functional movements in subjects with chronic pain.1, 2 Although the role of electromyography (EMG) in pain research is still largely unknown, findings of EMG studies in pericranial muscles in patients with chronic headaches have demonstrated increased resting amplitude of cranial muscles.3–5 However, some researchers did not find such difference in resting EMG levels.3
Some evidence illustrated significant changes in the timing and recruitment pattern of muscular activity in subjects with chronic pain in painful status.6 Therefore, it has been suggested that temporal variables of EMG such as onset and duration rather than amplitude could be more reliable to identify altered activity pattern of muscles. However, little EMG data for assessment of the timing pattern is available in patients with headache.
The main purpose of the present study was to investigate the effect of tension-type headache (TTH) and migraine without aura (MOA) on the timing pattern of the masseter muscle, during the open-close-clench cycle (OCC), in both ictal and interictal phases. It was hypothesized that the timing pattern activity of masseter would be various in different sources of pain (TTH and MOA) and phases (ictally and interictally).
Materials and Methods
Participants
The present study was a case-control study carried out at Tehran University of Medical Science and Health Services. A total of 35 adult females with primary headaches participated in this study. Patients were diagnosed by neurologist according to the criteria of the International Headache Society and were categorized in two groups TTH and MOA.7
All of participants had the following characteristics: 1) No surgery or trauma such as whiplash injury in the craniocervical region; 2) No history of orthodontic treatment; 3) No missing teeth, except for the second and/or the third molars.8
All subjects were examined for the occlusion class and the presence of TMJ sounds. The class of occlusion was determined based on Angle's classification method in which the relationship between upper and lower first molar and upper and lower canine was considered.9 For examination of TMJ sounds (click or crepitation) bilateral posterior palpation of TMJ through the external auditory meatus was performed by fingertips, and the participants was requested to open and close her jaw rhythmically in functional range (2-3 cm), so that the teeth did not collide. Those joints which had repeatable sounds or abnormal grinding were considered as having TMJ sounds.9, 10 The control group was free from signs and symptoms of headaches and temporomandibular disorders (TMD). They were matched with headache patients on the bases of sex, age, occlusion class and TMJ sounds. All subjects were fully informed about the nature of the study by signing on approval forms given by the university.
At interictal stage, the patients had no headache at least for 3 days before the test and at the time of EMG recording.8 Eighteen patients with MOA (31 ± 11 years old) and 12 patients (21 ± 2 years old) with TTH participated. They were matched with 22 (24 ± 7 years old) control women. Mean duration of headache was 12.2 ± 8.1 years for MOA and 4.8 ± 3 years for TTH group.
At ictal stage, study groups consisted of seven patients with MOA (38 ± 10 years old), and nine patients with TTH (23 ± 4 years old) who had pain during the test. To measure the pain intensity of headache visual analogue scale (VAS) was used. Score 0 corresponded to “no pain at all” and score 100 to “the worst imaginable pain”.11 Mean duration of headache was 14.7 ± 9.5 years for MOA and 5.1 ± 5.8 years for TTH group. The matched control group consisted of 15 women (25 ± 7 years old). Six MOA and 5 TTH patients participated in both stages.
EMG recording
The skin was cleaned by alcohol. Bipolar surface electrodes (Ag/Ag-Cl) with a 20 mm inter-electrode distance were filled by a specific gel and were placed on a line parallel to the masseter muscle fibers. They were then fixed with adhesive tape to reduce electrode impedance under 5 kΩ.8 The ground electrode was attached around the wrist. The EMG signal of the masseter muscle was recorded by using a 4-channel, amplified, differential electromyography (Premiere model, Medelec, with a CMRR > 100 db). The parameters of the recording were gain settings 500 µV/Div; sweep 1sec/Div. and band pass filter 10-500 Hz.
Motion recording
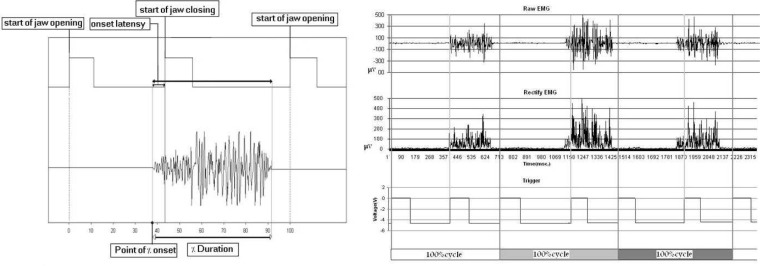
Mandibular motion in the sagittal plane was recorded by the mandibular motion recorder which was fixed on mandible and moved between two plates which adjusted between upper and lower mandible. The details of this device have been previously reported (Figure 1).12
Figure 1.
The method used to analyze timing of the masseter EMG; A) Desirable investigatory variables of masseter activity in one open-close-clench cycle and B) A sample of signal processing in one subject
Procedure
The participants were asked to sit on a chair with an arm rest and without a head rest. After placing and fixation devices (EMG electrodes and mandibular motion recording), they were instructed to maintain their head in the Frankfort horizontal position, the line parallel to the horizontal from the anterior cartilage of the ear to the inferior prominence of the orbit. To record the rest activity, the participants opened their mouth 1 cm in order to relax and minimize her masseter activity without lips contact.13 They were asked to complete cycles of jaw opening, closing and clenching the teeth. The speed of motion was standardized by a voice metronome (80 cycles/minute). The two EMG signals were recorded from both sides for 15 s (around 20 cycles) with 3 minutes intervals.
Analyzing process
The two signals (EMG and mandibular motion) were transferred synchronously to the computer by a 12-bite A/D board with a 1000 Hz sampling rate. The mean of 10 optimal cycles (with minimum noises and artifacts) from 40 cycles were selected for analysis.
To detect onset of masseter muscle, the rectified unfiltered EMG data was used by visual checking to ensure that the start time of masseter activity were not obscured by movement artifacts or other noises. Onset of muscle activity was defined the point at which its intensity was equal to the average of 200 ms of baseline plus two standard deviations.
The dependent variables included both absolute and normalized data: 1) Onset latency which means the interval between starting time of masseter activity and beginning of jaw closing, if the muscle activity proceeded jaw closing was considered as positive and vice versa; 2) Duration of the masseter activity in each OCC cycle; 3) Percentage of duration of masseter activity in each OCC cycle; and 4) Percentage of onset which means the point of the start time of masseter activity in OCC cycle (Figure 1). Last two variables were considered as normalized data in which variables were measured with regard to the OCC cycle (one cycle corresponds to 100%).
Statistics
One-way ANOVA and Tukey's post hoc tests were performed to analyze the EMG variables between 3 groups (control, TTH, MOA). Chi-Square was used to determine independency of occlusion and TMJ sounds in the study groups. The alpha level was set at 0.05.
Results
Chi-Square test did not illustrate significant difference in the distribution of TMJ sounds and classes of occlusion in three groups (control, TTH, MOA).
Interictal phase
The mean values and standard errors of timing factors of EMG in three groups during non-headache period are depicted in Table 1. As shown, no significant difference was appeared between MOA, TTH and control groups in the timing factors of EMG.
Table 1.
The mean and standard error of duration, onset, percentage of the duration, and onset of the masseter activity in open-close-clench cycle in left and right masseter in the interictal phase
| Duration (msec)† | Onset (msec)† | Percent duration (%)† | Percent onset (%)† | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Control (N = 22) | 351.6 ± 11 | 355.6 ± 10 | -2.2 ± 8.7 | -1.1 ± 8.5 | 46.6 ± 1.4 | 47.6 ± 1.6 | 41 ± 1.7 | 40.4 ± 1.6 |
| MOA‡ (N = 18) | 365.1 ± 11 | 387 ± 14 | 10 ± 8.3 | 15.8 ± 7.3 | 49.4 ± 1.4 | 51.6 ± 1.6 | 38 ± 2 | 36.5 ± 2.2 |
| TTH‡‡ (N = 12) | 375.9 ± 21 | 395.9 ± 20 | 7.8 ± 11 | 13.3 ± 8 | 50.6 ± 2.7 | 53.2 ± 2 | 35.1 ± 2 | 35.6 ± 2 |
Data are is presented as mean ± standard error; P < 0.05 by one-way ANOVA: Tukey's post-hoc comparison
Migraine without aura
Tension-type headache
The ictal phase
The mean values and standard errors of timing factors of EMG during headache period are demonstrated in Table 2. The analysis showed that both masseter muscles in the TTH and left masseter muscle in MOA patients in existence of pain activated significantly earlier than the control. The duration of left masseter was also significantly greater in the TTH than in the control group.
Table 2.
The mean and standard error of duration, onset, percentage of the duration and onset of the masseter activity in open-close-clench cycle in left and right masseter in the ictal phase
| Duration (msec)† | Onset (msec)† | Percent duration (%)† | Percent onset (%)† | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Control (N = 14) | 3326 ± 10 | 348.4 ± 15 | -19.2 ± 10.5 | -10.4 ± 9.4 | 44 ± 1.6 | 46.4 ± 2 | 44.5 ± 2 | 42 ± 2 |
| MOA‡ (N = 7) | 341.1 ± 13 | 347.5 ± 22 | 19.6 ± 10* | 26.2 ± 9 | 47.9 ± 1 | 48.5 ± 2 | 41.1 ± 2 | 39.2 ± 2 |
| TTH‡‡ (N = 9) | 405.6 ± 21** | 388.6 ± 14 | 29.7 ± 11** | 26 ± 11* | 53.7 ± 2.7* | 51.2 ± 2 | 35.3 ± 2* | 34 ± 2* |
Data are presented as mean ± standard error
P < 0.05
P < 0.005 by one-way ANOVA: Tukey's post-hoc comparison
Migraine without aura
Tension-type headache
Discussion
The aim of the present study was to investigate the timing pattern of EMG of the masseter muscles in three groups, TTH, MOA and control in two stages, interictally and ictally. The results showed that there was no significant difference between three groups in all variables interictally while in the ictal phase, the onset and percentage of onset of the both masseter muscle activity in TTH and onset of left side in MOA were significantly earlier. Investigation of the duration and percentage of duration of EMG revealed that they were longer only in left side of TTH group than in the control group. Therefore, it was demonstrated that alternation timing pattern of masseter muscle influenced by the existence of pain.
The physiologic mechanisms responsible for the alternation of masticatory muscles in headaches patients are poorly understood. The origin of pain in chronic tension type headache and migraine has still remained controversial.14–16 However, it is apparent that nociceptive input can have potent effects on the motor control functions of the central nervous system. Both peripheral and central mechanisms have been proposed as explanations of the physiologic changes that occur in the motor system as a result of pain.17
The results of this study confirmed previous ones, which stated muscles are more involved in TTH than in migraine based on the supraspinal-myogenic model.3, 18 Earlier and greater activity of masseter muscle during OCC in the headache patients might be direct effect of pain input on coordination between muscle spindle, interneurons of the central pattern generator (CPG) and trigeminal motor neuron as well as higher centers.17, 19
In contradiction to studies that have declared peripheral nociceptive mechanisms does not have a direct effect on amplitude of muscle activity,3, 20, 21 our results showed that the peripheral nociceptor inputs may have a temporary and selective modulation role in the pattern of muscle activity, because some changes in motor function of masticatory muscles that occur in the presence of pain in headaches patients were absent during headache-free periods.
According to the results of the current study, it seems onset of activity of masseter muscle could be more sensitive and reliable to evaluate the effect of pain on motor activity system than duration and amplitude of muscle contraction. Further studies need to be carried out to investigate timing activity pattern during functional movement prior to, during, and following headaches to evaluate underlying mechanisms involved in different kinds of headaches.
Conclusion
This study showed that headaches such as tension-type headache and migraine without aura ictally could change the timing of the masseter muscle activity especially the onset of EMG, but TTH indicated more obvious changes.
Acknowledgements
The authors gratefully thank Prof. Yaghoob Fathollahi PhD for his scientific assistance and Mehdi Hatef MSc for editing. We also appreciate the financial support of Rehabilitation Faculty of Tehran Medical University which made this project possible.
Conflict of interest statement: The authors attest that there is no conflict of interests on this article.
Grant Support: Physical Therapy Department, Rehabilitation Faculty, Tehran University of Medical Science and Health Services.
References
- 1.Vogt L, Pfeifer K, Banzer W. Neuromuscular control of walking with chronic low-back pain. Man Ther. 2003;8(1):21–8. doi: 10.1054/math.2002.0476. [DOI] [PubMed] [Google Scholar]
- 2.Munro RR. Electromyography of the masseter and anterior temporalis muscles in the open-close-clench cycle in temporomandibular joint dysfunction. Monogr Oral Sci. 1975;4:117–25. doi: 10.1159/000397869. [DOI] [PubMed] [Google Scholar]
- 3.Jensen R, Fuglsang-Frederiksen A, Olesen J. Quantitative surface EMG of pericranial muscles in headache. A population study. Electroencephalogr Clin Neurophysiol. 1994;93(5):335–44. doi: 10.1016/0168-5597(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia R, Dureja GP, Tripathi M, et al. Role of temporalis muscle over activity in chronic tension type headache: effect of yoga based management. Indian J Physiol Pharmacol. 2007;51(4):333–44. [PubMed] [Google Scholar]
- 5.Peterson AL, Talcott GW, Kelleher WJ, et al. Site specificity of pain and tension in tension-type headaches. Headache. 1995;35(2):89–92. doi: 10.1111/j.1526-4610.1995.hed3502089.x. [DOI] [PubMed] [Google Scholar]
- 6.Shumway-Cook A, Woollacott MH. Motor Control: Translating Research Into Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 7.The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 8.Burnett CA, Fartash L, Murray B, et al. Masseter and temporalis muscle EMG levels and bite force in migraineurs. Headache. 2000;40(10):813–7. doi: 10.1046/j.1526-4610.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- 9.Jagger RG, Bates JF, Kopp JF. Temporomandibular joint dysfunction: the essentials. 1st ed. Costa Mesa, CA: Wright; 1994. [Google Scholar]
- 10.Hertling D, Kessler RM. Management of Common Musculoskeletal Disorders: Physical Therapy Principles and Methods. 3rd ed. Philadelphia, PA: Lippincott Williams&Wilki; 1996. p. 15. [Google Scholar]
- 11.Huskisson EC. Measurement of pain. Lancet. 1974;2(7889):1127–31. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 12.Hatef B, Talebain S, Oliyae GR, et al. Effect of Tempromandibular Joint Sounds on Timing of the Masseter Muscle Activity in the Open-close-clench Cycle. Journal of Medical Sciences. 2007;7(3):339–46. [Google Scholar]
- 13.Manns A, Miralles R, Guerrero F. The changes in electrical activity of the postural muscles of the mandible upon varying the vertical dimension. J Prosthet Dent. 1981;45(4):438–45. doi: 10.1016/0022-3913(81)90109-8. [DOI] [PubMed] [Google Scholar]
- 14.Vargas BB. Tension-type headache and migraine: two points on a continuum? Curr Pain Headache Rep. 2008;12(6):433–6. doi: 10.1007/s11916-008-0073-7. [DOI] [PubMed] [Google Scholar]
- 15.Jensen R. Pathophysiological mechanisms of tension-type headache: a review of epidemiological and experimental studies. Cephalalgia. 1999;19(6):602–21. doi: 10.1046/j.1468-2982.1999.019006602.x. [DOI] [PubMed] [Google Scholar]
- 16.Cutrer FM. Pathophysiology of migraine. Semin Neurol. 2010;30(2):120–30. doi: 10.1055/s-0030-1249222. [DOI] [PubMed] [Google Scholar]
- 17.Sterling M, Jull G, Wright A. The effect of musculoskeletal pain on motor activity and control. J Pain. 2001;2(3):135–45. doi: 10.1054/jpai.2001.19951. [DOI] [PubMed] [Google Scholar]
- 18.Olesen J. Clinical and pathophysiological observations in migraine and tension-type headache explained by integration of vascular, supraspinal and myofascial inputs. Pain. 1991;46(2):125–32. doi: 10.1016/0304-3959(91)90066-7. [DOI] [PubMed] [Google Scholar]
- 19.Westberg KG, Clavelou P, Schwartz G, et al. Effects of chemical stimulation of masseter muscle nociceptors on trigeminal motoneuron and interneuron activities during fictive mastication in the rabbit. Pain. 1997;73(3):295–308. doi: 10.1016/S0304-3959(97)00103-6. [DOI] [PubMed] [Google Scholar]
- 20.Lipchik GL, Holroyd KA, O'Donnell FJ, et al. Exteroceptive suppression periods and pericranial muscle tenderness in chronic tension-type headache: effects of psychopathology, chronicity and disability. Cephalalgia. 2000;20(7):638–46. doi: 10.1111/j.1468-2982.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- 21.Peddireddy A, Wang K, Svensson P, Arendt-Nielsen L. Stretch reflex and pressure pain thresholds in chronic tension-type headache patients and healthy controls. Cephalalgia. 2009;29(5):556–65. doi: 10.1111/j.1468-2982.2008.01772.x. [DOI] [PubMed] [Google Scholar]