Background: The causal relationship between tubulin acetylation and microtubule stability has remained poorly understood.
Results: Pharmacological inhibition of HDAC6 increased HDAC6 binding to microtubules, enhanced microtubule stability, and suppressed dynamics.
Conclusion: Increased binding of HDAC6, rather than acetylation per se, causes microtubule stability.
Significance: The study indicates a MAP-like function of HDAC6 that extends beyond its tubulin deacetylase function.
Keywords: Histone Deacetylase, Histone Deacetylase Inhibitors, MAPs, Microtubules, Post-translational Modification, Histone Deacetylase 6, Microtubule Dynamics, Tubastatin A, Tubulin Acetylation
Abstract
The post-translational modification of tubulin appears to be a highly controlled mechanism that regulates microtubule functioning. Acetylation of the ϵ-amino group of Lys-40 of α-tubulin marks stable microtubules, although the causal relationship between tubulin acetylation and microtubule stability has remained poorly understood. HDAC6, the tubulin deacetylase, plays a key role in maintaining typical distribution of acetylated microtubules in cells. Here, by using tubastatin A, an HDAC6-specific inhibitor, and siRNA-mediated depletion of HDAC6, we have explored whether tubulin acetylation has a role in regulating microtubule stability. We found that whereas both pharmacological inhibition of HDAC6 as well as its depletion enhance microtubule acetylation, only pharmacological inhibition of HDAC6 activity leads to an increase in microtubule stability against cold and nocodazole-induced depolymerizing conditions. Tubastatin A treatment suppressed the dynamics of individual microtubules in MCF-7 cells and delayed the reassembly of depolymerized microtubules. Interestingly, both the localization of HDAC6 on microtubules and the amount of HDAC6 associated with polymeric fraction of tubulin were found to increase in the tubastatin A-treated cells compared with the control cells, suggesting that the pharmacological inhibition of HDAC6 enhances the binding of HDAC6 to microtubules. The evidence presented in this study indicated that the increased binding of HDAC6, rather than the acetylation per se, causes microtubule stability. The results are in support of a hypothesis that in addition to its deacetylase function, HDAC6 might function as a MAP that regulates microtubule dynamics under certain conditions.
Introduction
Tubulin, the major constituent of microtubules, undergoes several post-translational modifications. It has been recently emerging that these post-translational modifications create functionally distinct microtubules and mark them for specialized functions (1). Acetylation at Lys-40 of α-tubulin is mediated by acetyltransferase (2) whereas histone deacetylase 6 (HDAC6)2 and sirtuin2 (SIRT2) catalyze the removal of the acetyl group (3–6). HDAC6 is a unique class IIb mammalian HDAC that contains two homologous catalytic domains compared with one catalytic domain in other HDACs (7). HDAC6 localizes predominantly in cytoplasm and exerts the deacetylase enzymatic activity mainly on nonhistone substrates in cells, although it can deacetylate histones in vitro (3). Apart from α-tubulin, the other cellular substrates of HDAC6 include heat shock protein-90 (Hsp90) and cortactin (8–10). HDAC6 is known to associate directly with microtubules (3). In addition, it is now evident that HDAC6 interacts with the ubiquitin group of polyubiquitinated misfolded proteins and recruits them to dynein motor protein which subsequently transports them to the aggresomes through microtubules (11). In vitro, polymerized microtubules are preferential substrate for HDAC6 with little or no deacetylase activity toward free αβ-tubulin dimers (3). However, in cells, free tubulin can be efficiently deacetylated by HDAC6 (4). The functional significance of microtubule acetylation has remained largely unknown. Tubulin acetylation, most likely, acts as guidance cues for the recruitment of proteins such as kinesins (12, 13), Hsp90 (14) (which in turn, increases the binding and activity of two of its client proteins namely the kinase Akt/PKB and the transcription factor p53 to microtubules) or microtubule-severing enzymes (15). Tubulin acetylation may also have an important role in maintaining the microtubule protofilament number in cells (16). Moreover, the stabilization of microtubules has usually been found to be associated with increased acetylation. Microtubules that are acetylated turn over slowly compared with the nonacetylated microtubules (17). Neuronal axons that possess extremely stable population of microtubules, or stable microtubules, oriented toward a particular direction during migration of certain cell types, are particularly enriched in tubulin acetylation (18–23). Acetylated microtubules have been found to be less sensitive toward treatment with microtubule-damaging agents, suggesting that acetylation of tubulin may impart stability to microtubules (4, 24–26). On the other hand, several studies suggest that acetylation does not affect microtubule stability per se (22, 27). Also, by inducing acetylation of purified brain tubulin by Chlamydomonas α-tubulin acetyltransferase, it was shown that acetylation status of tubulin in vitro does not affect the microtubule polymerization or depolymerization (28). The rate and extent of assembly and disassembly of the acetylated and nonacetylated tubulin were found to be similar in vitro (28). Thus, whether the acetylation of tubulin really affects microtubule assembly dynamics still remains unclear.
In this study, we investigated whether the increase in acetylation resulting from compromised HDAC6 functioning caused microtubule stabilization. The enzyme was inhibited using three different approaches: trichostatin A (TSA, a nonspecific inhibitor of HDAC6), tubastatin A (a specific inhibitor of HDAC6), and siRNA. TSA has been used previously to elucidate the functions of HDAC6 (25, 27, 29) but has a disadvantage in that it is a general inhibitor of class I and II HDACs. Tubastatin A, a hydroxamic acid-based inhibitor, exhibits ∼1000-fold more selectivity against almost all HDAC isozymes compared with tubacin, making it one of the most selective HDAC6 inhibitors known (30). We found that catalytic inactivation of HDAC6, but not its depletion, is associated with suppression of microtubule assembly dynamics and renders microtubules resistant toward depolymerizing conditions, though both treatments cause an increase in microtubule acetylation level. By using immunofluorescence and Western blot analysis, we further show that the inhibition of HDAC6 enzymatic activity increases its binding to microtubules, which possibly enhances microtubule stability.
EXPERIMENTAL PROCEDURES
Materials
TSA, mouse monoclonal anti-α-tubulin IgG, anti-acetylated tubulin IgG, alkaline phosphatase-conjugated anti-mouse IgG, anti-HDAC6 IgG, HRP-conjugated anti-mouse and anti-rabbit IgG and bovine serum albumin (BSA) were purchased from Sigma. An Enhanced Chemiluminescence kit was purchased from GE Healthcare. Anti-detyrosinated tubulin IgG was purchased from Millipore. Lipofectamine LTX, Lipofectamine RNAimax, luciferase siRNA, Opti-MEM I, RNase- and DNase-free water were purchased from Invitrogen. HDAC6 siRNA and anti-phosphohistone H3 serine 10 IgG was purchased from Santa Cruz Biotechnology. Tubastatin A was purchased from Biovision (Mountain View, CA). All other reagents were of analytical grade.
Cell Culture
Human breast cancer cells (MCF-7) were cultured as described earlier (31, 32). To determine the effect of tubastatin A and TSA on the proliferation of cells, MCF-7 cells were seeded at a density of 1 × 105 cells/ml in 96-well plates. After 24 h, cells were incubated with either vehicle or different concentrations of TSA or tubastatin A for 48 h. The effect on proliferation was measured by sulforhodamine B assay (23, 33).
Light Scattering Assay
Purified goat brain tubulin (10 μm) was incubated without or with 30 μm tubastatin A or 10 μm TSA in PEM buffer (25 mm PIPES, pH 6.8, 3 mm MgCl2, and 1 mm EGTA) in the presence of 1 m glutamate for 10 min on ice. Then, 1 mm GTP was added in the reaction mixtures. The assembly of tubulin was monitored by light scattering (350 nm) at 37 °C using FP-6500 spectrofluorometer (JASCO, Tokyo, Japan).
Measurement of Microtubule Dynamics
MCF-7 cells were transfected with EGFP-tubulin plasmid using Lipofectamine LTX plus and Opti-MEM I reduced serum medium using the manufacturer's protocol (23). Effects of tubastatin A and TSA on the dynamics of individual microtubules in EGFP-tubulin-expressing MCF-7 cells were determined as described recently (23). EGFP-tubulin-expressing MCF-7 cells were seeded at a density of 0.5 × 105 cells/ml on glass coverslips in 24-well plates. After 24 h, cells were incubated with either vehicle or 15 μm tubastatin A for 24 h. Microtubules in the peripheral region of control and treated cells were observed in 60× oil immersion objective in an FV500 laser scanning confocal microscope (Olympus, Tokyo, Japan). The end points of microtubules were tracked using ImageJ software. Life history plots of the microtubules were plotted, and different parameters of the dynamic instability were calculated (23, 34). Similarly, the effects of 30 and 60 nm TSA on microtubule dynamics were monitored.
Depletion of HDAC6 with siRNA
One day before transfection with siRNA, MCF-7 cells were seeded at a density of 0.5 × 105 cells/ml. For Western blotting, cells were seeded in T-25 tissue culture flasks. Cells were then transfected with luciferase siRNA or HDAC6 siRNA (200 nm) using Lipofectamine RNAimax and Opti-MEM I reduced serum medium. The cells were incubated at 37 °C in CO2 incubator for 24 h.
Immunostaining
MCF-7 cells were seeded at a density of 0.5 × 105 cells/ml on glass coverslips in 24-well plates and incubated for 24 h. After incubation, cells were treated with siRNA, tubastatin A, or TSA for 24 h and then fixed with 4% formaldehyde and permeabilized with ice-cold methanol. For co-localization of HDAC6 and microtubules, the cells were fixed with ice-cold methanol containing 1 mm EGTA for 10 min and then incubated with 0.5% Triton X-100 for 10 min to remove the soluble proteins and washed three times with PBS. The coverslips were incubated with 2% (w/v) BSA/PBS for 30 min to block nonspecific sites and then incubated with primary antibodies for 2 h. Immunostaining was done using α-tubulin (1:300), acetylated tubulin (1:400), detyrosinated tubulin (1:200), HDAC6 (1:200), or phosphohistone H3 Ser-10 (1:300) antibody. The cells were incubated with secondary antibodies for 1 h and washed three times with PBS. The coverslips were mounted using the mounting medium on glass slides. Cells were examined using a confocal laser scanning microscope (Olympus) or Nikon Eclipse TE2000-U fluorescence microscope. The images were captured and analyzed using Image-Pro Plus 4.5.
Cold-induced and Nocodazole-induced Microtubule Depolymerization Assay
MCF-7 cells were seeded at a density of 0.5 × 105 cells/ml on glass coverslips in 24-well plates and incubated for 24 h. After the cells were attached on the coverslips, they were either used for cold-induced or nocodazole-induced microtubule depolymerization assay. For cold treatment, cells were first incubated with tubastatin A (30 μm) or TSA (240 nm) or transfected with HDAC6 siRNA for 24 h. After treatment, cells were incubated on ice for different time intervals (0, 10, and 20 min) and then fixed. For determining the effect of nocodazole, cells were treated with 200 nm nocodazole alone or together with tubastatin A (15 μm), TSA (240 nm), or siRNA (200 nm) for 24 h. The cells were then fixed and processed for immunostaining.
Microtubule Reassembly Assay
MCF-7 cells were incubated with 500 nm nocodazole for 4 h (interphase cells) or with 300 nm nocodazole for 24 h to block the cells in mitosis (mitotic cells). After incubation, nocodazole was washed out from cells by rinsing with fresh medium five or six times. Cells were then incubated in fresh medium without or with 15 or 30 μm tubastatin A for 1.5 h in an incubator and then fixed and processed for immunostaining. Interphase cells were immunostained with antibodies against α-tubulin and acetylated tubulin whereas mitotic cells were immunostained with antibodies against α-tubulin and phosphohistone H3 Ser-10. The PH-H3 (Ser-10) antibody was used to show chromosomes. The antibody detects Ser-10 phosphorylation of histone H3 that occurs during mitosis. For the reassembly of microtubules after cold treatment, microtubules were depolymerized by incubating the cells on ice for 45 min. Then, cells were incubated with prewarmed medium, without or with 30 μm tubastatin A, at 37 °C in incubator for different time intervals (0, 20, 40, 120, and 180 min) and then fixed and processed for immunostaining. For quantification, 300 cells were counted for each experimental condition. The number of cells in which complete microtubule network was visible was determined. In a separate experiment, cells were transfected with luciferase siRNA or HDAC6 siRNA for 24 h and then used for microtubule reassembly assay as described above.
Western Blotting
MCF-7 cells were transfected with HDAC6 siRNA and incubated for 24 h. In separate experiments, MCF-7 cells were treated with vehicle, TSA (240 nm), or tubastatin A (30 μm) for 24 h. After incubation, whole cell lysates or tubulin polymeric fractions were prepared, separated on SDS-PAGE, and transferred on a PVDF membrane as described previously (32). To detect the level of acetylated tubulin or HDAC6 in polymeric tubulin, polymer fractions of cellular tubulin were prepared and were probed with antibodies against acetylated tubulin or HDAC6 and α-tubulin separately, as described above. The intensity of the bands was measured by densitometry using ImageJ.
RESULTS
Inhibition of HDAC6 by Tubastatin A and TSA Increased Microtubule Acetylation
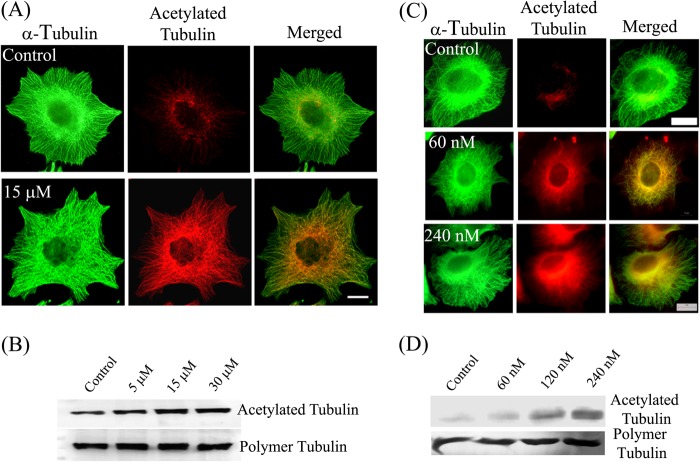
Tubastatin A and TSA were found to inhibit the proliferation of MCF-7 cells in a concentration-dependent manner (supplemental Fig. S1). The half-maximal inhibitory concentrations of tubastatin A and TSA were found to be 15 ± 1 μm and 54 ± 2 nm, respectively. The inhibition of catalytic activity of HDAC6 has been shown to increase the acetylation level of microtubules (3, 25). We determined the effect of tubastatin A treatment on the acetylation of cytoplasmic microtubules. The immunofluorescence images showed that in vehicle-treated cells, only a subset of total microtubules was acetylated and the acetylated microtubules were mainly restricted to the perinuclear region (Fig. 1A). In contrast, in tubastatin A-treated cells, a majority of the microtubules were acetylated. Although no apparent change in the morphology of microtubule cytoskeleton was observed, there was a clear increase in the acetylation level of microtubules upon tubastatin A treatment (Fig. 1A). The increase in the level of acetylation was further confirmed by determining the level of polymeric and acetylated tubulin in cells by Western blotting. The amount of acetylated tubulin in cells increased in a tubastatin A concentration-dependent manner (Fig. 1B). For instance, there was a 40 and 70% increase in the acetylation level of tubulin in the presence of 5 and 30 μm tubastatin A, respectively, whereas the level of polymeric tubulin remained unchanged. A similar increase in the acetylation level was also observed upon treating the cells with TSA (Fig. 1, C and D). For instance, the acetylation level increased by 2.5-fold in 120 nm TSA-treated cells compared with the control cells (Fig. 1D).
FIGURE 1.
Tubastatin A and TSA increased the microtubule acetylation level. Effects of tubastatin A (A and B) and TSA (C and D) on microtubules and microtubule acetylation level are shown. A and C, MCF-7 cells were treated with different concentrations of tubastatin A (A) or of TSA (C) for 24 h and processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). Scale bars, 10 μm. B and D, MCF-7 cells were treated with different concentrations of tubastatin A (B) or of TSA (D) for 24 h. Polymeric fractions of tubulin were isolated, processed for Western blotting, and were probed for α-tubulin and acetylated tubulin.
Inhibition of HDAC6 by Tubastatin A and TSA Stabilized Microtubules against Cold and Nocodazole-induced Depolymerization
We next determined whether the increase in acetylation level of microtubules due to pharmacological inhibition of HDAC6 was accompanied by an increase in stability of microtubules. MCF-7 cells were treated with either vehicle, tubastatin A, or TSA for 24 h and were then subjected to cold treatment. The cells were kept on ice, and the kinetics of microtubule depolymerization was monitored by fixing the cells at varying time points. As shown in Fig. 2, in vehicle-treated cells, the microtubules depolymerized rapidly. After 10 min of incubation on ice, hardly any microtubules could be observed, indicating that most of the microtubules were depolymerized. In contrast, in tubastatin A- and TSA-treated cells, the content of the acetylated microtubules was observed to be much higher than the control cells, and most of the filaments were found to be intact, indicating that the microtubule depolymerization rate was significantly reduced. Even after 20 min of cold treatment, several microtubules were observed in tubastatin A- and TSA-treated cells.
FIGURE 2.
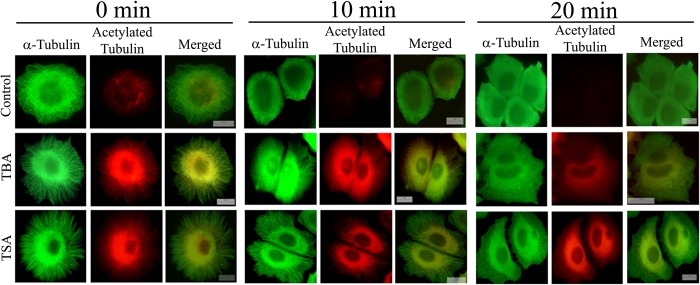
Tubastatin A and TSA stabilized microtubules against cold-induced depolymerization. MCF-7 cells were treated with vehicle, 30 μm tubastatin A (TBA), or 240 nm TSA for 24 h. Then cells were incubated on ice to depolymerize microtubules and were fixed at the indicated time points (0, 10, and 20 min). Fixed cells were processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). Scale bars, 10 μm.
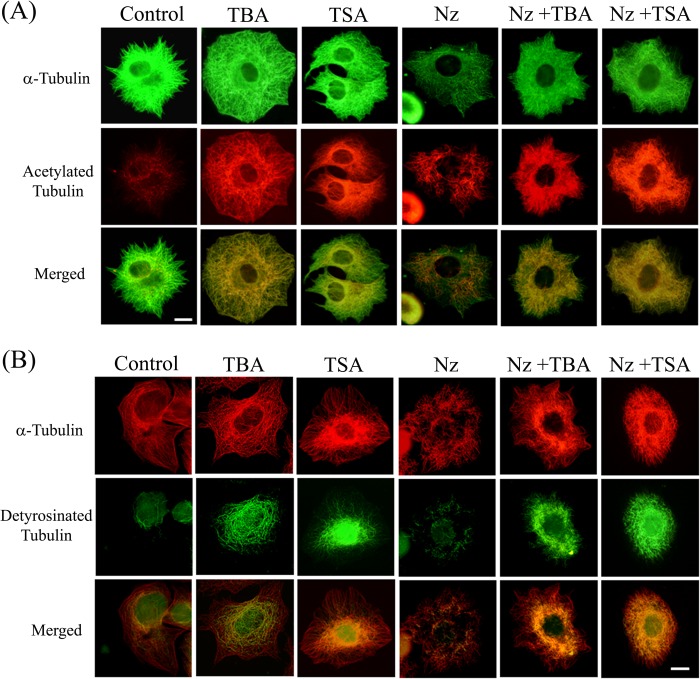
To further probe the increase in microtubule stability, we treated MCF-7 cells with 200 nm nocodazole, a known microtubule-depolymerizing agent, alone or in combination with tubastatin A or TSA. In the cells treated with nocodazole, microtubules were extensively depolymerized, as expected, and the remaining microtubules were found to be acetylated (Fig. 3A, Nz). On the other hand, the microtubules in cells treated with nocodazole along with tubastatin A or TSA displayed an increase in acetylation, and much less microtubule depolymerization was observed in these cells (Fig. 3A).
FIGURE 3.
Tubastatin A and TSA stabilized microtubules against nocodazole-induced disassembly. A, MCF-7 cells were treated with either vehicle, 15 μm tubastatin A (TBA), 240 nm TSA, or 200 nm nocodazole (Nz) individually or with a combination of nocodazole (200 nm) and tubastatin A (15 μm) or nocodazole (200 nm) and TSA (240 nm) for 24 h and processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). Scale bar, 10 μm. B, MCF-7 cells were treated with either vehicle, 15 μm tubastatin A (TBA), 240 nm TSA, 200 nm nocodazole (Nz) individually or with a combination of nocodazole (200 nm) and tubastatin A (15 μm) or nocodazole (200 nm) and TSA (240 nm) for 24 h and immunostained with antibodies against α-tubulin (red) and detyrosinated tubulin (green). Scale bar, 10 μm.
To assess whether an increase in microtubule acetylation is coupled with enhanced microtubule stability, we looked at the status of detyrosination of microtubules. Detyrosination is a post-translational modification that results from removal of the ultimate Tyr from tubulin subunits assembled into microtubule lattice (35). Detyrosination is found to accumulate in stable microtubules and thus can be used to quantify microtubule stability independently (36). Co-immunostaining with antibodies against total α-tubulin and detyrosinated tubulin showed that in control cells only a few of the total microtubules were detyrosinated whereas in tubastatin A- and TSA-treated cells, the proportion of detyrosinated microtubules was significantly higher in the interphase microtubule array (Fig. 3B), although the increase in detyrosination content was more prominent in TSA-treated cells. Moreover, in the cells treated with TSA or tubastatin A and nocodazole together, the microtubule depolymerization was greatly reduced compared with the cells treated with nocodazole alone, and all of the remaining microtubules in cells where nocodazole treatment was accompanied by inhibition of HDAC6 were found to be enriched in detyrosination (Fig. 3B).
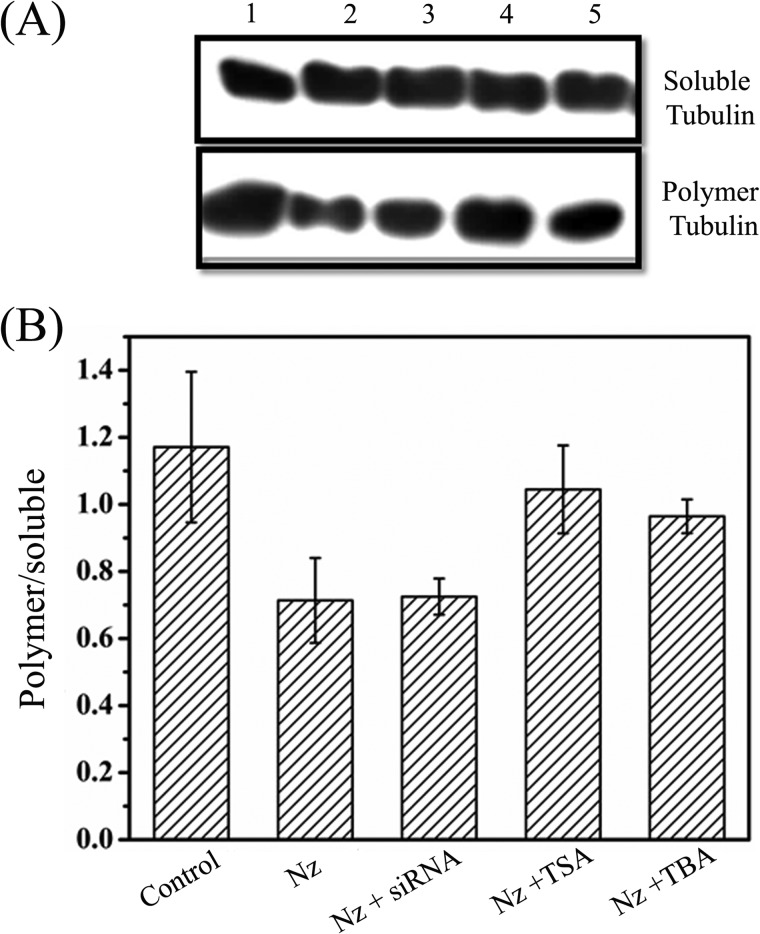
The effect of tubastatin A and TSA on nocodazole-induced disassembly of microtubules in MCF-7 cells was also examined by Western blotting (Fig. 4). Nocodazole treatment decreased the ratio of polymerized tubulin to soluble tubulin by 40% compared with that of the vehicle-treated cells. However, when the cells were treated with nocodazole in combination with TSA or tubastatin A, the ratio of polymerized tubulin to soluble tubulin was found to decrease by 11 and 18%, respectively (Fig. 4, A and B). The results suggested that microtubules resisted the depolymerizing effect of nocodazole in the presence of TSA and tubastatin A. Because the cells were incubated with tubastatin A or TSA at the same time that nocodazole was added, the finding also indicated that the inhibition of HDAC6 activity by tubastatin A or TSA is fast enough to counter the depolymerizing effect of nocodazole.
FIGURE 4.
Effects of tubastatin A, TSA, and HDAC6 siRNA on nocodazole-induced depolymerization of microtubules. A, polymeric and soluble tubulin fractions of MCF-7 cells treated with vehicle (lane 1), 200 nm nocodazole (Nz) alone (lane 2), or 200 nm nocodazole in combination with HDAC6 siRNA, 240 nm TSA, or 15 μm tubastatin A (TBA) (lanes 3, 4 and 5, respectively) for 24 h were isolated as described, and equal amounts of proteins were resolved by SDS-PAGE followed by immunoblotting with anti-α-tubulin antibody. B, the ratio of polymer to soluble fraction of tubulin in cells treated as in A was measured from the intensity of the bands in blot. Data were an average of three independent experiments and represent mean ± S.D. (error bars).
We determined whether tubastatin A and TSA had any effect on the assembly of purified tubulin in vitro. No discernible effect of either tubastatin A (30 μm) or TSA (10 μm) on the rate and extent of tubulin polymerization was observed (supplemental Fig. S2). The data indicated that tubastatin A and TSA do not exert any direct effect on microtubules.
Inhibition of HDAC6 by Tubastatin A and TSA Altered Assembly Dynamics of Interphase Microtubules in MCF-7 Cells
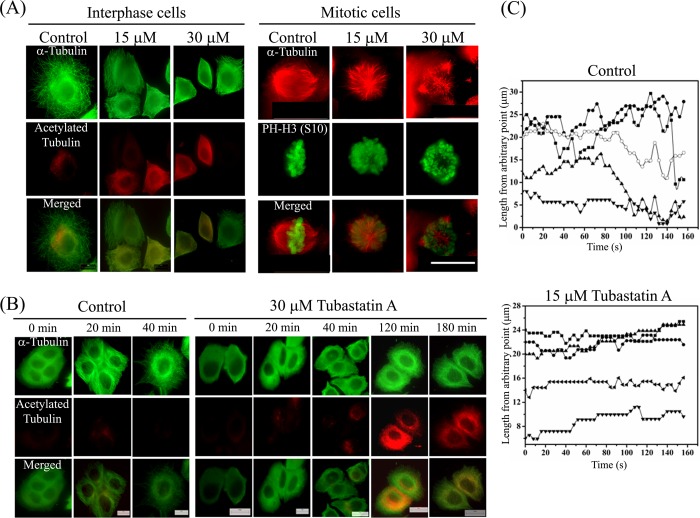
To determine whether the inhibition of HDAC6 activity alters kinetic properties of microtubules, we determined the reassembly kinetics of microtubules after depolymerizing the microtubules in cells by nocodazole. After the required incubation, nocodazole was carefully removed by repeated washing with medium, and microtubules were allowed to reassemble in the absence or presence of 15 or 30 μm tubastatin A. We found that within 1.5 h of removal of nocodazole, >90% (96 ± 1.5%) control interphase cells exhibited typical microtubule network, indicating complete regrowth of microtubules (Fig. 5A, interphase cells). On the other hand, there was a significant delay in the reassembly of microtubules in the presence of tubastatin A. For instance, only 45 ± 3 and 27 ± 4.5% cells treated with 15 and 30 μm tubastatin A, respectively, were found to have microtubule network whereas the remaining cells showed only partially grown microtubule filaments (Fig. 5A, interphase cells) even after 90 min. Similarly, formation of a typical bipolar spindle was observed and chromosomes (indicated by a mitotic marker PH-H3 (Ser-10)) were found to be aligned accurately on the metaphase plate in mitotic cells, which were allowed to reassemble the microtubules in the presence of vehicle alone. In contrast, the cells treated with tubastatin A showed aberrant monopolar or multipolar spindles after 90 min of reassembly. Moreover, the chromosomes were not aligned on the metaphase plate and appeared disorganized. The data indicated that assembly properties and functioning of microtubules were severely affected (Fig. 5A, mitotic cells). A similar delay in the assembly of cold depolymerized microtubules was seen in cells treated with tubastatin A. When cold-depolymerized microtubules were allowed to reassemble in the presence of vehicle alone, 81 ± 3.6 and 96 ± 1.5% cells displayed complete reassembly of microtubules in 20 and 40 min, respectively (Fig. 5B). In contrast, when microtubule regrowth was allowed in the presence of tubastatin A, 11 ± 3 and 17 ± 3.5% cells showed the formation of complete network in 20 and 40 min, respectively. Even after 180 min, microtubule array was observed in only 78 ± 2.5% of cells, indicating substantial reduction in the assembly of microtubules in the presence of tubastatin A. Together, the data indicated that the inhibition of HDAC6 extensively affected the assembly and disassembly properties of microtubules.
FIGURE 5.
Tubastatin A altered the assembly dynamics of interphase microtubules in MCF-7 cells. A, tubastatin A delayed the reassembly of nocodazole-depolymerized interphase and mitotic microtubules. Cells were treated with either 500 nm nocodazole for 4 h (interphase cells) or 300 nm nocodazole for 24 h (mitotic cells). Nocodazole was then removed by repeated washing, and microtubules were allowed to reassemble in the absence (control) or presence of 15 or 30 μm tubastatin A for 90 min. Cells were then fixed and processed for co-immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red) (interphase cells) or α-tubulin (red) and phosphohistone-H3 (Ser-10) (green) (mitotic cells). PH-H3 (Ser-10), a mitotic marker, was used to indicate the chromosomes. Scale bar, 10 μm. B, tubastatin A delayed the reassembly of cold-depolymerized interphase microtubules. Microtubules were depolymerized by incubating the cells on ice for 45 min. Then cells were incubated with prewarmed medium, without or with 30 μm tubastatin A, at 37 °C in incubator for different time intervals (0, 20, 40, 120, and 180 min) and then fixed and processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). Scale bars, 10 μm. C, life history plots of the individual microtubules in EGFP-tubulin-expressing MCF-7 cells treated with vehicle or 15 μm tubastatin A for 24 h. The initial length represents length from an arbitrary fixed point of microtubule to the end of the microtubule.
Effects of Tubastatin A and TSA on the Dynamic Instability of Individual Microtubules in MCF-7 Cells
We then determined the effect of inhibition of HDAC6 activity on microtubule dynamic instability parameters in MCF-7 cells. We transfected MCF-7 cells with EGFP-tubulin and followed the plus ends of microtubules near the cell periphery using time-lapse confocal fluorescence microscopy. The lengths of the microtubules were plotted as a function of time to obtain the life history traces of microtubules (Fig. 5C) and the dynamic instability parameters were measured from these traces. The microtubules in vehicle-treated cells displayed characteristic rapid growth and shortening of the ends. The periods of extensive length changes were interspersed with short phases of pauses during the course of imaging. Microtubules of cells treated with 15 μm tubastatin A for 24 h exhibited a marked reduction in the dynamics of microtubules. The mean growth and shortening rates of microtubules were reduced from 16.0 ± 3.5 and 19.5 ± 6.0 μm/min in control cells to 12.0 ± 2.0 (p ≤ 0.001) and 13.0 ± 3.0 μm/min (p ≤ 0.001), respectively, in 15 μm tubastatin A-treated cells (Table 1). Tubastatin A treatment decreased the time-based catastrophe (a transition from a growing or a pause state to a shortening state) frequency whereas it increased the rescue (a transition from a shortening to a growing or a pause state) frequency. The most prominent effect of inhibition of HDAC6 activity was observed on the time spent by microtubules in the pause state. The percentage of duration of the pause state of microtubules was found to increase from 37.0 ± 15.0 in control cells to 68.0 ± 7.4 (p ≤ 0.001) in the tubastatin A-treated cells. In addition, the dynamicity (the total length grown and shortened per unit time) of microtubules was diminished by 64% from 11.0 ± 4.0 μm/min in control to 4.0 ± 1.0 μm/min (p ≤ 0.001) in the presence of 15 μm tubastatin A. The data clearly suggested that microtubule plus ends were less dynamic after treatment with tubastatin A. In separate experiments, TSA was found to have a similar concentration-dependent suppression of microtubule dynamics in MCF-7 cells. The microtubule growth rate was decreased by 28% whereas the shortening rate was reduced by 31% in presence of 30 nm TSA. The percentage of time that microtubules spent in the pause state increased from 33.3% in control cells to 62 and 80% in cells treated with 30 and 60 nm TSA, respectively. The percentage of time that microtubules spent in the growth phase was reduced by 47 and 73% whereas the percentage of time spent by microtubules in the shortening phase was reduced by 37 and 66% in the presence of 30 and 60 nm TSA, respectively. Further, the dynamicity of microtubules was decreased by 63 and 79% after treatment with 30 and 60 nm TSA, respectively. In a previous study (25), TSA was found to suppress microtubule dynamics in B16F1 cells by reducing the rates of microtubule growth and shortening by 46 and 55%, respectively.
TABLE 1.
Tubastatin A suppresses dynamics and alters the parameters of dynamic instability of interphase microtubules in MCF-7 cells
Data represent mean ± S.D., n = 20 microtubules in each case.
| Parameters | Control | Tubastatin A (15 μm) |
|---|---|---|
| Growth rate (μm/min) | 16.0 ± 3.5 | 12.0 ± 2.0a |
| Growth length (μm) | 1.7 ± 0.6 | 1.1 ± 0.3a |
| Shortening rate (μm/min) | 19.5 ± 6.0 | 13.0 ± 3.0a |
| Shortening length (μm) | 2.1 ± 0.9 | 1.1 ± 0.3a |
| % time spent in growing | 33.5 ± 8.7 | 18.6 ± 4.6a |
| % time spent in shortening | 29.5 ± 6.8 | 12.8 ± 5.0a |
| % time spent in pause | 37.0 ± 15.0 | 68.0 ± 7.4a |
| Dynamicity (μm/min) | 11.0 ± 4.0 | 4.0 ± 1.0a |
| Catastrophe frequency (events/min) | 4.0 ± 1.3 | 1.8 ± 0.9a |
| Rescue frequency (events/min) | 9.4 ± 2.2 | 11.7 ± 3.5b |
| Catastrophe frequency (events/μm) | 0.54 ± 0.23 | 0.69 ± 0.32c |
| Rescue frequency (events/μm) | 0.52 ± 0.18 | 0.94 ± 0.30a |
a p ≤ 0.001.
b p ≤ 0.05.
c Not significant.
Depletion of HDAC6 by siRNA Did Not Stabilize Microtubules
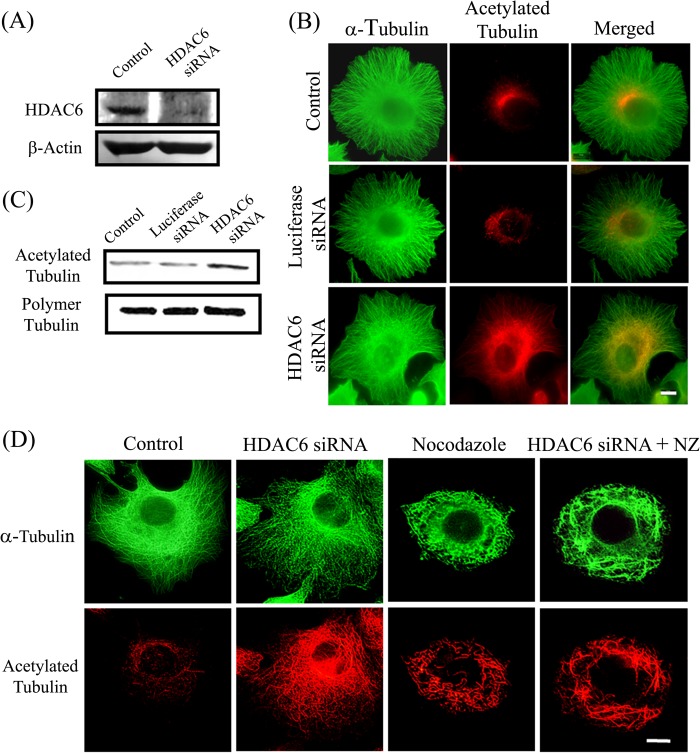
To further probe the effect of microtubule acetylation on the stability of microtubules, we depleted HDAC6 from MCF-7 cells by using an siRNA-based knockdown method. As indicated by Western blotting, the treatment of cells with HDAC6 siRNA for 24 h was successful in depleting approximately 90% of HDAC6 (Fig. 6A). To rule out the off-target effects, we used luciferase siRNA as a control. Microtubule arrays of both HDAC6 and luciferase siRNA-treated cells were intact and resembled that of the control cells (Fig. 6B). The depletion of HDAC6 resulted in an increase in the acetylation of microtubules whereas luciferase siRNA treatment did not cause a change in the acetylation level of microtubules (Fig. 6B). There was no discernible change in the polymeric α-tubulin level after treatment with any of the siRNAs (Fig. 6C). However, a significant increase (∼40%) in the acetylation level of polymeric α-tubulin fraction after treatment with HDAC6 siRNA was found whereas no change in the acetylation level was observed in luciferase siRNA-treated cells.
FIGURE 6.
Effect of depletion of HDAC6 on microtubule stability. A, levels of HDAC6 in cells treated with luciferase siRNA (control) and siRNA against HDAC6. β-Actin was used as loading control. B, effect of depletion of HDAC6 on microtubules and acetylated tubulin. MCF-7 cells were kept untreated or were treated with siRNA against luciferase or HDAC6 and were then processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). Scale bar, 10 μm. C, MCF-7 cells untreated or treated with siRNA against luciferase or HDAC6. The polymeric fractions of tubulin were isolated from the cells. The samples were processed for Western blotting and were probed for α-tubulin and acetylated tubulin. D, effect of depletion of HDAC6 on nocodazole-induced microtubule depolymerization. MCF-7 cells were treated with HDAC6 siRNA and 200 nm nocodazole alone or together with HDAC6 siRNA for 24 h. The cells were then fixed and processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). Scale bar, 10 μm.
We then compared the status of microtubule stability in the control and the HDAC6 siRNA- treated cells. The cells were treated with 200 nm nocodazole alone or in combination with siRNA for 24 h and observed for microtubules and acetylated microtubules by immunofluorescence. The treatment with nocodazole resulted in significant microtubule depolymerization (Fig. 6D). Interestingly, although there was an increase in the acetylation of the microtubules in HDAC6 siRNA-treated cells, the microtubules did not exhibit any increase in stability. The extent of microtubule depolymerization by nocodazole was similar in the absence and presence of HDAC6 siRNA (Fig. 6D). Following nocodazole treatment, the ratio of polymer to soluble tubulin in HDAC6-depleted and undepleted cells was found to be same (Fig. 4, A and B), suggesting that HDAC6 depletion by siRNA did not result in microtubule stabilization.
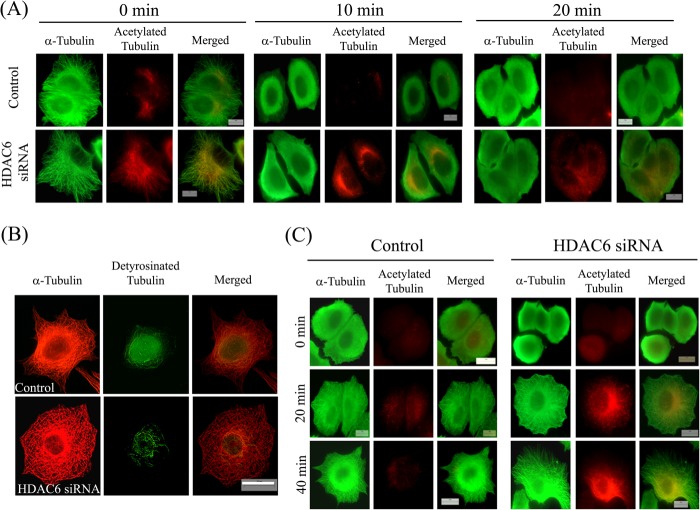
The sensitivity of microtubules toward cold treatment after depleting HDAC6 was determined. Microtubules of siRNA-treated cells were found to depolymerize similarly upon cold treatment as that of control cells (Fig. 7A). Moreover, siRNA-treated cells did not exhibit any increase in the detyrosination level of microtubules compared with the control cells (Fig. 7B). Further, the effect of the depletion of HDAC6 on the growth rate of microtubules was examined. The cells were treated with HDAC6 siRNA for 24 h. The microtubules were then depolymerized by cold treatment and allowed to reassemble at 37 °C. We did not observe any delay in the reassembly of microtubules (Fig. 7C) as was observed in case of cells treated with tubastatin A. The percentage of siRNA-treated cells that exhibited a well formed microtubule network after 20 and 40 min of reassembly was found to be 76 ± 2 and 88 ± 5%, respectively, which was similar to that in control cells (79 ± 4 and 90 ± 2%). It has been reported that the depletion of HDAC6 does not affect the dynamic instability of microtubules (25). The results of this study and the previous report together suggested that the increase in acetylation per se does not affect microtubule assembly dynamics and may not provide stability to microtubules.
FIGURE 7.
Depletion of HDAC6 did not stabilize microtubules. A, effect of depletion of HDAC6 on stability of microtubules against cold-induced depolymerization. MCF-7 cells were transfected with luciferase siRNA (control) or HDAC6 siRNA for 24 h. Then cells were incubated on ice to depolymerize microtubules and were fixed at the indicated time points (0, 10, and 20 min). Fixed cells were processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). Scale bars, 10 μm. B, MCF-7 cells treated with luciferase siRNA (control) or HDAC6 siRNA for 24 h. After treatment, cells were fixed and processed for immunostaining with antibodies against α-tubulin (red) and detyrosinated tubulin (green). Scale bar, 10 μm. C, depletion of HDAC6 not delaying reassembly of cold-depolymerized interphase microtubules. MCF-7 cells were treated with luciferase siRNA (control) or HDAC6 siRNA for 24 h. After treatment, microtubules were depolymerized by incubating the cells on ice for 45 min. Then cells were incubated with prewarmed medium at 37 °C in an incubator for different time intervals and then fixed and processed for immunostaining with antibodies against α-tubulin (green) and acetylated tubulin (red). The status of microtubules and their acetylation level in cells at 0, 20, and 40 min is shown. Scale bars, 10 μm.
Inhibition of HDAC6 Activity by Tubastatin A and TSA Increased Its Binding with Interphase Microtubules in MCF-7 Cells
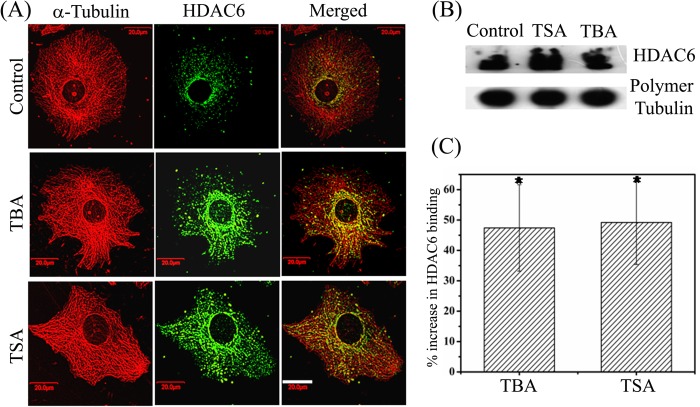
Our results indicated that only the inhibition of deacetylase activity of HDAC6 but not its depletion enhances microtubule stability. Several microtubule-associated proteins are known to bind to microtubules and regulate microtubule dynamics. We hypothesized that the interaction of HDAC6 with microtubules might have a role in stabilizing microtubules. Therefore, we examined whether the localization of HDAC6 on microtubules was affected after treatment with inhibitors of HDAC6. For this, the cells incubated with vehicle, tubastatin A, or TSA were fixed and treated with 0.5% Triton X-100 to remove the cytoplasmic proteins. This allowed us to examine the microtubule-bound HDAC6 in cells. As reported previously (3), HDAC6 was found to localize on microtubules in control cells (Fig. 8A). Interestingly, both tubastatin A and TSA treatment produced more intense staining of HDAC6 on microtubules than the vehicle-treated cells, indicating that the localization of HDAC6 on microtubules was increased in the presence of these inhibitors (Fig. 8A). We did not find any change in the expression level of total HDAC6 after treatment with either TSA or tubastatin A (supplemental Fig. S3). We then determined the effect of tubastatin A and TSA on the binding of HDAC6 with microtubules (Fig. 8B). The amount of HDAC6 bound with microtubules was significantly increased in the presence of both TSA as well as tubastatin A compared with the control cells (Fig. 8B). The amount of HDAC6 in polymeric tubulin fraction isolated from cells treated with tubastatin A and TSA was found to be 47.4 ± 14.2 and 49.2 ± 13.8% higher (p ≤ 0.001), respectively, compared with the control (Fig. 8C). The results together suggested that the inhibition of HDAC6 activity by tubastatin A and TSA increased the binding of HDAC6 to microtubules.
FIGURE 8.
Tubastatin A and TSA increased the binding of HDAC6 with interphase microtubules in MCF-7 cells. A, cells treated with vehicle, 30 μm tubastatin A (TBA), or 240 nm TSA for 24 h and fixed and processed to visualize microtubules (red) and HDAC6 (green). Scale bar, 20 μm. B, Western blot analysis of HDAC6 bound with the polymeric fraction of tubulin in cells. MCF-7 cells were treated with vehicle, 240 nm TSA, or 30 μm tubastatin A for 24 h. The polymeric fraction of tubulin was isolated and probed with antibodies against α-tubulin and HDAC6. C, histogram showing the percentage increase in the binding of HDAC6 with polymeric tubulin after treatment of cells with tubastatin A and TSA with respect to control as quantified from B. Data are average of five independent set of experiments and represent mean ± S.D. (error bars). *, p ≤ 0.001.
DISCUSSION
In this study, the pharmacological inhibition of the activity of HDAC6 was found to alter the assembly dynamics of interphase microtubules in MCF-7 cells and to provide them stability toward cold- and nocodazole-induced depolymerization. Interestingly, when HDAC6 was depleted, microtubules did not exhibit any stability toward the depolymerizing conditions. Microtubules in cells treated with tubastatin A and TSA displayed an increase in the detyrosination level whereas there was no change in the detyrosination level of microtubules after the depletion of HDAC6. The data together indicated that an increase in acetylation per se does not lead to an increase in the stability of microtubules.
How Might Inhibition of Activity of HDAC6 Increase Its Binding to Microtubules?
Microtubules are found to be the preferred substrate of HDAC6 compared with free tubulin heterodimers (3). The structural data suggest that Lys-40 lies in the lumen of microtubules but may be accessible through the walls of the microtubule lattice (37, 38). HDAC6 was shown to interact with the end-tracking proteins EB1 and Arp1, which might facilitate its localization at the plus end of microtubules (25). It was also suggested that the binding of HDAC6 at the plus end may form a cap-like structure, and the capping activity of a catalytically inactive HDAC6 is more prominent than that of an active HDAC6 (25). Upon treatment with either tubastatin A or TSA, the localization of HDAC6 not only increased at the plus ends of microtubules but was also found to increase along the length of the microtubules (Fig. 8A). As indicated earlier (25), it is possible that HDAC6 binds to microtubules, deacetylates them, and leaves the microtubule surface. However, when its activity is inhibited, a conformational change might be induced in HDAC6, due to which either more HDAC6 can bind to microtubules or HDAC6 may remain bound to microtubules for a longer duration than usual. This might, in turn, reduce the exchange of tubulin dimers at the microtubule ends, resulting in the suppression of microtubule dynamics together with an increase in the stability of microtubules.
Why Is There an Increase in the Microtubule Stability When HDAC6 Is Pharmacologically Inhibited?
It was reported that the overexpression of HDAC6 mutants with impaired enzymatic activity, but not of wild type HDAC6, altered microtubule dynamics, suggesting that the presence of catalytically inactive HDAC6 can inhibit microtubule growth (25). Our results showed that the inhibition of catalytic activity of HDAC6 alters microtubule dynamics. Based on our data and previous reports, we raised a hypothesis that the inhibition of HDAC6 activity alters its binding to microtubules which might be the cause of increased stability. In support of this, we found that when cells are treated with tubastatin A or TSA, there is an increase in the localization of HDAC6 on microtubules. In addition, the amount of HDAC6 bound to the polymeric fraction of tubulin increased after treatment with TSA as well as tubastatin A. The results suggested that the inhibition of activity of HDAC6 somehow increases its binding to microtubules which, in effect, dampens microtubule dynamics and increases microtubule stability. We did not observe any increase in the microtubule stability when HDAC6 was depleted from the cells, which further corroborates with the idea that the physical interaction of catalytically inactive HDAC6 is important for microtubule stability.
Does HDAC6 Act as a Microtubule-associated Protein (MAP)?
It is now becoming increasingly evident that several of the microtubule-associated proteins that were previously known to have one particular function have multiple roles and function differently under different conditions. For instance, several kinesins whose sole function was thought to be to carry the cargo have now been shown to regulate microtubule dynamics independently of their motor functions (39). Recently, LC8, a light chain of cytoplasmic dynein, has been found to promote tubulin polymerization and to stabilize interphase microtubule network in cells (40). In another study, it was shown that αTAT1, an α-tubulin acetyltransferase, destabilizes microtubules and accelerates microtubule dynamics in mammalian cells (41). Interestingly, the microtubule dynamics regulating function of αTAT1 is independent of its tubulin acetylation activity as αTAT1 mutant lacking acetyltransferase activity is equally effective in regulating microtubule dynamics as its wild type counterpart (41). These studies indicate unconventional roles of some MAPs that extend beyond their catalytic functions. This work indicated that in addition to its deacetylase function, HDAC6 might function as a MAP that regulates microtubule dynamics under certain conditions.
Acknowledgment
We thank the Centre for Research in Nanotechnology and Science, Indian Institute of Technology Bombay, for use of the confocal microscopy facility.
This work was supported by a grant from the Department of Biotechnology, Government of India.

This article contains supplemental Figs. S1–S3.
- HDAC6
- histone deacetylase 6
- MAP
- microtubule-associated protein
- TSA
- trichostatin A.
REFERENCES
- 1. Janke C., Bulinski J. C. (2011) Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 12, 773–786 [DOI] [PubMed] [Google Scholar]
- 2. Akella J. S., Wloga D., Kim J., Starostina N. G., Lyons-Abbott S., Morrissette N. S., Dougan S. T., Kipreos E. T., Gaertig J. (2010) MEC-17 is an α-tubulin acetyltransferase. Nature 467, 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., Yao T. P. (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 [DOI] [PubMed] [Google Scholar]
- 4. Matsuyama A., Shimazu T., Sumida Y., Saito A., Yoshimatsu Y., Seigneurin-Berny D., Osada H., Komatsu Y., Nishino N., Khochbin S., Horinouchi S., Yoshida M. (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 21, 6820–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. North B. J., Marshall B. L., Borra M. T., Denu J. M., Verdin E. (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437–444 [DOI] [PubMed] [Google Scholar]
- 6. Garnham C. P., Roll-Mecak A. (2012) The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton 69, 442–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grozinger C. M., Hassig C. A., Schreiber S. L. (1999) Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. U.S.A. 96, 4868–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bali P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Seto E., Bhalla K. (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 280, 26729–26734 [DOI] [PubMed] [Google Scholar]
- 9. Kovacs J. J., Murphy P. J., Gaillard S., Zhao X., Wu J. T., Nicchitta C. V., Yoshida M., Toft D. O., Pratt W. B., Yao T. P. (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell 18, 601–607 [DOI] [PubMed] [Google Scholar]
- 10. Zhang X., Yuan Z., Zhang Y., Yong S., Salas-Burgos A., Koomen J., Olashaw N., Parsons J. T., Yang X. J., Dent S. R., Yao T. P., Lane W. S., Seto E. (2007) HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell 27, 197–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hideshima T., Bradner J. E., Wong J., Chauhan D., Richardson P., Schreiber S. L., Anderson K. C. (2005) Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl. Acad. Sci. U.S.A. 102, 8567–8572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reed N. A., Cai D., Blasius T. L., Jih G. T., Meyhofer E., Gaertig J., Verhey K. J. (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166–2172 [DOI] [PubMed] [Google Scholar]
- 13. Hammond J. W., Huang C. F., Kaech S., Jacobson C., Banker G., Verhey K. J. (2010) Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giustiniani J., Daire V., Cantaloube I., Durand G., Poüs C., Perdiz D., Baillet A. (2009) Tubulin acetylation favors Hsp90 recruitment to microtubules and stimulates the signaling function of the Hsp90 clients Akt/PKB and p53. Cell. Signal. 21, 529–539 [DOI] [PubMed] [Google Scholar]
- 15. Sudo H., Baas P. W. (2010) Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 30, 7215–7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cueva J. G., Hsin J., Huang K. C., Goodman M. B. (2012) Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules. Curr. Biol. 22, 1066–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Webster D. R., Borisy G. G. (1989) Microtubules are acetylated in domains that turn over slowly. J. Cell Sci. 92, 57–65 [DOI] [PubMed] [Google Scholar]
- 18. Watson D. F., Hoffman P. N., Griffin J. W. (1990) The cold stability of microtubules increases during axonal maturation. J. Neurosci. 10, 3344–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baas P. W., Slaughter T., Brown A., Black M. M. (1991) Microtubule dynamics in axons and dendrites. J. Neurosci. Res. 30, 134–153 [DOI] [PubMed] [Google Scholar]
- 20. Ahmad F. J., Pienkowski T. P., Baas P. W. (1993) Regional differences in microtubule dynamics in the axon. J. Neurosci. 13, 856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gundersen G. G., Bulinski J. C. (1988) Selective stabilization of microtubules oriented toward the direction of cell migration. Proc. Natl. Acad. Sci. U.S.A. 85, 5946–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palazzo A., Ackerman B., Gundersen G. G. (2003) Cell biology: tubulin acetylation and cell motility. Nature 421, 230. [DOI] [PubMed] [Google Scholar]
- 23. Kapoor S., Panda D. (2012) Kinetic stabilization of microtubule dynamics by indanocine perturbs EB1 localization, induces defects in cell polarity and inhibits migration of MDA-MB-231 cells. Biochem. Pharmacol. 83, 1495–1506 [DOI] [PubMed] [Google Scholar]
- 24. Tran A. D., Marmo T. P., Salam A. A., Che S., Finkelstein E., Kabarriti R., Xenias H. S., Mazitschek R., Hubbert C., Kawaguchi Y., Sheetz M. P., Yao T. P., Bulinski J. C. (2007) HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J. Cell Sci. 120, 1469–1479 [DOI] [PubMed] [Google Scholar]
- 25. Zilberman Y., Ballestrem C., Carramusa L., Mazitschek R., Khochbin S., Bershadsky A. (2009) Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J. Cell Sci. 122, 3531–3541 [DOI] [PubMed] [Google Scholar]
- 26. Matov A., Applegate K., Kumar P., Thoma C., Krek W., Danuser G., Wittmann T. (2010) Analysis of microtubule dynamic instability using a plus-end growth marker. Nat. Methods 7, 761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haggarty S. J., Koeller K. M., Wong J. C., Grozinger C. M., Schreiber S. L. (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci. U.S.A. 100, 4389–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maruta H., Greer K., Rosenbaum J. L. (1986) The acetylation of α-tubulin and its relationship to the assembly and disassembly of microtubules. J. Cell Biol. 103, 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y., Li N., Caron C., Matthias G., Hess D., Khochbin S., Matthias P. (2003) HDAC6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 22, 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler K. V., Kalin J., Brochier C., Vistoli G., Langley B., Kozikowski A. P. (2010) Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc. 132, 10842–10846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohan R., Panda D. (2008) Kinetic stabilization of microtubule dynamics by estramustine is associated with tubulin acetylation, spindle abnormalities, and mitotic arrest. Cancer Res. 68, 6181–6189 [DOI] [PubMed] [Google Scholar]
- 32. Rathinasamy K., Panda D. (2008) Kinetic stabilization of microtubule dynamic instability by benomyl increases the nuclear transport of p53. Biochem. Pharmacol. 76, 1669–1680 [DOI] [PubMed] [Google Scholar]
- 33. Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 34. Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. (1988) Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J. Cell Biol. 107, 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. (1977) Release of tyrosine from tyrosinated tubulin: some common factors that affect this process and the assembly of tubulin. FEBS Lett. 73, 147–150 [DOI] [PubMed] [Google Scholar]
- 36. Webster D. R., Gundersen G. G., Bulinski J. C., Borisy G. G. (1987) Differential turnover of tyrosinated and detyrosinated microtubules. Proc. Natl. Acad. Sci. U.S.A. 84, 9040–9044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nogales E., Whittaker M., Milligan R. A., Downing K. H. (1999) High-resolution model of the microtubule. Cell 96, 79–88 [DOI] [PubMed] [Google Scholar]
- 38. Perdiz D., Mackeh R., Poüs C., Baillet A. (2011) The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell. Signal. 23, 763–771 [DOI] [PubMed] [Google Scholar]
- 39. Drummond D. R. (2011) Regulation of microtubule dynamics by kinesins. Semin. Cell Dev. Biol. 22, 927–934 [DOI] [PubMed] [Google Scholar]
- 40. Asthana J., Kuchibhatla A., Jana S. C., Ray K., Panda D. (2012) Dynein light chain 1 (LC8) association enhances microtubule stability and promotes microtubule bundling. J. Biol. Chem. 287, 40793–40805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kalebic N., Martinez C., Perlas E., Hublitz P., Bilbao-Cortes D., Fiedorczuk K., Andolfo A., Heppenstall P. A. (2013) Tubulin acetyltransferase αTAT1 destabilizes microtubules independently of its acetylation activity. Mol. Cell. Biol. 33, 1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]