Abstract
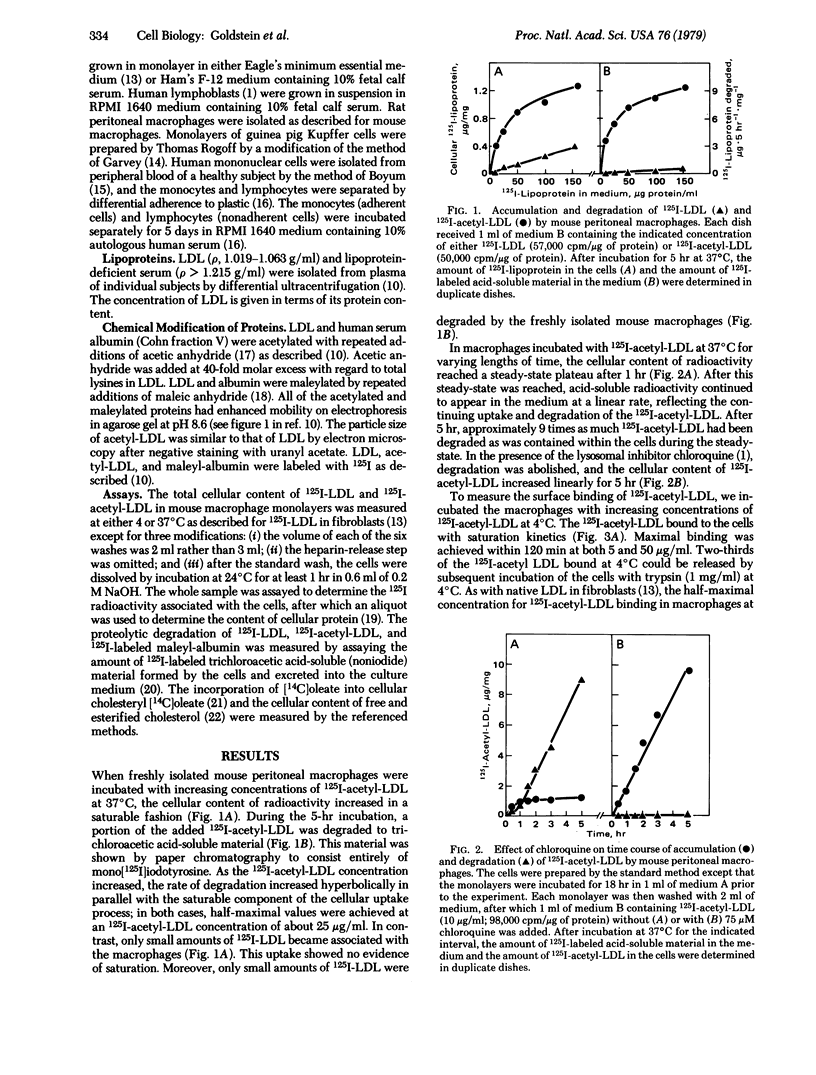
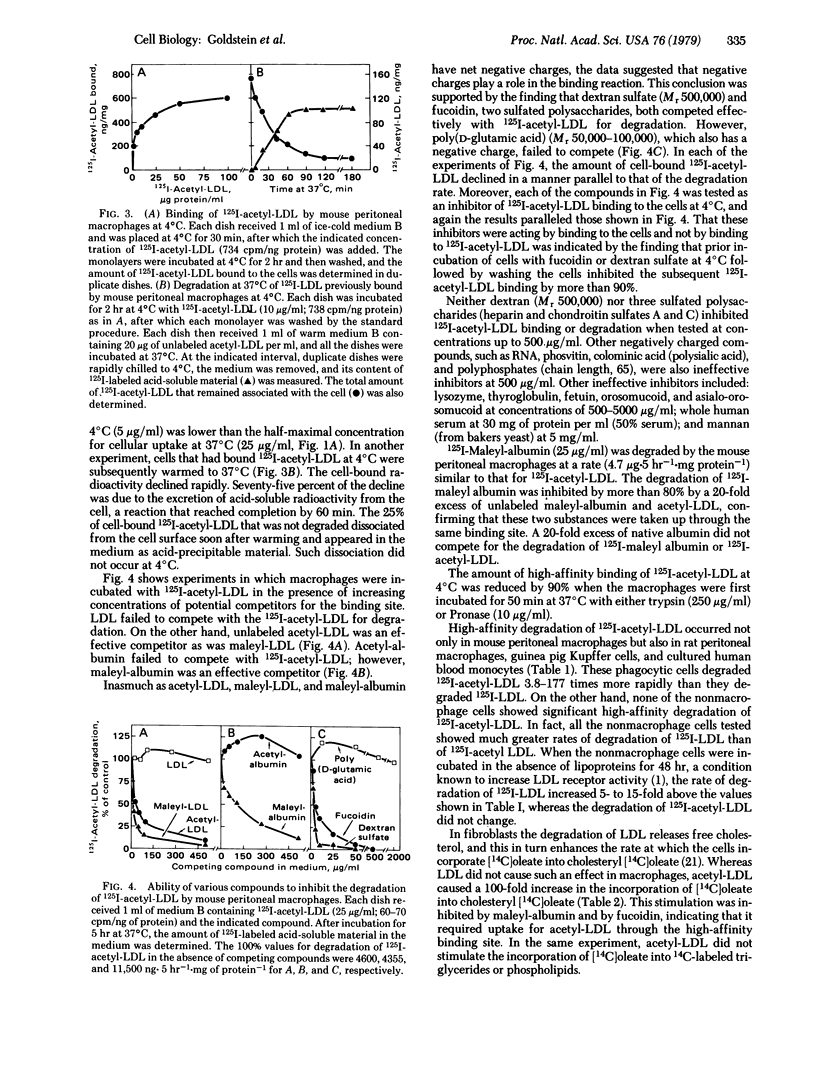
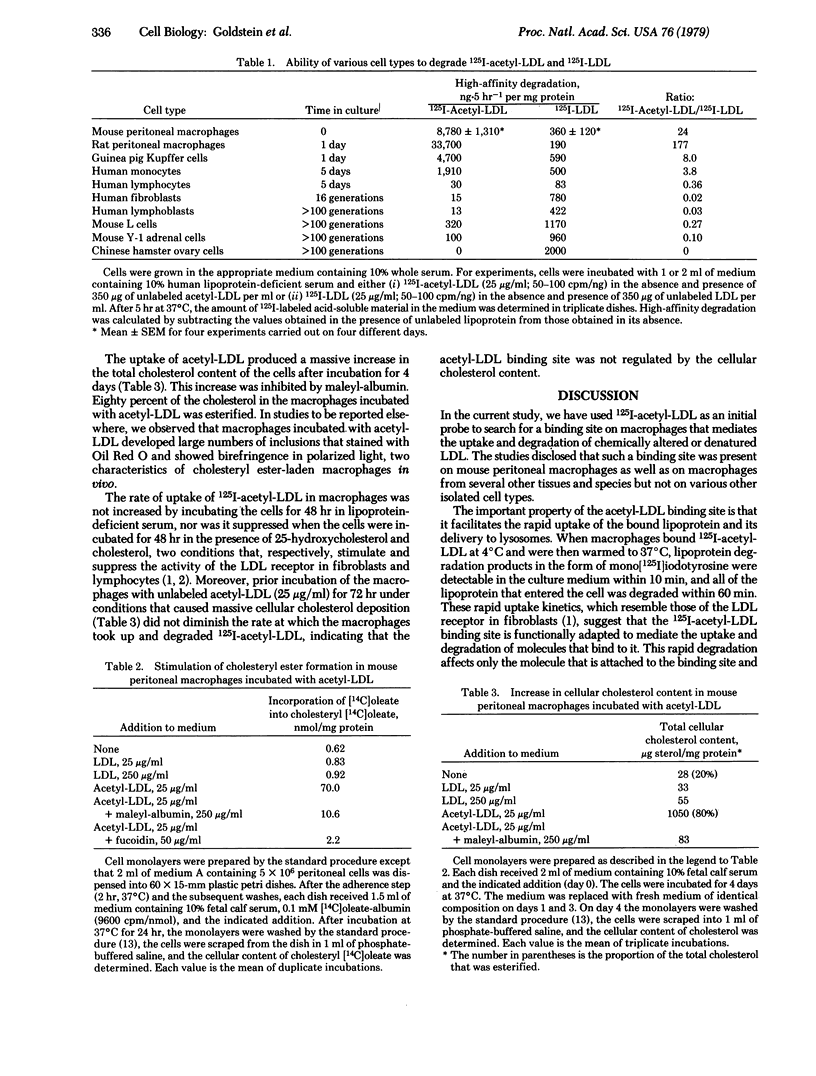
Resident mouse peritoneal macrophages were shown to take up and degrade acetylated 125I-labeled low density lipoprotein (125I-acetyl-LDL) in vitro at rates that were 20-fold greater than those for the uptake and degradation of 125I-LDL. The uptake of 125I-acetyl-LDL and its subsequent degradation in lysosomes were attributable to a high-affinity, trypsin-sensitive, surface binding site that recognized acetyl-LDL but not native LDL. When 125I-acetyl-LDL was bound to this site at 4°C and the macrophages were subsequently warmed to 37°C, 75% of the cell-bound radioactivity was degraded to mono[125I]iodotyrosine within 1 hr. The macrophage binding site also recognized maleylated LDL, maleylated albumin, and two sulfated polysaccharides (fucoidin and dextran sulfate) indicating that negative charges were important in the binding reaction. A similar binding site was present on rat peritoneal macrophages, guinea pig Kupffer cells, and cultured human monocytes but not on human lymphocytes or fibroblasts, mouse L cells or Y-1 adrenal cells, or Chinese hamster ovary cells. Uptake and degradation of acetyl-LDL via this binding site stimulated cholesterol esterification 100-fold and produced a 38-fold increase in the cellular content of cholesterol in mouse peritoneal macrophages. Although the physiologic significance, if any, of this macrophage uptake mechanism is not yet known, we hypothesize that it may mediate the degradation of denatured LDL in the body and thus serve as a “backup” mechanism for the previously described receptor-mediated degradation of native LDL that occurs in parenchymal cells. Such a scavenger pathway might account for the widespread deposition of LDL-derived cholesteryl esters in macrophages of patients with familial hypercholesterolemia in whom the parenchymal cell pathway for LDL degradation is blocked, owing to a genetic deficiency of receptors for native LDL.
Keywords: cell surface receptors, denatured proteins, cultured cells, lysosomes, familial hypercholesterolemia
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Brown M. S. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975 Dec;56(6):1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys C. H., Dejong A. S., Bouma J. M., Gruber M. Rapid uptake by liver sinusoidal cells of serum albumin modified with retention of its compact conformation. Biochim Biophys Acta. 1975 May 5;392(1):95–100. doi: 10.1016/0304-4165(75)90169-5. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomette G., de Gennes J. L., Delcourt A., Hammou J. C., Perie G. La xanthomatose cutanéo-tendineuse hypercholestérolémique familiale. Etude anatomo-clinique. A propos de deux cas. Ann Anat Pathol (Paris) 1971 Jul-Sep;16(3):233–250. [PubMed] [Google Scholar]
- GARVEY J. S. Separation and in vitro culture of cells from liver tissue. Nature. 1961 Sep 2;191:972–974. doi: 10.1038/191972a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Atherosclerosis: the low-density lipoprotein receptor hypothesis. Metabolism. 1977 Nov;26(11):1257–1275. doi: 10.1016/0026-0495(77)90119-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia: pathogenesis of a receptor disease. Johns Hopkins Med J. 1978 Jul;143(1):8–16. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brown M. S. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEGO J. L., MCQUEEN J. D. THE UPTAKE AND DEGRADATION OF INJECTED LABELED PROTEINS BY MOUSE-LIVER PARTICLES. Biochim Biophys Acta. 1965 Apr 12;100:136–143. doi: 10.1016/0304-4165(65)90436-8. [DOI] [PubMed] [Google Scholar]
- Moore A. T., Williams K. E., Lloyd J. B. The effect of chemical treatments of albumin and orosomucoid on rate of clearance from the rat bloodstream and rate of pinocytic capture of rat yolk sac cultured in vitro. Biochem J. 1977 Jun 15;164(3):607–616. doi: 10.1042/bj1640607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M., Berg T. Uptake and degradation of formaldehyde-treated 125I-labelled human serum albumin in rat liver cells in vivo and in vitro. Biochim Biophys Acta. 1977 Mar 29;497(1):171–182. doi: 10.1016/0304-4165(77)90150-7. [DOI] [PubMed] [Google Scholar]
- Pratten M. K., Williams K. E., Lloyd J. B. A quantitative study of pinocytosis and intracellular proteolysis in rat peritoneal macrophages. Biochem J. 1977 Dec 15;168(3):365–372. doi: 10.1042/bj1680365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tanaka K., Yanai N. Essential familial hypercholesteremic xanthomatosis--an autopsy case with special reference to the pathogensis of cardiovascular lipidosis. Acta Pathol Jpn. 1968 Aug;18(3):319–331. [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Cholesterol metabolism in the macrophage. 3. Ingestion and intracellular fate of cholesterol and cholesterol esters. J Exp Med. 1972 Jan;135(1):21–44. doi: 10.1084/jem.135.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]


