Abstract
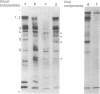
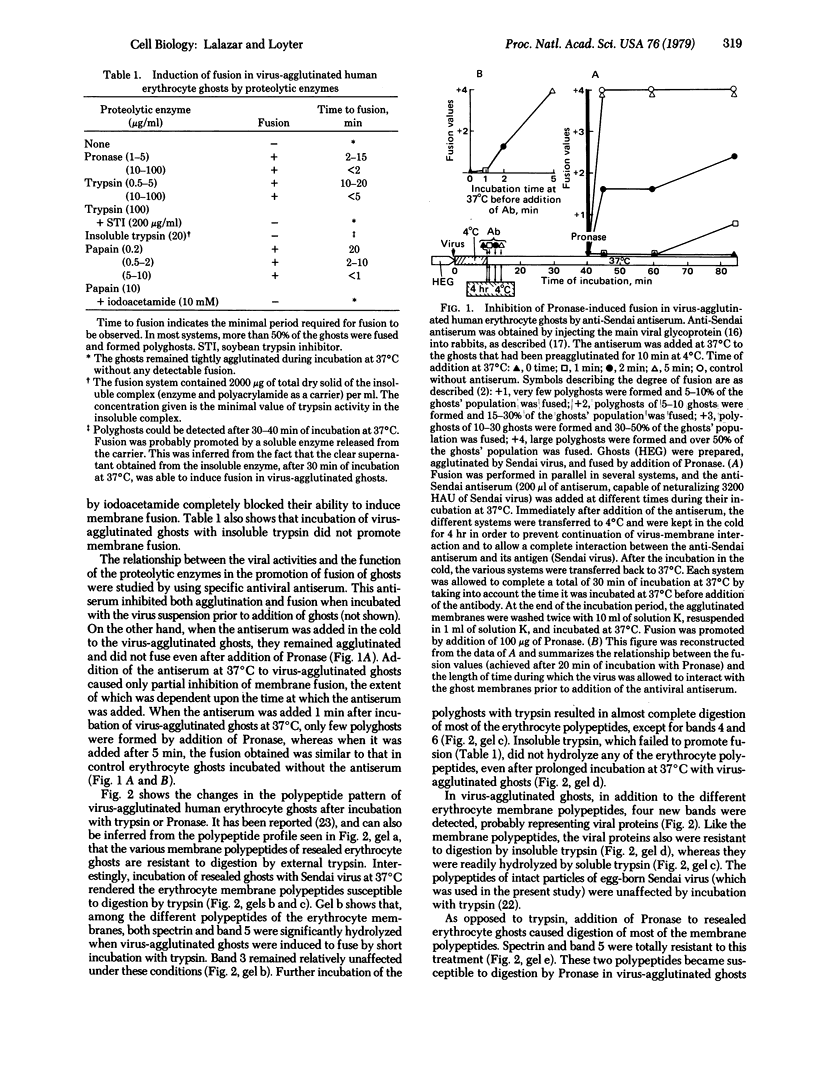
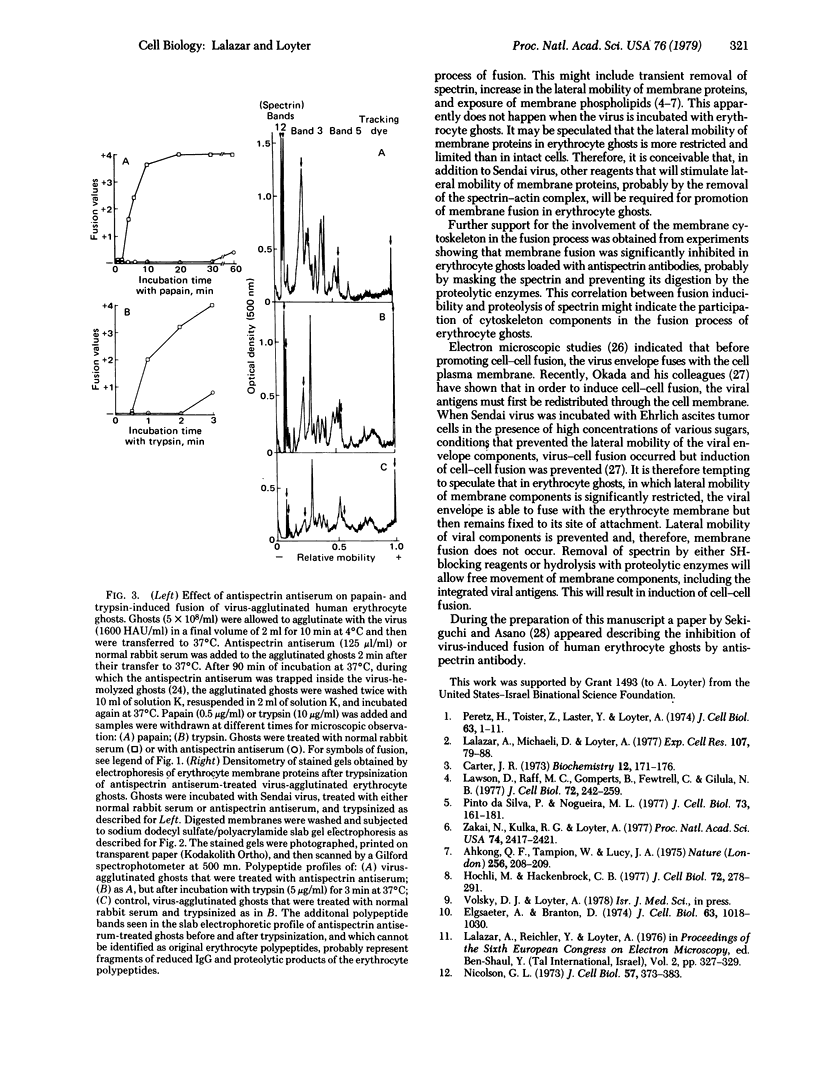
In contrast to intact human erythrocytes, human erythrocyte ghosts can be agglutinated but not fused by Sendai virus. Membrane fusion can, however, be induced in virus-agglutinated erythrocyte ghosts by addition of proteolytic enzymes such as trypsin, papain, or Pronase. When erythrocyte ghosts were reacted with antispectrin antiserum, the antiserum inhibited both the induction of fusion and the proteolysis of the membrane spectrin. The correlation between the membrane fusion process and the membrane cytoskeleton is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Tampion W., Lucy J. A. Promotion of cell fusion by divalent cation ionophores. Nature. 1975 Jul 17;256(5514):208–209. doi: 10.1038/256208a0. [DOI] [PubMed] [Google Scholar]
- Carter J. R., Jr Role of sulfhydryl groups in erythrocyte membrane structure. Biochemistry. 1973 Jan 2;12(1):171–176. doi: 10.1021/bi00725a028. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. I. The effects of protein removal. J Cell Biol. 1974 Dec;63(3):1018–1036. doi: 10.1083/jcb.63.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höchli M., Hackenbrock C. R. Thermotropic lateral translational motion of intramembrane particles in the inner mitochondrial membrane and its inhibition by artificial peripheral proteins. J Cell Biol. 1977 Feb;72(2):278–291. doi: 10.1083/jcb.72.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalazar A., Michaeli D., Loyter A. Restoration of the fusion capacity of human erythrocyte ghosts by SH blocking reagents. Exp Cell Res. 1977 Jun;107(1):79–88. doi: 10.1016/0014-4827(77)90388-3. [DOI] [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepke S., Passow H. Effects of incorporated trypsin on anion exchange and membrane proteins in human red blood cell ghosts. Biochim Biophys Acta. 1976 Dec 2;455(2):353–370. doi: 10.1016/0005-2736(76)90311-4. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Kim J., Koseki I., Mekada E., Shiokawa Y., Okada Y. Modification of cell membranes with viral envelopes during fusion of cells with HVJ (Sendai virus). III. Effects of mono- and di-saccharides on cell fusion and membrane movement of fused cells. Exp Cell Res. 1977 Aug;108(1):95–106. doi: 10.1016/s0014-4827(77)80014-1. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Isolation of spectrin from erythrocyte membranes. Methods Enzymol. 1974;32:275–277. doi: 10.1016/0076-6879(74)32028-9. [DOI] [PubMed] [Google Scholar]
- Mueller T. J., Morrison M. The transmembrane proteins in the plasma membrane of normal human erythrocytes. Evaluation employing lactoperoxidase and proteases. Biochemistry. 1975 Dec 16;14(25):5512–5516. doi: 10.1021/bi00696a020. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Anionic sites of human erythrocyte membranes. I. Effects of trypsin, phospholipase C, and pH on the topography of bound positively charged colloidal particles. J Cell Biol. 1973 May;57(2):373–387. doi: 10.1083/jcb.57.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz H., Toister Z., Laster Y., Loyter A. Fusion of intact human erythrocytes and erythrocyte ghosts. J Cell Biol. 1974 Oct;63(1):1–11. doi: 10.1083/jcb.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Nogueira M. L. Membrane fusion during secretion. A hypothesis based on electron microscope observation of Phytophthora Palmivora zoospores during encystment. J Cell Biol. 1977 Apr;73(1):161–181. doi: 10.1083/jcb.73.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Asano A. Participation of spectrin in Sendai virus-induced fusion of human erythrocyte ghosts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1740–1744. doi: 10.1073/pnas.75.4.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto J. T., Becht H., Rott R. Effect of specific antibodies on biological functions of the envelope components of Newcastle disease virus. Virology. 1974 Oct;61(2):354–360. doi: 10.1016/0042-6822(74)90273-6. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Kirshner N., Schramm M. Non-parallel transport of membrane proteins and content proteins during assembly of the secretory granule in rat parotid gland. Biochim Biophys Acta. 1975 Jan 14;375(1):87–105. doi: 10.1016/0005-2736(75)90074-7. [DOI] [PubMed] [Google Scholar]
- Yu J., Branton D. Reconstitution of intramembrane particles in recombinants of erythrocyte protein band 3 and lipid: effects of spectrin-actin association. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3891–3895. doi: 10.1073/pnas.73.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakai N., Kulka R. G., Loyter A. Membrane ultrastructural changes during calcium phosphate-induced fusion of human erythrocyte ghosts. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2417–2421. doi: 10.1073/pnas.74.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]