Abstract
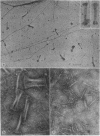
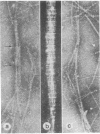
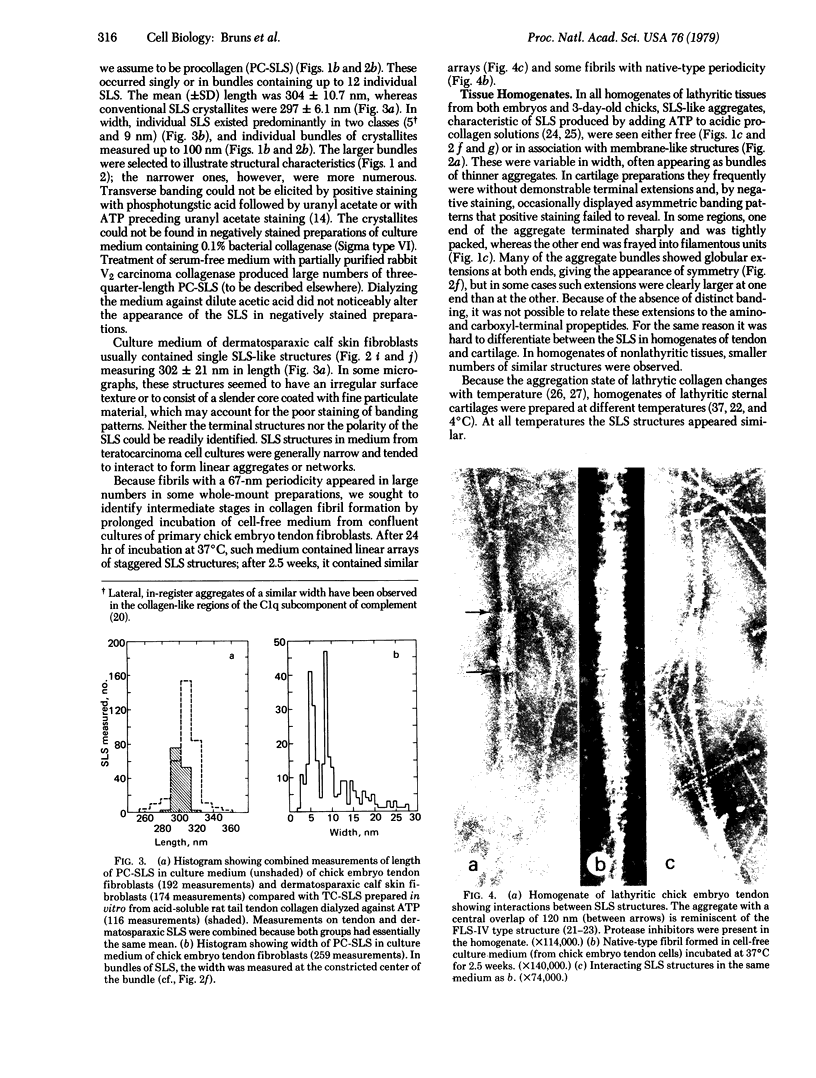
Naturally occurring segment-long-spacing crystallites of procollagen collagen have been found in the culture medium of fibroblasts from chick embryo tendon, human skin, and dermatosparaxic calf skin; in whole-mount preparations of cultured human skin fibroblasts; in homogenates of lathyritic chick embryo tendon, cartilage, and cornea; and in a partially purified preparation of procollagen. Bundles of similar aggregates occurred within secretory vacuoles of collagen-synthesizing fibroblasts and chondrocytes. These observations suggest that fibroblasts and chondrocytes secrete procollagen assemblies that are stable in the extracellular environment. We propose that subsequent enzymatic processing is accompanied by direct incorporation of such structures into the assembling fibril, which then may be considered as an n X 67 nm staggered array of segment-long-spacing crystallites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns R. R. A symmetrical, extracellular fibril. J Cell Biol. 1969 Aug;42(2):418–430. doi: 10.1083/jcb.42.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church R. L., Yaeger J. A., Tanzer M. L. Isolation and partial characterization of procollagen fractions produced by a clonal strain of calf dermatosparactic cells. J Mol Biol. 1974 Jul 15;86(4):785–799. doi: 10.1016/0022-2836(74)90354-4. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., McEneany L. S., Bornstein P. Intermediates in the conversion of procollagen to collagen. Evidence for stepwise limited proteolysis of the COOH-terminal peptide extensions. Eur J Biochem. 1977 Dec 1;81(2):349–355. doi: 10.1111/j.1432-1033.1977.tb11958.x. [DOI] [PubMed] [Google Scholar]
- Doyle B. B., Hukins D. W., Hulmes D. J., Miller A., Woodhead-Galloway J. Collagen polymorphism: its origins in the amino acid sequence. J Mol Biol. 1975 Jan 5;91(1):79–99. doi: 10.1016/0022-2836(75)90373-3. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- GROSS J. THERMAL DENATURATION OF COLLAGEN IN THE DISPERSED AND SOLID STATE. Science. 1964 Feb 28;143(3609):960–961. doi: 10.1126/science.143.3609.960. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The route of secretion of procollagen. The influence of alphaalpha'-bipyridyl, colchicine and antimycin A on the secretory process in embryonic-chick tendon and cartilage cells. Biochem J. 1976 Apr 15;156(1):81–90. doi: 10.1042/bj1560081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H. P., Olsen B. R., Chen H. T., Prockop D. J. Segment-long-spacing aggregates and isolation of COOH-terminal peptides from type I procollagen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4304–4308. doi: 10.1073/pnas.73.12.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura S., Tanaka S., Takase B. Intracytoplasmic segment long spacing fibrils in chondrosarcoma. J Electron Microsc (Tokyo) 1975;24(2):87–95. [PubMed] [Google Scholar]
- Lichtenstein J. R., Byers P. H., Smith B. D., Martin G. R. Identification of the collagenous proteins synthesized by cultured cells from human skin. Biochemistry. 1975 Apr 22;14(8):1589–1594. doi: 10.1021/bi00679a007. [DOI] [PubMed] [Google Scholar]
- Olsen B. R. Electron microscope studies on collagen. IV. Structure of vitrosin fibrils and interaction properties of vitrosin molecules. J Ultrastruct Res. 1965 Aug;13(1):172–191. doi: 10.1016/s0022-5320(65)80095-8. [DOI] [PubMed] [Google Scholar]
- PORTER K. R. Observations on a submicroscopic basophilic component of cytoplasm. J Exp Med. 1953 May;97(5):727–750. doi: 10.1084/jem.97.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. I., Bissell M. J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton E., Yonemasu K., Stroud R. M. Ultrastructure of the human complement component, Clq (negative staining-glutamine synthetase-biologically active Clq). Proc Natl Acad Sci U S A. 1972 Jan;69(1):65–68. doi: 10.1073/pnas.69.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Coulombre A. J. Morphogenesis of the collagenous stroma in the chick cornea. J Cell Biol. 1971 Sep;50(3):840–858. doi: 10.1083/jcb.50.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Gross J. Collagen fibrillogenesis: intermediate aggregates and suprafibrillar order. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4027–4031. doi: 10.1073/pnas.73.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Hoffmann H. P., Prockop D. J. Purification and partial characterization of the type II procollagen synthesized by embryonic cartilage cells. Arch Biochem Biophys. 1977 Mar;179(2):654–662. doi: 10.1016/0003-9861(77)90154-0. [DOI] [PubMed] [Google Scholar]
- Veis A., Brownell A. G. Collagen biosynthesis. CRC Crit Rev Biochem. 1975 Feb;2(4):417–453. doi: 10.3109/10409237509102549. [DOI] [PubMed] [Google Scholar]
- Warshawsky H. The presence of atypical collagen fibrils in EDTA decalcified predentine and dentine of rat incisors. Arch Oral Biol. 1972 Dec;17(12):1745–1754. doi: 10.1016/0003-9969(72)90238-5. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Centrosymmetrical cross-banded structures in the matrix of rat incisor predentin and dentin. J Ultrastruct Res. 1977 Nov;61(2):218–229. doi: 10.1016/s0022-5320(77)80089-0. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Synthesis, migration, and release of precursor collagen by odontoblasts as visualized by radioautography after (3H)proline administration. J Cell Biol. 1974 Jan;60(1):92–127. doi: 10.1083/jcb.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]