Background: Nrf2 is required for normal dendritic cell immune functions.
Results: Loss of Nrf2 alters DC function and results in hyperphosphorylation of CREB/ATF1 transcription factors that are responsive to p38 MAPK inhibition.
Conclusion: The p38 MAPK-CREB/ATF1 axis contributes to Nrf2-mediated regulation of DC function.
Significance: Defining the relevance of p38-CREB/ATF1 in Nrf2 signaling expands understanding of DC biology.
Keywords: Dendritic Cells, Heme Oxygenase, Nrf2, p38 MAPK, Reactive Oxygen Species (ROS), CREB/ATF1, Co-stimulation
Abstract
Nrf2 is a redox-responsive transcription factor that has been implicated in the regulation of DC immune function. Loss of Nrf2 results in increased co-stimulatory molecule expression, enhanced T cell stimulatory capacity, and increased reactive oxygen species (ROS) levels in murine immature DCs (iDCs). It is unknown whether altered immune function of Nrf2-deficient DCs (Nrf2−/− iDCs) is due to elevated ROS levels. Furthermore, it is unclear which intracellular signaling pathways are involved in Nrf2-mediated regulation of DC function. Using antioxidant vitamins to reset ROS levels in Nrf2−/− iDCs, we show that elevated ROS is not responsible for the altered phenotype and function of these DCs. Pharmacological inhibitors were used to explore the role of key MAPKs in mediating the altered phenotype and function in Nrf2−/− iDCs. We demonstrate that the increased co-stimulatory molecule expression (MHC II and CD86) and antigen-specific T cell activation capacity observed in Nrf2−/− iDCs was reversed by inhibition of p38 MAPK but not JNK. Importantly, we provide evidence for increased phosphorylation of cAMP-responsive element binding protein (CREB) and activating transcription factor 1 (ATF1), transcription factors that are downstream of p38 MAPK. The increased phosphorylation of CREB/ATF1 in Nrf2−/− iDCs was sensitive to p38 MAPK inhibition. We also show data to implicate heme oxygenase-1 as a potential molecular link between Nrf2 and CREB/ATF1. These results indicate that dysregulation of p38 MAPK-CREB/ATF1 signaling axis underlies the altered function and phenotype in Nrf2-deficient DCs. Our findings provide new insights into the mechanisms by which Nrf2 mediates regulation of DC function.
Introduction
Dendritic cells (DCs)3 are antigen-presenting cells pivotal for the induction of primary adaptive immune responses. Immature DCs (iDCs) express low levels of MHC II and other co-stimulatory molecules such as CD80, CD86, and CD40, with limited capacity to induce antigen-specific T-cell activation. DC maturation is associated with up-regulation of co-stimulatory molecules and cytokine production, renders the DCs competent in T cell activation and elicitation of an immune response (1). The MAPKs represent vital intracellular signaling pathways that regulate a variety of cellular processes, including cell differentiation, proliferation, and apoptosis. There are at least three distinct MAPK pathways in mammals, including extracellular signal-regulated 1/2 kinases (ERK1/2), JNK, and p38 MAPK (2, 3). MAPKs activation is important for regulation of DC maturation, survival, and cytokine secretion (4). Importantly, the p38 MAPK pathway has been shown to regulate DC co-stimulatory receptor expression, T cell proliferative capabilities, and cytokine production (5, 6). Signaling through the p38 MAPK pathway results in the downstream activation of cAMP-responsive element binding protein (CREB) and activating transcription factor 1 (ATF1) (7). Phosphorylation of CREB is known to be associated with up-regulation of CD86 and secretion of the cytokine, IL-10 (8, 9).
Redox homeostasis is important for a variety of cellular functions such as proliferation, apoptosis, and intracellular signaling pathways, including MAPK signaling (10–12). In the context of DCs, alterations in cellular reactive oxygen species (ROS) and redox status result in changes in immune function such as maturation and cytokine production, which subsequently impacts on the type of T cell immune response elicited (13, 14). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox-sensitive, basic-leucine zipper transcription factor (15, 16). Nrf2 is expressed in a variety of cell types, including DCs, where it contributes to maintenance of redox homeostasis (17, 18) by regulating key cytoprotective/antioxidant genes, including glutathione (GSH), heme oxygenase-1 (HO-1), NAD(P)H:quinine oxidoreductase 1, and superoxide dismutases (19). Hemo-oxygenase-1 is a rate-limiting enzyme in the catabolism of heme and exhibits antioxidant, anti-inflammatory, anti-apoptotic and immunomodulatory properties, and has been implicated in DC differentiation and maturation (20–25).
We and others (14, 26) have shown that loss of Nrf2 in iDCs results in increased intracellular ROS, enhanced co-stimulatory molecules expression, impaired antigen capture capacity, and enhanced capacity for antigen-specific CD8 T cell stimulation. However, it is not known whether the elevated ROS level, in the absence of Nrf2, is responsible for the changes in the DC immune function. Furthermore, it is not clear what signaling pathways are involved in mediating the changes observed in the absence of Nrf2. In this study, using Nrf2-deficient iDCs, we demonstrate that elevated ROS levels do not underlie altered immune function in these DCs. We also show that functional changes associated with the loss of Nrf2 in iDCs is sensitive to pharmacological inhibition of p38 MAPK but not JNK activity. Importantly, we demonstrate that CREB and ATF1 are hyperphosphorylated in the absence of Nrf2. Our results also show that CREB and ATF1 hyperphosphorylation can be induced through inhibition of HO-1 activity. Our findings highlight the importance of the p38 MAPK-CREB signaling axis in Nrf2-mediated regulation of DC immune function.
EXPERIMENTAL PROCEDURES
Reagents
All reagents were from Sigma-Aldrich unless otherwise stated. FCS (Invitrogen), SB203580 and PD98059 (Cell Signaling Technology, Danvers, MA), and tin protoporphyrin IX dichloride (Tocris Bioscience, Bristol, UK) were also purchased for the study.
Mice
Nrf2+/+ and Nrf2−/− mice were purchased from Riken BioResource Center (Ibaraki, Japan) and maintained at the Biomedical Services Unit, University of Liverpool (27). Mice transgenic for the H-2Db-restricted TCR-αβ transgene, F5, were a kind gift from Dr. James Matthews (Cardiff, Wales, UK). Protocols described herein were undertaken in accordance with criteria outlined in license granted under the Animals (Scientific Procedures) Act 1986 (PPL 40/3379).
Generation of Bone Marrow-derived DCs
Bone marrow-derived iDCs were generated from Nrf2+/+ and Nrf2−/− mice according to published protocol (28). On day 6 cells were harvested, and CD11c-positive DCs were isolated using magnetic beads by positive selection according to the manufacturer's instructions (MiltenyiBiotec, Surrey, UK). The purity of the isolated DC population was >90% as determined by flow cytometry on a BD FACSCanto II flow cytometer (BD Biosciences, Oxford, UK).
Cell Surface Receptor Expression
DCs were stained with fluorescent αCD11cTC (Invitrogen) and αCD86FITC, or αMHCIIPE (BD Biosciences) antibodies for 30 min on ice, washed, acquired on a BD FACSCanto II flow cytometer (BD Biosciences), and analyzed using Cyflogic software (version 1.2.1, CyFlo, Ltd.).
Measurement of ROS
Basal or vitamin-treated iDCs from Nrf2+/+ and Nrf2−/− mice were stained using fluorescent ROS indicator, dihydroethidium, according to Ref. 29, and analyzed by flow cytometry.
F5 CD8 T Cell Proliferation
F5 CD8 T cell proliferation was quantified as described previously (30). Briefly, Nrf2+/+ and Nrf2−/− iDCs were pulsed with a dose range of antigenic peptide (NP68), washed, and co-cultured with F5 CD8 T cells for 72 h. [3H]Thymidine was added for the last 16 h. Cells were harvested onto glass fiber filter mats and read on a scintillation counter (MicroBetaTrilux; PerkinElmer Life Sciences, Buckinghamshire, UK).
Gel Electrophoresis and Western Immunoblotting
Nrf2+/+ and Nrf2−/− iDCs were lysed, and 20 μg of lysate protein was resolved by SDS-PAGE, transferred to nitrocellulose membranes (GE Healthcare), blocked, and probed for the indicated proteins using the appropriate primary antibodies; phospho-p38, p38, phospho-CREB, CREB (Cell Signaling Technology, Danvers, MA); and α-tubulin (Santa Cruz Biotechnology) followed by horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) and visualized using the ECL system (PerkinElmer Life Sciences).
ELISA
Cell-free culture supernatants were used for measuring IL-10 cytokine concentrations by sandwich ELISA (Quantikine, R&D Systems, Abingdon, UK). On day 6, DCs were seeded onto 24-well plates in duplicates at a density of 8 × 105 DCs per ml at 2 ml/well. After 48 h, cell-free supernatants from SB203580 untreated and treated groups with or without the Toll-like receptor agonist LPS were collected and stored at −20 °C until further analysis.
Statistics
Raw data obtained were analyzed using the unpaired t test or one-way ANOVA. p values < 0.05 were considered to be statistically significant.
RESULTS
Altered Immature DC Function Due to the Loss of Nrf2 Is Not Dependent on Elevated ROS
Loss of Nrf2 leads to increased co-stimulatory molecule expression, T cell stimulatory potential, and elevated ROS levels in iDCs (14, 26). We investigated whether the elevated ROS contributed to increased co-stimulatory molecules expression by reducing ROS to normal levels using antioxidants in these cells. Vitamins C and E have antioxidant activity and are known to reduce ROS levels (31, 32). Nrf2+/+ and Nrf2−/− iDCs were treated with vitamins C and E for 48 h, and ROS levels measured by flow cytometry using the fluorescent ROS indicator dihydroethidium. A significant reduction in ROS levels was observed in vitamin-treated Nrf2−/− iDCs compared with untreated controls (mean fluorescence intensity, 2079 versus 938, p < 0.05) as shown in Fig. 1A. A slight reduction in the ROS levels was also seen in vitamin-treated Nrf2+/+ iDCs, which was not statistically significant (mean fluorescence intensity 1230 versus 878, p > 0.05) (Fig. 1A). It is important to note that the ROS levels in vitamin-treated Nrf2−/− iDCs was equivalent to that in untreated Nrf2+/+ iDCs (Fig. 1A). To test whether elevated intracellular redox levels is responsible for increased DC co-stimulatory molecule expression, we measured MHC II and CD86 expression in Nrf2−/− iDCs following vitamins treatment. As shown in Fig. 1B, the expression of MHC II and CD86 under basal condition was significantly higher in the Nrf2−/− iDCs compared with Nrf2+/+ iDCs (MHC II 43.2% versus 23.1%, p < 0.05; CD86 34.8% versus 18.4%, p < 0.05). However, there was no significant difference in the co-stimulatory molecules expression between untreated controls and vitamins treatment groups in both Nrf2+/+ (MHC II 23.1% versus 21.2% p > 0.05; CD86 18.4% versus 17.2%, p > 0.05) and Nrf2−/− iDCs (MHC II 43.2% versus 42.6% p > 0.05; CD86 34.8% versus 35.2%, p > 0.05). This result indicates that restoring ROS levels in Nrf2−/− to Nrf2+/+ status did not reverse co-stimulatory molecule expression. We further investigated whether the lack of change in co-stimulatory molecule expression in Nrf2−/− iDCs to ROS reset is also reflected in its ability to induce antigen-specific T cell activation. To determine this, we utilized a TCR transgenic mouse model wherein the CD8 T cells express a T cell receptor (F5 TCR) that responds to an antigenic peptide, NP68, when presented by DCs (33). Using this system, we have previously shown that NP68-bearing Nrf2−/− iDCs stimulated F5 CD8 T cell proliferation more potently than its wild type counterpart (26). Consistent with our previous findings, antigenic peptide-bearing Nrf2−/− iDCs induced higher F5 CD8 T cell proliferation compared with Nrf2+/+ iDCs (Fig. 1C). Lowering of ROS levels by vitamin treatment did not reduce the potential of NP68-bearing Nrf2−/− iDCs to stimulate F5 CD8 T cell proliferation (Fig. 1C). These results demonstrate that altered ROS status associated with loss of Nrf2 does not contribute to increased co-stimulatory molecules expression and T cell stimulatory potential of DCs.
FIGURE 1.
Reducing ROS levels does not restore altered phenotype and function of Nrf2−/− iDCs. Nrf2+/+ and Nrf2−/− iDCs were treated with or without vitamins C (1 mm) and E (100 μm) for 48 h. A, cells were incubated with the ROS indicator dihydroethidium and analyzed by flow cytometry. Data are presented as average mean fluorescence intensity ± S.D. and derived from three independent experiments. B, DCs were labeled with fluorescent conjugated antibodies against MHC II and CD86 co-stimulatory molecules. Co-stimulatory molecule expression was determined by flow cytometry. The percentages of iDCs expressing high levels MHC II (i) and CD86 (ii) are indicated above the marker. Representative histograms are presented with average percentage ± S.D. Data are derived from three independent experiments. C, Nrf2+/+ (i) or Nrf2−/− (ii) iDCs were pulsed with increasing concentrations of NP68 antigenic peptide and then co-cultured with F5 CD8 T cells for 72 h. [3H]Thymidine (3H-Thy) was added for the last 16 h. Proliferation of T cells was determined by scintillation counting of incorporated [3H]thymidine. Data are presented as average [3H]thymidine scintillation counts ± S.D. Statistical significance was assessed using unpaired Student's t test or one-way ANOVA. Data are representative of three independent experiments (*, p < 0.05; NS, not significant).
Contribution of p38 MAPK Signaling to Nrf2-dependent Regulation of DC Immune Function
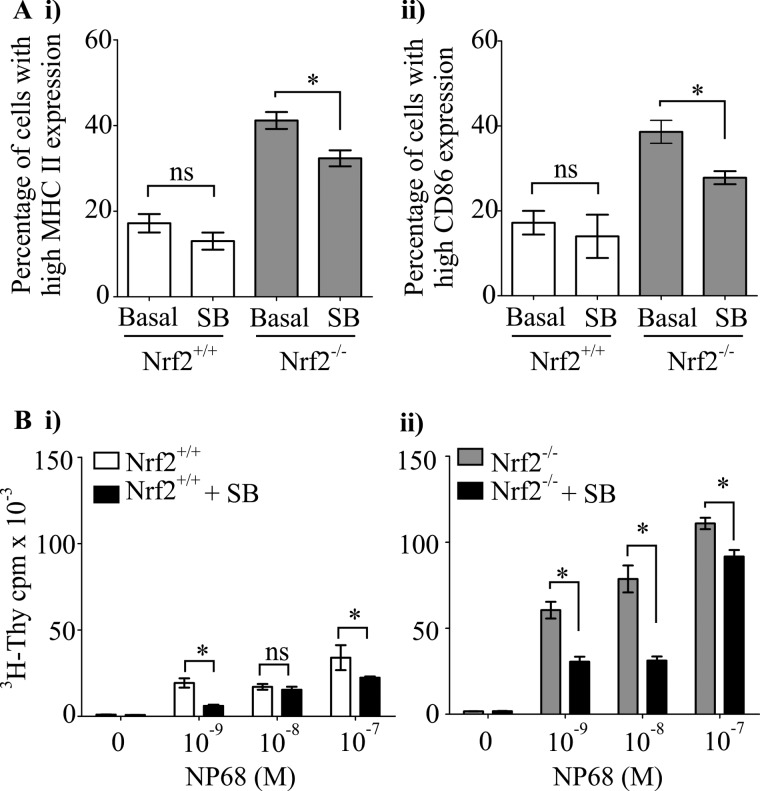
Mitogen-activated protein kinase signaling comprising of ERK1/2, p38 MAPK, and JNK pathways coordinate DCs co-stimulatory molecules expression and DC-mediated T cell activation (5, 34, 35). Using pharmacological approaches, we have previously shown that ERK1/2 was not critical in mediating Nrf2-dependent modulation of DC function (26). In the current study, we investigated the role of p38 MAPK and JNK in this context using synthetic inhibitors of JNK and p38 MAPK (JNK-specific inhibitor, SP600125; p38 activity inhibitor, SB203580). JNK inhibition did not reverse the enhanced co-stimulatory molecules expression in Nrf2−/− iDCs to that of the wild type levels (MHC II, 42.8% versus 47.0%; p > 0.05; CD86, 44.0% versus 51.8%; p > 0.05 in Nrf2−/− iDCs; and MHC II, 21.3% versus 20.5%, p > 0.05; CD86, 20.0% versus 21.0%, p > 0.05 in Nrf2+/+ iDCs) as indicated in Fig. 2A. The iDC-mediated antigen-specific CD8 T cell proliferation also remained unaltered following JNK inhibition in Nrf2−/− and Nrf2+/+ iDCs (Fig. 2B). However, inhibition of p38 MAPK activity caused a significant reduction in co-stimulatory molecule expression in Nrf2−/− iDCs (Fig. 3A) (MHC II, 41.0% versus 32.3%, p < 0.05; CD86, 38.6% versus 27.8%, p < 0.05). Inhibition of p38 MAPK in Nrf2+/+ iDCs resulted in only a slight, statistically insignificant reduction in co-stimulatory molecules expression (MHC II, 17.2% versus 13.0%, p > 0.05; CD86, 17.2% versus 14.0%, p > 0.05). Consistent with the changes in co-stimulatory molecule expression, inhibition of p38 MAPK resulted in significant reductions in DC-mediated antigen-specific CD8 T cell proliferation in Nrf2−/− iDCs with less pronounced effects on Nrf2+/+ iDCs (Fig. 3B). These observations suggest the contribution of p38 MAPK but not JNK in the Nrf2-dependent modulation of DC immune functions.
FIGURE 2.
JNK activity is not required for increased co-stimulatory molecule expression of Nrf2−/− iDCs and T cell activation. Nrf2+/+ and Nrf2−/− iDCs treated with or without 10 μm of JNK inhibitor, SP600125 (SP) for 48 h. A, MHC II (i) and CD86 (ii) expression was determined by flow cytometry and presented as percentage of cells expressing high MHC II or CD86. Data derived from three independent experiments are presented as average percentage ± S.D. B, Nrf2+/+ (i) and Nrf2−/− (ii) iDCs were pulsed with increasing concentrations of NP68 antigenic peptide and co-cultured with F5 CD8 T cells for 72 h. [3H]Thymidine (3H-Thy) was added for the last 16 h. Proliferation of T cells was determined by scintillation counting of incorporated [3H]thymidine. Data are presented as average [3H]thymidine scintillation counts ± S.D. Statistical significance was assessed using unpaired Student's t test or one-way ANOVA. Data are representative of three independent experiments (NS, not significant).
FIGURE 3.
Contribution of p38 MAPK activity toward altered phenotype and function of Nrf2−/− iDCs. Nrf2+/+ and Nrf2−/− iDCs treated with or without 20 μm of p38 MAPK inhibitor, SB203580 (SB) for 48 h. A, MHC II (i) and CD86 (ii) expression was determined by flow cytometry and presented as percentage of cells expressing high MHC II or CD86. Data derived from three independent experiments are presented as average percentage ± S.D. B, Nrf2+/+ (i) and Nrf2−/− (ii) iDCs were pulsed with increasing concentrations of NP68 antigenic peptide and co-cultured with F5 CD8 T cells for 72 h. [3H]Thymidine (3H-Thy) was added for the last 16 h. Proliferation of T cells was determined by scintillation counting of incorporated [3H]thymidine. Data are presented as average [3H]thymidine scintillation counts ± S.D. Statistical significance was assessed using unpaired Student's t test and one-way ANOVA. Data are representative of three independent experiments (*, p < 0.05; NS, not significant).
Loss of Nrf2 Leads to Increased Basal Phosphorylation of CREB and ATF1 Transcription Factors in Immature DCs
As the activity of p38 MAPK is regulated by phosphorylation, we assessed the phosphorylation state of p38 MAPK. Western blotting analysis revealed marginal differences in p38 MAPK phosphorylation between Nrf2−/− and Nrf2+/+ iDCs (Fig. 4A). Major downstream effectors of p38 MAPK are the transcription factors, CREB and ATF1. Serine phosphorylation of CREB and ATF1 by upstream MAPKs is required for their activation. We measured the phosphorylation state of CREB and ATF1 and observed that CREB and ATF1 were hyperphosphorylated under basal conditions in Nrf2−/− iDCs (Fig. 4B). A significant reduction in the phosphorylation of CREB and ATF1 was observed upon inhibiting p38 MAPK activity using a pharmacological inhibitor (SB203580) as seen in Fig. 4C. This suggests that CREB and ATF1 phosphorylation in DCs are dependent on p38 MAPK activity. There is evidence in other cell types that in addition to p38 MAPK, ERK1/2 can also mediate CREB/ATF1 phosphorylation. We tested the requirement of ERK1/2 for phosphorylation of CREB and ATF1 in the DCs under basal and LPS-stimulated conditions using an ERK1/2 inhibitor (PD98059). As seen in Fig. 4D, inhibition of ERK1/2 did not reduce the level of phospho-CREB/ATF1 under basal conditions. LPS stimulation markedly increased the phosphorylation of CREB/ATF1 in Nrf2+/+ and Nrf2−/− iDCs (Fig. 4D). Inhibition of ERK1/2 did not reduce the phosphorylation of CREB/ATF1 under LPS-stimulated conditions in both Nrf2+/+ and Nrf2−/− iDCs (Fig. 4D). Consistent with the previous result (Fig. 4C), p38 MAPK inhibition reduced CREB/ATF1 phosphorylation levels both basally and upon LPS stimulation. To test whether the basal hyperphosphorylation of CREB/ATF1 in Nrf2−/− iDCs could be the result of elevated ROS (36), we treated the DCs with vitamins and show that decreasing elevated ROS did not reverse the increased CREB/ATF1 phosphorylation (Fig. 4E). These results indicate that Nrf2 is required for controlling the activity of the p38 MAPK-CREB/ATF1 signaling axis in DCs.
FIGURE 4.
Loss of Nrf2 perturbs p38 MAPK-CREB/ATF1 signaling in iDCs. Whole cell lysates from Nrf2+/+ and Nrf2−/− iDCs were subjected to SDS-PAGE. Western immunoblotting was used to determine the levels of phospho-p38 (p-p38) total p38 (p38), and tubulin (A). B, phospho-CREB (p-CREB), phospho-ATF1 (p-ATF1), and total CREB (CREB). C, whole cell lysates from Nrf2+/+and Nrf2−/− iDCs treated with or without 20 μm of p38 activity inhibitor SB203580 (SB) for 1 h were subjected to SDS-PAGE. Phosphorylation of CREB and ATF1 (p-CREB and p-ATF1) and total CREB (CREB) were assessed. D, whole cell lysates from Nrf2+/+ and Nrf2−/− iDCs treated with or without 50 μm of ERK1/2 inhibitor, PD98059 (PD), or 20 μm of p38 MAPK inhibitor, SB203580 (SB) for 1 h in the presence or absence of LPS (1 μg/ml) for the last 30 min were subjected to SDS-PAGE. Phosphorylation of CREB and ATF1 (p-CREB and p-ATF1) and total tubulin (as loading control) were assessed. E, whole cell lysates from Nrf2+/+ and Nrf2−/− iDCs treated with or without vitamins C (1 mm) and E (100 μm) for 48 h were subjected to SDS-PAGE. Phosphorylation of CREB (p-CREB) and ATF1 (p-ATF1) and total CREB (CREB) were assessed. Data are representative of three independent experiments.
Loss of Nrf2 Leads to the Dysregulated IL-10 Production in DCs
Transcription of the anti-inflammatory cytokine IL-10 is regulated by CREB/ATF1 activity (37). We therefore measured the levels of IL-10 secreted by the iDCs. As shown in Fig. 5, Nrf2−/− iDCs produce higher levels of IL-10 in comparison to Nrf2+/+ DCs under basal conditions (40.1 pg/ml versus 25.7 pg/ml p < 0.05). Furthermore, upon LPS stimulation, Nrf2−/− iDCs produced levels of IL-10, which was greater than that produced by LPS-stimulated Nrf2+/+ iDCs (96.3 pg/ml versus 73.3 pg/ml p < 0.05). Although basal production of IL-10 was not sensitive to p38 MAPK inhibition, a significant reduction in LPS-induced IL-10 production (Nrf2+/+, 73.3 pg/ml to 31.3 pg/ml, p < 0.05; Nrf2−/−, 96.3 pg/ml to 41.2 pg/ml, p < 0.05) was observed in both Nrf2+/+ and Nrf2−/−iDCs treated with SB203580 (Fig. 5). This result suggests that LPS-stimulated but not basal IL-10 production in iDCs is dependent on p38 MAPK-CREB activity. Taken together, our findings suggest that the p38 MAPK-CREB/ATF1 signaling axis contributes to the Nrf2-mediated regulation of DC immune function.
FIGURE 5.
Loss of Nrf2 results in elevated IL-10 production by DCs. Nrf2+/+ and Nrf2−/− DCs were incubated with or without 20 μm p38 MAPK inhibitor, SB203580 (SB) and/or LPS (1 μg/ml) for 48 h. Levels of IL-10 in supernatants were measured by ELISA. Data derived from two independent experiments are presented as average pg/ml ± S.D. Statistical significance was tested by one-way ANOVA (*, p < 0.05; NS, not significant).
Inhibition of HO-1 Activity Leads to Increased DC Co-stimulatory Receptor Expression and Hyperphosphorylation of CREB/ATF1
A key Nrf2-transcribed gene involved in cellular homeostasis is HO-1. To test whether HO-1 is involved in Nrf2-mediated regulation of co-stimulatory molecule expression, Nrf2+/+ iDCs were treated with HO-1 inhibitor, tin protoporphyrin IX dichloride. As shown in Fig. 6A, tin protoporphyrin IX dichloride treatment increases cell surface expression of MHC II and CD86 in Nrf2+/+ iDCs compared with untreated Nrf2+/+ iDCs (MHC II 59.2% versus 18.1%, p < 0.05; CD86 70.7% versus 11.5%, p < 0.05). In addition, inhibition of HO-1 activity was shown to increase the phosphorylation of CREB/ATF1 in Nrf2+/+ iDCs (Fig. 6B). This observation suggests that one of the mechanisms by which Nrf2 modulates co-stimulatory molecule expression and CREB/ATF phosphorylation is through its effect on HO-1 function.
FIGURE 6.
HO-1 activity modulates DC phenotype and CREB/ATF phosphorylation. Nrf2+/+ iDCs were treated with or without tin protoporphyrin IX dichloride (SnPP-IX, 5 μm) for 14 h. A, expression of MHC II (i) and CD86 (ii) was determined by flow cytometry and presented as percentage of cells expressing high MHC II or CD86. Data derived from three independent experiments are presented as average percentage ± S.D. Statistical significance was assessed using unpaired Student's t test (*, p < 0.05). B, whole cell lysates from Nrf2+/+ iDCs treated with or without tin protoporphyrin IX dichloride (5 μm) for 2 h and from untreated Nrf2−/− iDCs were subjected to SDS-PAGE and phosphorylation status of CREB and ATF1 (p-CREB and p-ATF1) assessed by Western blotting. Lysate from Nrf2+/+ iDCs treated with LPS (1 μg/ml, 30 min) was used as positive control. Total CREB was assessed for equal loading of lanes.
DISCUSSION
Understanding the role of Nrf2 in DC biology requires the definition of critical molecular pathways that are subject to modulation by Nrf2 activity. Nrf2 is central to redox homeostasis, and it has been shown that in the absence of Nrf2, there is elevated ROS in DCs (14, 26). Although there is evidence suggesting that increased ROS is associated with elevated co-stimulatory changes (38, 39), our results demonstrate that in the context of Nrf2 deficiency, ROS does not directly underlie these changes in immature DCs. A possible explanation for this is that increased co-stimulatory molecule expression in response to physiological stimuli is usually due to transient and not persistent elevation of ROS (40). Sustained elevations of ROS levels as seen in Nrf2−/− iDCs are therefore likely to result in cellular adaptive changes that cannot be simply reversed by rebalancing ROS levels but require more complex cellular reprogramming (41, 42). Our findings suggest that Nrf2-mediated regulation of DC function is not solely dependent on its role in redox homeostasis. This is consistent with our previous results wherein lowering the levels of the redox-regulating molecule, GSH in Nrf2+/+ iDCs does not recapitulate the altered phenotype and function of Nrf2−/− iDCs.
Enhanced Nrf2 activity through the use of sulforaphane has been shown to suppress p38 MAPK pathway in endothelial cells (43). Our observations from this and an earlier report (26) also suggest that the p38 but not ERK1/2 or JNK is the main MAPK that is involved in Nrf2-mediated regulation of immature DC function. The inhibition of p38 MAPK causes a marked but incomplete reversal of DC function in Nrf2-deficient DCs, indicating that other pathways or factors are also involved. Indeed, histone deacetylases have also been implicated in the Nrf2-associated changes in phenotype (26).
The downstream target of p38 MAPK, CREB, has been associated with the regulation of key DC immune functions, including the expression of co-stimulatory molecules (8). Our finding of constitutive hyperphosphorylation of CREB and ATF1 in Nrf2−/− iDCs highlights the requirement of Nrf2 in maintaining the integrity of the p38 MAPK-CREB/ATF1 pathway in iDCs. Using pharmacological inhibitors, we confirmed that CREB and ATF1 hyperphosphorylation was selectively mediated by p38 MAPK and not ERK1/2. The other MAPK, JNK is not involved in CREB/ATF1 phosphorylation (44) and hence was not examined in this study. In keeping with the lack of involvement of ROS in altered co-stimulatory molecule expression, reducing ROS did not alter CREB/ATF1 phosphorylation levels. These observations confirm the selective involvement of p38-CREB/ATF1 axis in Nrf2-mediated regulation of DC function.
In addition to MAPKs, the phosphorylation state of CREB can be regulated by protein phosphatases (45). Key phosphatases that act on CREB and regulate its phosphorylation are PP1 (46) and PP2A (7). It is possible that changes in PP1 and/or PP2A activity could also contribute to the increased CREB phosphorylation in Nrf2−/− iDCs. It will be interesting to measure PP1 and PP2A activity in the DCs to test this possibility.
There is evidence that stimuli-induced IL-10 production requires CREB/ATF1 activation (37). Surprisingly, we found that the basal IL-10 production could not be reduced by inhibition of the p38-CREB/ATF1 pathway. However, LPS-induced IL-10 secretion was sensitive to inhibition of this pathway. This suggests that there are differential requirements for p38-CREB/ATF1 in the transcriptional regulation of IL-10 under basal versus stimulated conditions. It is pertinent to note that although the level of CREB/ATF1 phosphorylation in Nrf2−/− iDCs under basal conditions is comparable with that in LPS-stimulated iDCs, the amount of IL-10 secreted basally is only half of that in LPS-stimulated iDCs. This indicates that CREB/ATF1 phosphorylation alone is not sufficient for IL-10 synthesis. To test the direct functional consequence of increased CREB/ATF1 phosphorylation in Nrf2−/− iDCs, other readouts of CREB/ATF1 function such as levels of Bcl2 (47) or degree of cell survival could be measured.
Transactivation by CREB/ATF1 requires its association with cofactors such as CREB binding protein (CBP) (48). There are data demonstrating that availability of cellular CBP is limited and that CBP is also utilized by other transcription factors, including Nrf2 and NF-κB (49). It is therefore suggested that Nrf2 and NF-κB (in addition to CREB) compete for the available CBP (50–52). In the absence of Nrf2, more CBP is available for use by NF-κB and CREB. This could partly explain the increased levels of a number of genes transcribed by these transcription factors in Nrf2−/− iDCs (53, 54). A candidate gene product of Nrf2 transactivation that could potentially account for the influence of Nrf2 in DC function and intracellular signaling is HO-1. Our results indicate that HO-1 activity is required to maintain iDC phenotype and prevent basal hyperphosphorylation of CREB/ATF. Evidence implicating HO-1 in modulating DC maturation comes from studies that demonstrate inhibition of activation-induced DC maturation when HO-1 is overexpressed (25). The molecular mechanism/s through which HO-1 modulates CREB/ATF1 phosphorylation and DC maturation are unclear and is the focus of our ongoing investigations. Our data, in conjunction with other studies, suggest that Nrf2 activity can affect cell signaling both at the level of p38-CREB/ATF1 and at the level of transcriptional cofactors (i.e. CBP).
In summary, our data implicates p38 MAPK-CREB/ATF1 axis as a key signaling pathway that is regulated by Nrf2 in DCs. The molecules within this pathway, as well as HO-1, could represent targets for pharmacological intervention in disease states that arise from dysregulated Nrf2 function.
This work was supported in whole or in part by studentships from the Iraqi Ministry of Higher Education and Scientific Research (to L. M. A. A), the Saudi Ministry of Higher Education and King Abdullah Scholarships Program (to N. A), and the Biotechnology and Biological Sciences Research Council (to J. H.).
- DC
- dendritic cell
- Nrf2
- nuclear factor-erythroid 2 (NF-E2) p45-related factor-2
- iDC
- immature DC
- ROS
- reactive oxygen species
- NP68
- nucleoprotein 68
- CREB
- cAMP response element-binding protein
- ATF1
- activating transcription factor 1
- HO-1
- Heme oxygenase-1
- ANOVA
- analysis of variance
- CBP
- CREB binding protein
- PP1
- protein phosphatase 1
- PP2A
- protein phosphatase 2A.
REFERENCES
- 1. Steinman R. M. (2003) Some interfaces of dendritic cell biology. APMIS 111, 675–697 [DOI] [PubMed] [Google Scholar]
- 2. Chang L., Karin M. (2001) Mammalian MAP kinase signalling cascades. Nature 410, 37–40 [DOI] [PubMed] [Google Scholar]
- 3. Dong C., Davis R. J., Flavell R. A. (2002) MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 [DOI] [PubMed] [Google Scholar]
- 4. Nakahara T., Moroi Y., Uchi H., Furue M. (2006) Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J. Dermatol. Sci. 42, 1–11 [DOI] [PubMed] [Google Scholar]
- 5. Arrighi J. F., Rebsamen M., Rousset F., Kindler V., Hauser C. (2001) A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-α, and contact sensitizers. J. Immunol. 166, 3837–3845 [DOI] [PubMed] [Google Scholar]
- 6. Aiba S., Manome H., Nakagawa S., Mollah Z. U., Mizuashi M., Ohtani T., Yoshino Y., Tagami H. (2003) p38 Mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J. Invest. Dermatol. 120, 390–399 [DOI] [PubMed] [Google Scholar]
- 7. Shanware N. P., Zhan L., Hutchinson J. A., Kim S. H., Williams L. M., Tibbetts R. S. (2010) Conserved and distinct modes of CREB/ATF transcription factor regulation by PP2A/B56γ and genotoxic stress. PLoS One 5, e12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ardeshna K. M., Pizzey A. R., Devereux S., Khwaja A. (2000) The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96, 1039–1046 [PubMed] [Google Scholar]
- 9. Avni D., Ernst O., Philosoph A., Zor T. (2010) Role of CREB in modulation of TNFα and IL-10 expression in LPS-stimulated RAW264.7 macrophages. Mol. Immunol. 47, 1396–1403 [DOI] [PubMed] [Google Scholar]
- 10. Son Y., Cheong Y. K., Kim N. H., Chung H. T., Kang D. G., Pae H. O. (2011) Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Go Y. M., Gipp J. J., Mulcahy R. T., Jones D. P. (2004) H2O2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J. Biol. Chem. 279, 5837–5845 [DOI] [PubMed] [Google Scholar]
- 12. Riemann A., Schneider B., Ihling A., Nowak M., Sauvant C., Thews O., Gekle M. (2011) Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLoS One 6, e22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams M. A., Rangasamy T., Bauer S. M., Killedar S., Karp M., Kensler T. W., Yamamoto M., Breysse P., Biswal S., Georas S. N. (2008) Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J. Immunol. 181, 4545–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rangasamy T., Williams M. A., Bauer S., Trush M. A., Emo J., Georas S. N., Biswal S. (2010) Nuclear erythroid 2 p45-related factor 2 inhibits the maturation of murine dendritic cells by ragweed extract. Am. J. Respir. Cell Mol. Biol. 43, 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. (1993) Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature 362, 722–728 [DOI] [PubMed] [Google Scholar]
- 16. Nguyen T., Sherratt P. J., Pickett C. B. (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 43, 233–260 [DOI] [PubMed] [Google Scholar]
- 17. Motohashi H., Yamamoto M. (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10, 549–557 [DOI] [PubMed] [Google Scholar]
- 18. Li N., Alam J., Venkatesan M. I., Eiguren-Fernandez A., Schmitz D., Di Stefano E., Slaughter N., Killeen E., Wang X., Huang A., Wang M., Miguel A. H., Cho A., Sioutas C., Nel A. E. (2004) Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 173, 3467–3481 [DOI] [PubMed] [Google Scholar]
- 19. Copple I. M., Goldring C. E., Kitteringham N. R., Park B. K. (2010) The keap1-nrf2 cellular defense pathway: mechanisms of regulation and role in protection against drug-induced toxicity. Handb. Exp. Pharmacol. 196, 233–266 [DOI] [PubMed] [Google Scholar]
- 20. Wu B. J., Kathir K., Witting P. K., Beck K., Choy K., Li C., Croft K. D., Mori T. A., Tanous D., Adams M. R., Lau A. K., Stocker R. (2006) Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J. Exp. Med. 203, 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yet S. F., Melo L. G., Layne M. D., Perrella M. A. (2002) Heme oxygenase 1 in regulation of inflammation and oxidative damage. Methods Enzymol. 353, 163–176 [DOI] [PubMed] [Google Scholar]
- 22. Silva G., Cunha A., Grégoire I. P., Seldon M. P., Soares M. P. (2006) The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 alpha MAPK isoform. J. Immunol. 177, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 23. Schumacher A., Wafula P. O., Teles A., El-Mousleh T., Linzke N., Zenclussen M. L., Langwisch S., Heinze K., Wollenberg I., Casalis P. A., Volk H. D., Fest S., Zenclussen A. C. (2012) Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS One 7, e42301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng C., Noorderloos M., van Deel E. D., Tempel D., den Dekker W., Wagtmans K., Duncker D. J., Soares M. P., Laman J. D., Duckers H. J. (2010) Dendritic cell function in transplantation arteriosclerosis is regulated by heme oxygenase 1. Circ. Res. 106, 1656–1666 [DOI] [PubMed] [Google Scholar]
- 25. Chauveau C., Rémy S., Royer P. J., Hill M., Tanguy-Royer S., Hubert F. X., Tesson L., Brion R., Beriou G., Gregoire M., Josien R., Cuturi M. C., Anegon I. (2005) Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 106, 1694–1702 [DOI] [PubMed] [Google Scholar]
- 26. Aw Yeang H. X., Hamdam J. M., Al-Huseini L. M., Sethu S., Djouhri L., Walsh J., Kitteringham N., Park B. K., Goldring C. E., Sathish J. G. (2012) Loss of transcription factor nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) leads to dysregulation of immune functions, redox homeostasis, and intracellular signaling in dendritic cells. J. Biol. Chem. 287, 10556–10564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322 [DOI] [PubMed] [Google Scholar]
- 28. Lutz M. B., Kukutsch N., Ogilvie A. L., Rössner S., Koch F., Romani N., Schuler G. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 29. Bindokas V. P., Jordán J., Lee C. C., Miller R. J. (1996) Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 16, 1324–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson K. G., LeRoy F. G., Borysiewicz L. K., Matthews R. J. (1999) TCR signaling thresholds regulating T cell development and activation are dependent upon SHP-1. J. Immunol. 162, 3802–3813 [PubMed] [Google Scholar]
- 31. Jeong Y. J., Hong S. W., Kim J. H., Jin D. H., Kang J. S., Lee W. J., Hwang Y. I. (2011) Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naïve T cells into Th1 cells by increased IL-12 secretions. Cell Immunol. 266, 192–199 [DOI] [PubMed] [Google Scholar]
- 32. Roche M., Tarnus E., Rondeau P., Bourdon E. (2009) Effects of nutritional antioxidants on AAPH- or AGEs-induced oxidative stress in human SW872 liposarcoma cells. Cell Biol. Toxicol. 25, 635–644 [DOI] [PubMed] [Google Scholar]
- 33. Mamalaki C., Norton T., Tanaka Y., Townsend A. R., Chandler P., Simpson E., Kioussis D. (1992) Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T-cell receptor transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 89, 11342–11346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakahara T., Uchi H., Urabe K., Chen Q., Furue M., Moroi Y. (2004) Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int. Immunol. 16, 1701–1709 [DOI] [PubMed] [Google Scholar]
- 35. Sato K., Nagayama H., Tadokoro K., Juji T., Takahashi T. A. (1999) Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-α-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J. Immunol. 162, 3865–3872 [PubMed] [Google Scholar]
- 36. Kim S. S., Jang S. A., Seo S. R. (2013) CREB-mediated Bcl-2 expression contributes to RCAN1 protection from hydrogen peroxide-induced neuronal death. J. Cell Biochem. 114, 1115–1123 [DOI] [PubMed] [Google Scholar]
- 37. Platzer C., Fritsch E., Elsner T., Lehmann M. H., Volk H. D., Prösch S. (1999) Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 29, 3098–3104 [DOI] [PubMed] [Google Scholar]
- 38. Rutault K., Alderman C., Chain B. M., Katz D. R. (1999) Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic. Biol. Med. 26, 232–238 [DOI] [PubMed] [Google Scholar]
- 39. Kantengwa S., Jornot L., Devenoges C., Nicod L. P. (2003) Superoxide anions induce the maturation of human dendritic cells. Am. J. Respir. Crit. Care Med. 167, 431–437 [DOI] [PubMed] [Google Scholar]
- 40. Bergamo P., Maurano F., D'Arienzo R., David C., Rossi M. (2008) Association between activation of phase 2 enzymes and down-regulation of dendritic cell maturation by c9,t11-conjugated linoleic acid. Immunol. Lett. 117, 181–190 [DOI] [PubMed] [Google Scholar]
- 41. Trachootham D., Alexandre J., Huang P. (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 [DOI] [PubMed] [Google Scholar]
- 42. Boonstra J., Post J. A. (2004) Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337, 1–13 [DOI] [PubMed] [Google Scholar]
- 43. Zakkar M., Van der Heiden K., Luong le A., Chaudhury H., Cuhlmann S., Hamdulay S. S., Krams R., Edirisinghe I., Rahman I., Carlsen H., Haskard D. O., Mason J. C., Evans P. C. (2009) Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler. Thromb. Vasc. Biol. 29, 1851–1857 [DOI] [PubMed] [Google Scholar]
- 44. Vaishnav D., Jambal P., Reusch J. E., Pugazhenthi S. (2003) SP600125, an inhibitor of c-jun N-terminal kinase, activates CREB by a p38 MAPK-mediated pathway. Biochem. Biophys. Res. Commun. 307, 855–860 [DOI] [PubMed] [Google Scholar]
- 45. Johannessen M., Delghandi M. P., Moens U. (2004) What turns CREB on? Cell Signal. 16, 1211–1227 [DOI] [PubMed] [Google Scholar]
- 46. Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. (1992) Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell 70, 105–113 [DOI] [PubMed] [Google Scholar]
- 47. Sakamoto K. M., Frank D. A. (2009) CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin. Cancer Res. 15, 2583–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cardinaux J. R., Notis J. C., Zhang Q., Vo N., Craig J. C., Fass D. M., Brennan R. G., Goodman R. H. (2000) Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol. Cell Biol. 20, 1546–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alvarez Y., Municio C., Alonso S., Sánchez Crespo M., Fernández N. (2009) The induction of IL-10 by zymosan in dendritic cells depends on CREB activation by the coactivators CREB-binding protein and TORC2 and autocrine PGE2. J. Immunol. 183, 1471–1479 [DOI] [PubMed] [Google Scholar]
- 50. Katoh Y., Itoh K., Yoshida E., Miyagishi M., Fukamizu A., Yamamoto M. (2001) Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 6, 857–868 [DOI] [PubMed] [Google Scholar]
- 51. Gerritsen M. E., Williams A. J., Neish A. S., Moore S., Shi Y., Collins T. (1997) CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. U.S.A. 94, 2927–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vo N., Goodman R. H. (2001) CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276, 13505–13508 [DOI] [PubMed] [Google Scholar]
- 53. Jin W., Wang H., Yan W., Xu L., Wang X., Zhao X., Yang X., Chen G., Ji Y. (2008) Disruption of Nrf2 enhances upregulation of nuclear factor-κB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm. 2008, 725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thimmulappa R. K., Lee H., Rangasamy T., Reddy S. P., Yamamoto M., Kensler T. W., Biswal S. (2006) Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 116, 984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]