Background: Non-structural protein 5 (NS5) of dengue virus serotype 2 is predominantly localized in the nucleus.
Results: The cellular localization of NS5 differs for the four dengue virus serotypes.
Conclusion: NS5 nuclear localization is not a property shared by all dengue viruses.
Significance: The identification of serotypic differences in the properties of dengue virus proteins is important for understanding viral pathogenesis and developing antiviral strategies.
Keywords: Flaviviruses, Nuclear Transport, Positive Strand RNA Viruses, Viral Polymerase, Virology, Dengue
Abstract
The four serotypes of dengue virus (DENV-1 to -4) cause the most important arthropod-borne viral disease of humans. DENV non-structural protein 5 (NS5) contains enzymatic activities required for capping and replication of the viral RNA genome that occurs in the host cytoplasm. However, previous studies have shown that DENV-2 NS5 accumulates in the nucleus during infection. In this study, we examined the nuclear localization of NS5 for all four DENV serotypes. We demonstrate for the first time that there are serotypic differences in NS5 nuclear localization. Whereas the DENV-2 and -3 proteins accumulate in the nucleus, DENV-1 and -4 NS5 are predominantly if not exclusively localized to the cytoplasm. Comparative studies on the DENV-2 and -4 NS5 proteins revealed that the difference in DENV-4 NS5 nuclear localization was not due to rapid nuclear export but rather the lack of a functional nuclear localization sequence. Interaction studies using DENV-2 and -4 NS5 and human importin-α isoforms failed to identify an interaction that supported the differential nuclear localization of NS5. siRNA knockdown of the human importin-α isoform KPNA2, corresponding to the murine importin-α isoform previously shown to bind to DENV-2 NS5, did not substantially affect DENV-2 NS5 nuclear localization, whereas knockdown of importin-β did. The serotypic differences in NS5 nuclear localization did not correlate with differences in IL-8 gene expression. The results show that NS5 nuclear localization is not strictly required for virus replication but is more likely to have an auxiliary function in the life cycle of specific DENV serotypes.
Introduction
The four serotypes of dengue virus (DENV,2 types 1–4) cause the most important arthropod-borne viral disease of humans. Infection with DENV causes a range of clinical outcomes ranging from asymptomatic infection to classical dengue fever and the potentially fatal dengue hemorrhagic fever. It is estimated that 50–100 million cases of dengue fever and 250,000 cases of dengue hemorrhagic fever occur annually (1, 2).
DENV is a member of the Flavivirus genus in the Flaviviridae family and has a positive sense single-stranded RNA genome of ∼11 kb that contains a single long open reading frame (ORF). The ORF encodes a large polyprotein that is co- and post-translationally processed by host and the virally encoded NS2B/3 proteinase to yield the three structural proteins capsid, premembrane/membrane, and envelope and the seven non-structural (NS) proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (3). NS5 is the largest (∼105 kDa) and most well conserved of the flavivirus proteins. NS5 contains two domains. The N-terminal methyltransferase (MTase) domain contains N-7, 2′-O-methyltransferase (4, 5) and guanylyltransferase (6) enzymatic activities, all required for capping of the RNA genome. The C-terminal domain contains RNA-dependent RNA polymerase (POL) activity that is essential for replication of the RNA genome (7, 8). The enzymatic activities of NS5 are major targets for the development of antiviral compounds (9, 10).
In addition to its role in capping and replication of the RNA genome, the NS5 of a number of flaviviruses has been shown to perturb the host innate immune response. The NS5 proteins of Japanese encephalitis virus, Langat virus, tick-borne encephalitis virus, and West Nile virus have been shown to inhibit type I IFN signaling by interfering with STAT1 activation (11–14), whereas for DENV-2, NS5 binds to STAT2 and targets it for degradation (15, 16). The interaction is species-specific because DENV-2 NS5 binds to human but not murine STAT2 (17). In addition, 2′-O-methylation of the viral RNA cap structure by the West Nile virus NS5 protein modulates the antiviral effects of IFN-induced proteins with tetratricopeptide repeats (18). Specific host cells can also prevent the actions of NS5, as evidenced by the finding that the NS5 of the tick-borne viruses Langat virus and tick-borne encephalitis virus are actively targeted for degradation by tripartite motif protein 79α (19).
Flavivirus genome replication, which depends on the enzymatic activities of NS5, occurs in the cytoplasm of infected host cells in association with perinuclear endoplasmic reticulum membranes (20). However, NS5 of a number of flaviviruses, including DENV-2, Japanese encephalitis virus, yellow fever virus, and tick-borne encephalitis virus, have been detected in the nuclei of infected cells (reviewed in Ref. 21). Nuclear transport of proteins of >45 kDa is an active process that depends on proteins of the karyopherin-β family (also termed importins and exportins). Karyopherin family members may bind directly to cargo proteins via nuclear localization sequences (NLSs) or nuclear export sequences before interacting with proteins in the nuclear pore complex to mediate nuclear/cytoplasmic transport (22, 23). In the well characterized “classical nuclear import pathway,” an NLS in the cargo protein is first recognized by the adaptor protein importin-α (karyopherin-α (KPNA)), which in turn is bound by importin-β (karyopherin-β1 (KPNB1)) to form a complex that is transported through the nuclear pore complex. Once in the nucleus, the cargo protein is released when Ran-GTP binds to importin-β. Importin-α is known to bind to two different NLS sequences, a monopartite NLS consisting of a single stretch of basic amino acids or a bipartite NLS consisting of two stretches of basic amino acids separated by a 10–12-amino acid linker region (24–26).
Following the initial finding that DENV-2 NS5 accumulates in the nucleus during infection (27), subsequent studies defined a functional bipartite NLS (termed the a/bNLS) in a stretch of 37 amino acids (positions 369–405) in the NS5 POL domain (28). A murine importin-α/importin-β heterodimer was found to bind with high affinity to the a/bNLS fused to β-galactosidase or the full-length NS5 (28–30). Mutagenesis studies defined a “minimal” a/bNLS (amino acid residues 369–389) containing two basic amino acid clusters important for nuclear localization both in vitro (29) and in vivo in the context of the viral life cycle (30). Interestingly, mutations in the a/bNLS that delayed NS5 nuclear localization resulted in decreased virus replication but increased production of IL-8 (30). DENV-2 NS5 also contains a functional nuclear export sequence recognized by exportin 1 (CRM1) (31) in a stretch of amino acids (positions 320–368, termed the bNLS) that was found to bind directly to importin-β and DENV-2 NS3 (32). These studies suggest that DENV-2 NS5 shuttles between the cytoplasm and nucleus of infected cells and that this process is important for virus replication and the regulation of IL-8 amounts.
However, to date, all studies on NS5 nuclear localization have been done on DENV-2 NS5. In this study, we examined the nuclear localization of NS5 for all four DENV serotypes. We found serotypic differences in the nuclear localization of NS5, either when transiently expressed or produced during the virus life cycle. More detailed comparative studies on the DENV-2 and -4 NS5 proteins that were predominantly localized to the nucleus and cytoplasm, respectively, revealed that the differences in localization were due to the lack of an NLS in DENV-4 NS5. Surprisingly, siRNA knockdown of the human importin-α isoform KPNA2, homologous to the murine importin-α isoform previously shown to bind to DENV-2 NS5, in cells either infected with DENV-2 or expressing NS5 alone did not substantially affect NS5 nuclear localization, whereas siRNA knockdown of KPNB1 dramatically decreased NS5 nuclear localization. The results suggest that NS5 nuclear localization is not strictly required for virus replication but is more likely to have an auxiliary function in the life cycle of specific DENV serotypes.
EXPERIMENTAL PROCEDURES
Cell Lines and Viruses
Human lung carcinoma (A549), HEK293, and Huh-7 liver cells were cultured in DMEM (Invitrogen) supplemented with 0.1 nm non-essential amino acids and 10% FBS (Invitrogen). The cell line HEK-DV2-NS5F3 derived from Flp-InTM T-RExTM-293 cells (Invitrogen) was grown in the same medium supplemented with 15 μg/ml blasticidin and 150 μg/ml hygromycin. African green monkey kidney (Vero) cells, baby hamster kidney cells (BHK-21), and Aedes albopictus C6/36 cells were cultured as described previously (33, 34). Mammalian and insect cells were maintained at 37 and 28 °C, respectively, and 5% CO2 in a humidified atmosphere. The following viruses were used in this study: DENV-1 strain Hawaii (GenBankTM code EU848545), DENV-2 strain New Guinea C (GenBankTM code AF038403); DENV-3 strain H87 (GenBankTM code M93130), and DENV-4 strain H241 (GenBankTM code AY947539) (the DENV-1, -3, and -4 viruses were a kind gift from J. Mongkolsapaya (Imperial College London)). Viral stocks were prepared and titered, and cells were infected with DENV as described previously for DENV-2 (33, 35).
Plasmid Construction
pcDV-NS5F Constructs
The DENV-2 New Guinea C NS5 gene was simultaneously amplified by PCR from the DENV-2 strain New Guinea C genomic length cDNA clone pDVWS601 (33, 34) and C-terminally FLAG-tagged using the primers P1 and P2 (the sequences of the oligonucleotide primers used in this study are shown in supplemental Table 1). The DENV-1 strain Hawaii and DENV-4 strain H241 NS5 genes were simultaneously amplified by PCR from plasmid clones containing these sequences and C-terminally FLAG-tagged, using the primer combinations P3/P4 and P5/P6, respectively. The PCR fragments were cloned into pcDNA-3.1 (Invitrogen) to produce pcDV2-NS5F, pcDV1-NS5F, and pcDV4-NS5F, respectively. The DENV-3 strain Singapore (GenBankTM code AY662691) NS5 sequence was amplified by PCR from pcGFP-DV3-NS5F (see below) using the primers P7 and P8 and cloned into pcDNA3.1 to produce pcDV3-NS5F.
pcUb-DV-NS5F Constructs
The mouse ubiquitin gene (GenBankTM code AF118402) was amplified by PCR from an existing plasmid (a kind gift from R. Bartenschlager (University of Heidelberg)), using the primer combination P9/P11 (DENV-2), P10/P12 (DENV-3), or P9/P13 (DENV-4). The PCR products were used as co-templates together with pcDV2-NS5F, pcDV3-NS5F, or pcDV4-NS5F and the primer combinations P9/P14, P10/P15, and P9/P6, respectively, in PCRs to produce gene fusions between ubiquitin and the DENV-2, -3, and -4 NS5 genes. The PCR products were cloned into pcDV2-NS5F, pcDV3-NS5F, and pcDV4-NS5F to produce pcUb-DV2-NS5F, pcUb-DV3-NS5F, and pcUb-DV4-NS5F, respectively.
pcGFP-DV-NS5F Constructs
A gene construct encoding a DENV-2 GFP-NS5-FLAG fusion protein was constructed by overlap PCR (OL-PCR) using the plasmids pEGFP (Clontech) and pcDV2-NS5F as templates and the primer combinations P16/P17 and P18/P19, respectively. The OL-PCR product obtained using primers P16 and P19 was cloned into pcDV2-NS5F to produce pcGFP-DV2-NS5F. A plasmid encoding GFP fused to NS5 from the DENV-3 strain Singapore was constructed with a four-step OL-PCR strategy using as templates pcGFP-DV2-NS5F and two plasmids encoding the DENV-3 MTase and POL domains. Initially, an XbaI site in the MTase gene sequence was removed by OL-PCR mutagenesis without changing the amino acid sequence using primers P20, P21, P22, and P23. The GFP gene was PCR-amplified from pcGFP-DV2-NS5F using primers P16 and P24 and joined to the DENV-3 MTase gene fragment by OL-PCR. The DENV-3 POL gene was amplified from the appropriate plasmid and C-terminally FLAG-tagged using primers P25 and P26. The GFP-MTase- and POL-encoding fragments were joined in a stepwise cloning strategy and cloned into pcDNA3.1 to produce pcGFP-DV3-NS5F. Plasmids encoding GFP-NS5-FLAG fusion proteins for DENV-1 and -4 were constructed by amplifying the GFP gene from pcGFP-DV2-NS5F using primers P27 and P28 and cloning the fragments into pcDV1-NS5F and pcDV4-NS5F.
pcGFP-NS5-POL Constructs
A 900-bp fragment was amplified by PCR from pcGFP-DV2-NS5F using primers P29 and P30 and used as a co-template together with pcGFP-DV2-NS5F in a second PCR amplification, using primers P29 and P31. The resulting 1.9-kb fragment was digested with XbaI/EcoRV and exchanged with the corresponding fragment of pcGFP-DV2-NS5F to produce pcGFP-DV2-POLF (encoding DENV-2 NS5 amino acids 274–900). A 1-kb fragment was amplified from pcGFP-DV4-NS5F using oligonucleotides P32 and P33. The fragment was digested with NotI/EcoRV and exchanged with the corresponding fragment from pcGFP-DV4-NS5F to produce pcGFP-DV4-POLF (encoding DENV-4 NS5 amino acids 274–900).
pcGFP-NS5-MTase Constructs
Sequences encoding GFP N-terminally fused to the DENV-2 and -4 NS5 MTase domains (NS5 amino acids 1–273) were amplified by PCR from pcGFP-DV2-NS5F and pcGFP-DV4-NS5F using primers P34 and P35 (DENV-2) or P36 (DENV-4), respectively. The DENV-2 PCR product was digested with EcoRI/HindIII and exchanged with the EcoRI/HindIII fragment of pcGFP-DV2-NS5F, resulting in pcGFP-DV2-MT. The DENV-4 PCR product was cleaved with XcmI/HindIII and exchanged with the XcmI/HindIII fragment of pcGFP-DV4-NS5F, resulting in pcGFP-DV4-MT.
pc2xGFP-DV-NS5-a/bNLS Constructs
The sequence encoding GFP was amplified by PCR from pcGFP-DV4-NS5F using the primers P37 and either P38 or P39 so that the amplified PCR products contained the DENV-2 or -4 NS5 a/bNLS sequences. These products were subsequently used as co-templates in a second PCR amplification step together with either pcDV2-NS5F or pcDV4-NS5F and primers P37/P40 or P37/P41, respectively. The PCR products were digested with NotI/HindIII and exchanged with the NotI/HindIII-digested pcGFP-DV4-NS5F (which removed the DENV-4 NS5 sequence), resulting in the plasmids pc2xGFP-DV2-NLS and pc2xGFP-DV4-NLS.
pcGFP-DV2-NS5-DV4(370–390) and pcGFP-DV2-NS5-DV4(370–401), encoding GFP-DENV-2 NS5 fusion proteins containing DENV-4 NS5 amino acid residues 370–390 and 370–401 were constructed using a triple OL-PCR cloning strategy and the primer sets P27, P42, P43, P44, P45, P46 and P27, P42, P43, P47, P48, and P46, respectively. pcGFP-DV4-NS5-DV2(370–390) and pcGFP-DV4-NS5-DV2(370–401), encoding GFP-DENV-4 NS5 fusion proteins containing DENV-2 NS5 amino acid residues 370–390 and 370–401 were constructed using primer sets P27, P49, P50, P51, P52, P53 and P27, P49, P50, P54, P55, and P53, respectively. The final OL-PCR products were introduced into the respective plasmids using the restriction sites NheI and EcoRV. Sequences encoding DENV-4 NS5 amino acid residues 370–390 and 370–401 were isolated from pcGFP-DV2-NS5-DV4(370–390) and pcGFP-DV2-NS5-DV4(370–401) by AatII/MluI digest and cloned into the corresponding sites of the DENV-2 infectious cDNA clone pDVWS601, to produce pDV2/4-NS5(370–390) and pDV2/4-NS5(370–401), respectively.
Plasmids containing the coding sequences of KPNA1 (GenBankTM code NR_026698), KPNA2 (NM_002266), KPNA3 (NM_002267), KPNA4 (NM_002268), KPNA6 (NM_012316), and KPNB1 (NM_002265) were constructed by amplification of the respective ORFs from available plasmids (36, 37) (a kind gift from R. Depping (University of Lübeck, Germany)) by PCR using primers P56/P57, P58/P59, P60/P61, P62/P63, P64/P65, and P66/P67, respectively, which added a 5′ NheI (or ApaI for KPNB1) and a 3′ NotI site to the PCR fragments. The PCR fragments were digested with NheI/NotI (or ApaI/NotI for KPNB1) and ligated with NheI/XhoI- or ApaI/XhoI-digested pcDNA3.1 and a NotI/XhoI linker encoding a V5-epitope tag and a stop codon at the 3′-end. Because the expression levels of the V5-tagged KPNA1 to KPNA4 proteins were low, the corresponding ORFs of KPNA1, KPNA2, KPNA3, and KPNA4 were transferred into the vector pEFlink2 (38) (a kind gift from S. Goodbourn (University of London)) to enhance gene expression. All plasmids produced in the study were verified by sequencing before use.
Plasmid Transfection
A549, HEK293, BHK-21, and Vero cells were transfected using either Lipofectamine 2000 (Invitrogen) or TurboFect (Fermentas) according to the manufacturer's recommendations. For IL-8 luciferase reporter gene assays, HEK293 and A549 cells were transfected using GeneJuice (Novagen) and Lipofectamine LTX (Invitrogen), respectively.
Cellular Fractionation and Western Blotting
Huh-7 cells (1 × 107) were infected with DENV-2 or -4 at a multiplicity of infection of 3 or mock-infected. At 28 h postinfection, the cells were detached, washed twice with ice-cold PBS, and resuspended in hypotonic swelling buffer (10 mm Tris-HCl (pH 8.0), 10 mm NaCl, 1.5 mm MgCl2, and proteinase inhibitors (Complete, mini, EDTA-free; Roche Applied Science). After a 10-min incubation on ice, the cells were passed 20 times through both 20- and 26-gauge needles to lyse the cells and detach the perinuclear membranes from the nucleus, as described previously (39). The lysates were centrifuged at 800 × g for 10 min at 4 °C. This process was repeated, and the pellets were combined and taken as the nuclear fraction. The supernatant was then centrifuged at 16,000 × g for 20 min at 4 °C to produce a heavy membrane pellet (16K) and a soluble cytoplasmic fraction. The nuclear and 16K pellets were resuspended in swelling buffer. All fractions were adjusted to 1× Laemmli sample buffer before SDS-PAGE analysis. SDS-PAGE and Western blotting were done as previously described (30), using antibodies against the DENV NS5 and E proteins (monoclonal antibody 4G2) and histone H3 (Cell Signaling).
Immunofluorescence Assay (IFA) and Confocal Imaging
Cells grown on glass coverslips in 24-well trays were either transfected with expression plasmids, infected with DENV, or mock-transfected/infected. At an appropriate time post-transfection/infection, the cells were fixed with 4% formaldehyde in PBS for 5 min, followed by permeabilization in 1% (v/v) Triton X-100, and then analyzed by IFA as described previously (30), using primary antibodies against NS5 (30), the DENV E protein, the FLAG epitope (Sigma), GFP (Roche Applied Science), KPNA2 (Sigma), KPNB1 (Abcam), or the V5 epitope (Invitrogen/Sigma) and secondary antibodies coupled to Alexa-Fluor 568 or Alexa-Fluor 488 (Invitrogen). The affinity of the polyclonal rabbit antiserum raised against DENV-2 NS5 (30) to the DENV-1, -3, and -4 NS5 proteins was increased by cross-absorption following the method of Miller et al. (40). Cells were mounted in Vectashield containing DAPI (Vecta Laboratories). Confocal laser-scanning microscopy (CLSM) was done using a Leica confocal microscope (TCS-SP2). Fluorescence recovery after photobleaching (FRAP) analysis was done on living cells expressing GFP fusion proteins using a Leica DMI6000 microscope equipped with a spinning disc system (PerkinElmer Life Sciences) and a Hamamatsu EMCCD camera. Fluorescence was quantified, and data were analyzed using Volocity version 6.0 software (PerkinElmer Life Sciences).
Leptomycin B Assays
Vero and A549 cells were transfected with either pcGFP-DV2-NS5F or pcGFP-DV4-NS5F. At 16 h post-transfection, leptomycin B (LMB) (Sigma) was added to the culture medium to a final concentration of 15 μg/ml or not added, and the cellular localization of GFP was monitored at 5, 20, 60, 120, and 360 min post-treatment. At 360 min post-treatment, cells were fixed and analyzed by CLSM. The action of LMB was confirmed with a CRM1-dependant luciferase reporter assay. A549 cells were transfected with pRL-TK (Promega), pLUCSALRRE, and either pRev (41) (kind gifts of A. Whitehouse (University of Leeds)) or no plasmid and treated with 15 μg/ml LMB or left untreated. Dual luciferase assays were performed at 16 h post-treatment using a Dual-Luciferase reporter assay system (Promega), and luminescence was read on a Modulus single tube multimode reader (Turner Biosystems). At least two independent experiments were performed for each assay with all readings performed in triplicate.
Co-immunoprecipitation (Co-IP) Assays
HEK293 cells were co-transfected with plasmids encoding the proteins of interest. 24 h post-transfection, the cells were harvested, washed twice in cold PBS, and then lysed by incubation in either lysis buffer 1 (TBS containing 1% (w/v) Brij97 (Sigma) and proteinase inhibitors (Complete, Roche Applied Science)) or lysis buffer 2 (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Triton X-100, and proteinase inhibitors) for 30 min on ice followed by brief sonication. The lysates were centrifuged at 13,000 × g for 10 min at 4 °C, and supernatants were incubated with either anti-V5 magnetic beads (MBL International), anti-FLAG® M2 magnetic beads (Sigma), or GFP-Trap (Chromotek) for 16 h at 4 °C with rotation. The anti-V5 or anti-FLAG® M2 magnetic beads were then collected by magnetic separation and washed three times with either lysis buffer 1 or 2, respectively. Proteins were eluted from the beads by boiling in SDS-PAGE sample buffer for 5 min. The eluted proteins and input proteins in the total cell lysates were analyzed by SDS-PAGE and Western blotting as described above, using anti-FLAG and V5 antibodies. Eukaryotic expression plasmids encoding a FLAG-tagged Ras-related C3 botulinum toxin substrate 1 protein (Rac1-F (42), a kind gift of N. Klugbauer (University of Freiburg)) and human STAT2 (a kind gift of S. Goodbourn) were used for co-IP studies.
siRNA Knockdown Experiments
HEK293 cells were transfected with either AllStars Negative Control siRNA-488 (Qiagen) or a pool of four siRNAs against KPNA2 or KPNB1 (Dharmacon) using DharmaFECT1 (Dharmacon), or cells were left untreated as controls. At 72 h post-siRNA transfection, the cells were infected with DENV-2, mock-infected, or transfected with pcDV2-NS5F. Alternatively, HEK-DV2-NS5F cells were transfected with the respective siRNA pools as described above, and at 24 h post-siRNA transfection, they were incubated in medium containing 1 μg/ml doxycycline for a further 72 h. 96 h after siRNA transfection, the cells were either lysed in SDS-PAGE sample buffer and subjected to SDS-PAGE and Western blot analysis using antibodies against NS5, KPNA2 (Sigma), or KPNB1 (Abcam) or fixed and analyzed by IFA and CLSM as described above.
IL-8 Luciferase Reporter Gene Assays
HEK293 cells were co-transfected with either a plasmid encoding NS5 or the positive control pMal together with a plasmid containing a firefly luciferase reporter gene under control of the IL-8 promoter (43) (a kind gift of K. Fitzgerald (University of Massachusetts)) and pRL-TK (Promega). Dual luciferase assays were performed 16 h post-transfection as described above.
Quantitative RT-PCR (qRT-PCR)
RNA was extracted using an SV total RNA isolation kit (Promega) and reverse transcribed using ImPromII reverse transcriptase (Promega). qRT-PCR was performed using an ABsoluteTM QPCR SYBR® Green mix (ABgene) and a Stratagene MX3500 cycler. The primer pairs P68/P69 and P70/P71 were used for amplification of the IL-8 and neomycin resistance gene transcripts, respectively.
RESULTS
Differential Nuclear Localization of Transiently Expressed DENV-1 to -4 NS5
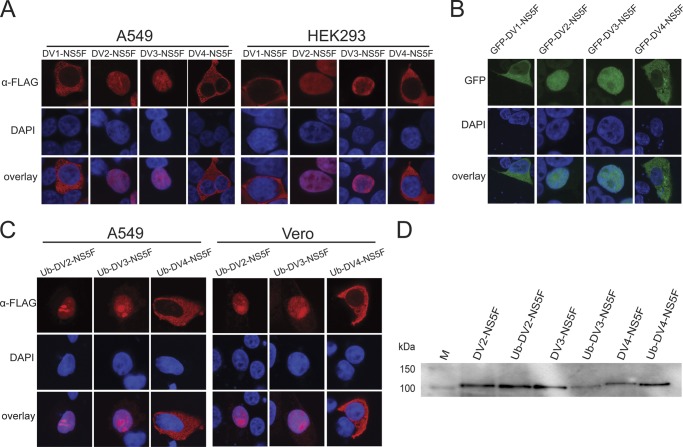
Previous studies have shown that DENV-2 NS5 is nucleus-localized in a range of cell types, both during infection and when expressed as a GFP-NS5 fusion protein (27, 30, 31, 40, 44–46). In order to determine if nuclear localization is a general property of DENV NS5, the localization of different recombinant forms of NS5, transiently expressed in cells, was examined for all four DENV serotypes. Plasmids encoding NS5, either N-terminally tagged with GFP and C-terminally tagged with the FLAG epitope (pcGFP-DV-NS5F plasmids) or C-terminally tagged with the FLAG epitope only (pcDV-NS5F plasmids) were constructed using the DENV-1 to -4 NS5 genes. The pcDV-NS5F plasmids were used to transfect human A549 and HEK293 cells, and the localization of NS5 was examined by IFA and CLSM (Fig. 1A). As previously reported, DENV-2 NS5 was nucleus-localized in both cell lines, as was DENV-3 NS5. Surprisingly, the DENV-1 and -4 NS5 proteins were confined to the cytoplasm. Similar results were obtained at 48 h after transfection and when the DENV-1 to -4 NS5 genes were transiently expressed in Vero and BHK-21 cells (data not shown). The same nuclear/cytoplasmic localization patterns for NS5 of the different DENV serotypes were observed when the pcGFP-DV-NS5F plasmids were transiently expressed in A549 cells (Fig. 1B) and Vero cells (data not shown), showing that fusion of GFP to the N terminus of the different NS5 proteins did not influence their cellular localization. Compared with the analysis of NS5 localization using the FLAG epitope and IFA, weak nuclear fluorescence was observed in cells transiently expressing GFP-DV1-NS5F and GFP-DV4-NS5F. It is possible that this fluorescence was mediated by GFP itself because it has been reported that GFP multimers with a size of >45 kDa accumulate in small amounts in the nucleus (47).
FIGURE 1.
Subcellular localization of transiently expressed DENV-1 to -4 NS5. A, CLSM images of A549 (left panels) or HEK293 cells (right panels) transfected with pcDV1-NS5F (DV1-NS5F), pcDV2-NS5F (DV2-NS5F), pcDV3-NS5F (DV3-NS5F), and pcDV4-NS5F (DV4-NS5F) and immunostained using an anti-FLAG antibody (α-FLAG) at 24 h post-transfection. B, CLSM images of A549 cells transfected with pcGFP-DV1-NS5F (GFP-DV1-NS5F), pcGFP-DV2-NS5F (GFP-DV2-NS5F), pcGFP-DV3-NS5F (GFP-DV3-NS5F), and pcGFP-DV4-NS5F (GFP-DV4-NS5F) and analyzed for GFP fluorescence at 24 h post-transfection. C, CLSM images of A549 (left panels) and Vero cells (right panels) transfected with pcUb-DV2-NS5F (Ub-DV2-NS5F), pcUb-DV3-NS5F (Ub-DV3-NS5F), and pcUb-DV4-NS5F (Ub-DV4-NS5F) and immunostained using an anti-FLAG antibody at 24 h post-transfection. Nuclear DNA was stained with DAPI in all experiments. D, HEK293 cells were transfected with the plasmids described in A and C. At 24 h post-transfection, total cell lysates were prepared and analyzed by SDS-PAGE and Western blotting. The respective NS5 proteins were detected using an anti-FLAG antibody. The positions of relevant molecular mass markers (M) are shown.
It has previously been shown that only NS5 generated by N-terminal proteolytic processing (either in the context of the viral polyprotein or when fused C-terminally to ubiquitin) could degrade STAT2, suggesting that the conformation of the N-terminal region of NS5 may affect its properties (15). The initial amino acid of the NS5 proteins C-terminally tagged with the FLAG epitope had been changed to methionine and was not generated by proteolytic processing. The importance of the N-terminal conformation of NS5 to nuclear localization was therefore examined by constructing plasmids that encoded NS5F C-terminally fused to ubiquitin (pcUb-DV-NS5F plasmids) and using them for transient expression assays. Despite repeated attempts, a plasmid encoding a ubiquitin DENV-1 NS5 fusion protein could not be obtained. The localization of the transiently expressed NS5 proteins generated by N-terminal ubiquitin cleavage was then examined in A549 and Vero cells (Fig. 1C). As for the NS5F proteins, the transiently expressed DENV-2 and -3 NS5F proteins that had been generated by N-terminal ubiquitin cleavage accumulated in the nucleus, whereas the DENV-4 NS5F protein was confined to the cytosol (Fig. 1C). The cleavage of ubiquitin from the N terminus of NS5 was verified by Western blot analysis (Fig. 1D). Collectively, the results demonstrated that there were serotype-specific differences in the nuclear localization of transiently expressed NS5 in a range of cell lines.
Differential Localization of DENV-1 to -4 NS5 during Virus Infection
To confirm that the transient expression experiments were an accurate representation of the events that occurred during virus infection, human A549 cells and insect C6/36 cells were infected with DENV-1 to -4, and at 24 h post-infection, the localization of the respective NS5 proteins was determined by IFA using an antibody against DENV-2 NS5. Because the specificity of the anti-NS5 antibody to the DENV-1 to -4 NS5 proteins varied, the antibody was preabsorbed to transfer membranes containing the respective NS5 proteins, to increase its serotype specificity. In support of the transient expression experiments, the DENV-2 and -3 NS5 proteins were localized predominantly to the nucleus, whereas the DENV-1 and -4 NS5 proteins were cytoplasmically localized in both human and insect cells (Fig. 2, A and B).
FIGURE 2.
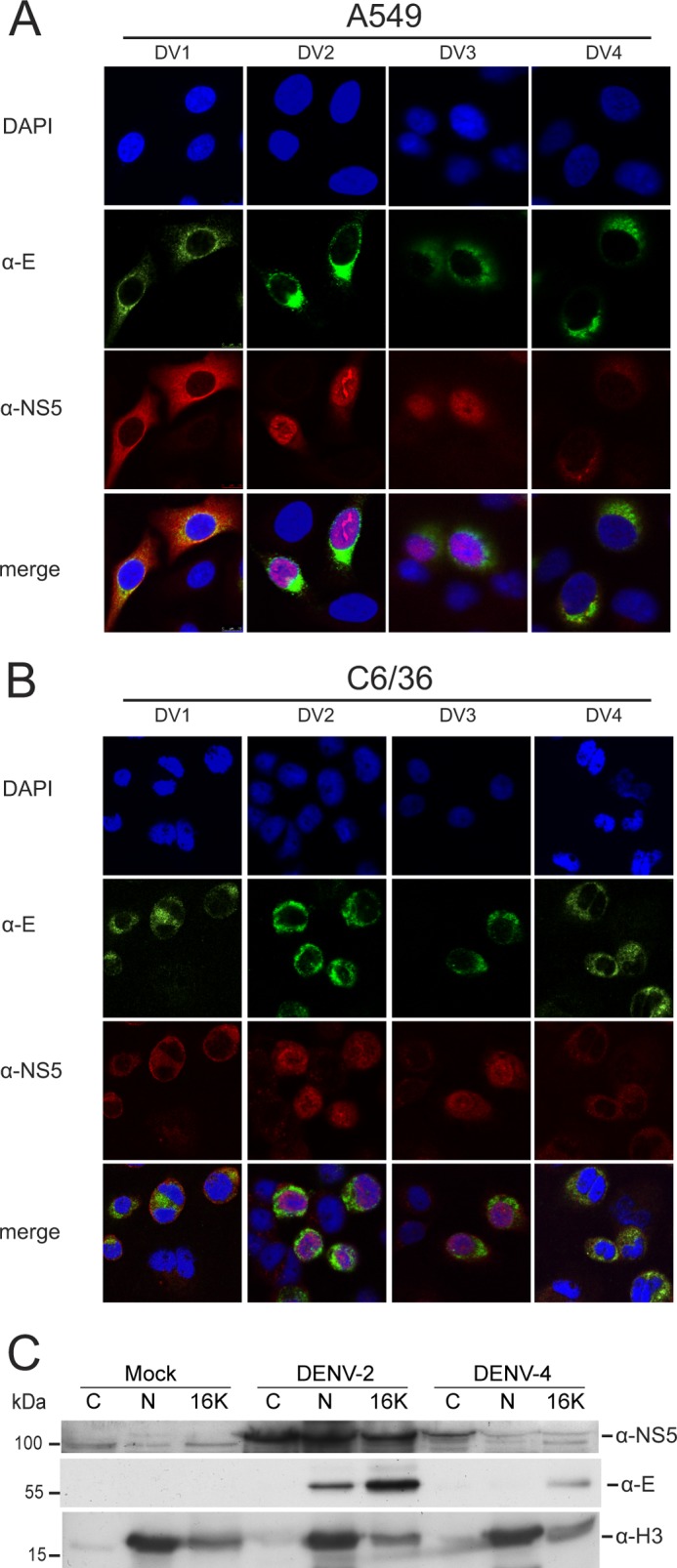
Subcellular localization of DENV-1 to -4 NS5 during viral infection. CLSM images of A549 cells (A) and insect C6/36 cells (B) infected with DENV-1, -2, -3 and -4 at a multiplicity of infection of 2.0 and immunostained using anti-E (α-E) and anti-NS5 (α-NS5) antibodies at 30 h postinfection. In each panel, a selection of infected (as evidenced by anti-E staining) and non-infected cells are shown as a control for nonspecific staining by the anti-NS5 antibody. Nuclear DNA was stained with DAPI. C, Western blot analysis of cytoplasmic (C), nuclear (N), and cytoplasmic membrane (16K) fractions prepared from Huh-7 cells at 30 h postinfection with DENV-2 or -4 (multiplicity of infection of 3) or mock-infected. 10 μg of protein from each cellular fraction was analyzed for the presence of the DENV NS5 and E proteins and histone H3 using specific antibodies by Western blotting. Histone H3 was used as a nuclear marker. The positions of relevant molecular mass markers are shown in kDa.
To complement the IFA analysis, the localization of NS5 in infected cells was then examined by cell fractionation followed by Western blotting. In this experiment and subsequent studies, we concentrated on the DENV-2 and -4 NS5 proteins as examples of proteins that did and did not localize to the nucleus, respectively. The cells were fractionated into nuclear, cytoplasmic, and a cytoplasmic heavy membrane fraction previously shown to contain flavivirus RdRp activity (39, 48). Overall, the cell fractionation experiments supported the IFA analysis because a major portion of DENV-2 NS5 was found in the nuclear fraction, whereas only trace amounts of DENV-4 NS5 were detected in the nuclear fraction (Fig. 2C). DENV replication is known to occur in intimate association with perinuclear membranes (20, 21, 40); therefore, the possibility could not be excluded that the low amount of DENV-4 NS5 present in the nuclear fraction was due to its association with perinuclear membranes that were not removed from the nuclear membrane, as suggested by the detection of the E protein in the nuclear fraction. Compared with the IFA analysis, a greater proportion of DENV-2 NS5 was found in the cytoplasm both in the soluble and heavy membrane fractions, suggesting that the analysis of DENV-2 NS5 localization by IFA exaggerates the proportion of NS5 found in the nucleus.
DENV-4 NS5 Does Not Shuttle between the Nucleus and the Cytoplasm
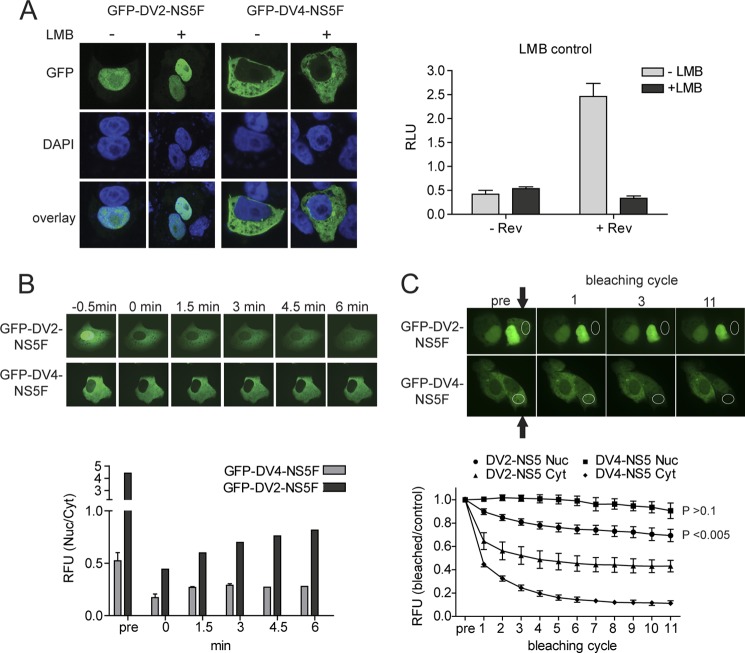
It has previously been reported that DENV-2 NS5 shuttles between the nucleus and cytoplasm using the importin-α/β import and the CRM1-mediated export pathways, respectively (31). To exclude the possibility that DENV-4 NS5 was imported into the nucleus and rapidly exported, preventing nuclear accumulation, Vero cells transiently expressing the DENV-2 and -4 GFP-NS5F proteins were treated with LMB, a specific inhibitor of CRM1-mediated nuclear export. LMB treatment did not increase the accumulation of DENV-4 NS5 to levels detectable by IFA and CLSM, providing further evidence that DENV-4 NS5 does not accumulate in the nucleus (Fig. 3A).
FIGURE 3.
Analysis of DENV-2 and -4 NS5 nuclear-cytoplasmic trafficking. A, Vero cells were transfected with either pcGFP-DV2-NS5F (GFP-DV2-NS5F) or pcGFP-DV4-NS5F (GFP-DV4-NS5F). At 16 h post-transfection, the cells were treated with either 15 μg/ml LMB (+) or DMSO (−). 6 h post-treatment, GFP fluorescence was analyzed by CLSM. Nuclear DNA was stained with DAPI. The inhibition of CRM1 by LMB was confirmed using a CRM1-dependant luciferase-based reporter system. Vero cells were transfected with either pLUCSALRRE, which expresses a transcript containing an HIV rev-responsive element and encodes luciferase (−Rev), or pLUCSALRRE and a plasmid encoding HIV rev (+Rev). The cells were treated with 15 μg/ml LMB after transfection. At 16 h post-transfection, the luciferase activity in the cells was measured and expressed as relative light units (RLU). Error bars, measurement averages of three independent experiments. B, Vero cells were transfected with either pcGFP-DV2-NS5F (GFP-DV2-NS5F) or pcGFP-DV4-NS5F (GFP-DV4-NS5F). At 24 h post-transfection, the movement of NS5 between the cytoplasm and nucleus of transfected cells was analyzed by FRAP. The nuclei of transfected cells (n = 5) were bleached, and the relative ratios of nuclear versus cytoplasmic fluorescence (RFU (Nuc/Cyt)) at the given times postbleaching were determined. Representative images of the photobleaching of one transfected cell are shown. The images of cells expressing GFP-DV2-NS5F were purposely overexposed to visualize the cytoplasmic fraction of NS5, whereas the original pictures were used for quantification. C, Vero cells were transfected as in B. For FRAP analysis, the indicated region (white circle) of the cytoplasm of a transfected cell was bleached, and CLSM images were taken 29 s later. This sequence was repeated 11 times. Representative images are shown prebleaching and for the first, third, and eleventh bleaching cycles. A bleached cell (arrow) is shown beside an untreated cell for comparison. The images were used to determine the relative fluorescence (RFU) intensities of the cytoplasm (triangles and diamonds) or nuclei (squares and circles) in bleached versus unbleached cells (n = 5; error bars, S.D.), with all prebleached RFU values set to 1. The significance of the variation between the nuclear fluorescence before bleaching and after 11 bleaching cycles was determined using a two-tailed Student's t test and is shown for cells expressing GFP-DV2-NS5F and GFP-DV4-NS5F.
Further analysis of NS5 nuclear shuttling was done by FRAP experiments. In order to conduct these experiments, we made use of the weak nuclear fluorescence observed in cells expressing GFP-DV4-NS5F (see above). We first investigated the nuclear import of DENV-2 and -4 GFP-NS5F. Vero cells transiently expressing either GFP-DV2-NS5F or GFP-DV4-NS5F were subjected to nuclear photobleaching, and the recovery of nuclear fluorescence and the corresponding change in cytoplasmic fluorescence were measured over a 6-min interval in 30-s increments (Fig. 3B). The relative nuclear fluorescence of cells expressing GFP-DV2-NS5F recovered in an almost linear fashion over the duration of the experiment. However, the recovery of DENV-2 NS5 nuclear fluorescence was slow and incomplete, suggesting that the major part of the cytoplasmic NS5 pool could not be rapidly transported to the nucleus to replenish the nuclear NS5 pool, supporting the earlier finding that phosphorylation differentially regulates the nuclear localization of DENV-2 NS5 (27, 28). By contrast, cells expressing GFP-DV4-NS5F showed only a slight recovery during the first 30 s after photobleaching, which could be attributed to spontaneous GFP recovery after photobleaching (49), with no further increase in nuclear fluorescence after this time.
The movement of NS5 from the nucleus to the cytoplasm was then investigated by cytoplasmic photobleaching of Vero cells expressing either GFP-DV2-NS5F or GFP-DV4-NS5F. During each bleaching cycle, about 30% of the cytoplasm of a GFP-expressing cell was photobleached; 29 s later, an image was taken, and the next bleaching cycle would start for a total of 11 cycles. Nuclear and cytoplasmic fluorescence values were normalized to the respective values of a transfected non-bleached neighboring cell (Fig. 3C). Over the course of 11 bleaching cycles, the nuclear fluorescence of cells expressing GFP-DV4-NS5F was not significantly decreased (p > 0.1), whereas the nuclear fluorescence in cells expressing GFP-DV2-NS5F was (p < 0.005). Taken together, these experiments provide evidence that transiently expressed DENV-4 NS5 does not shuttle between the cytoplasm and the nucleus.
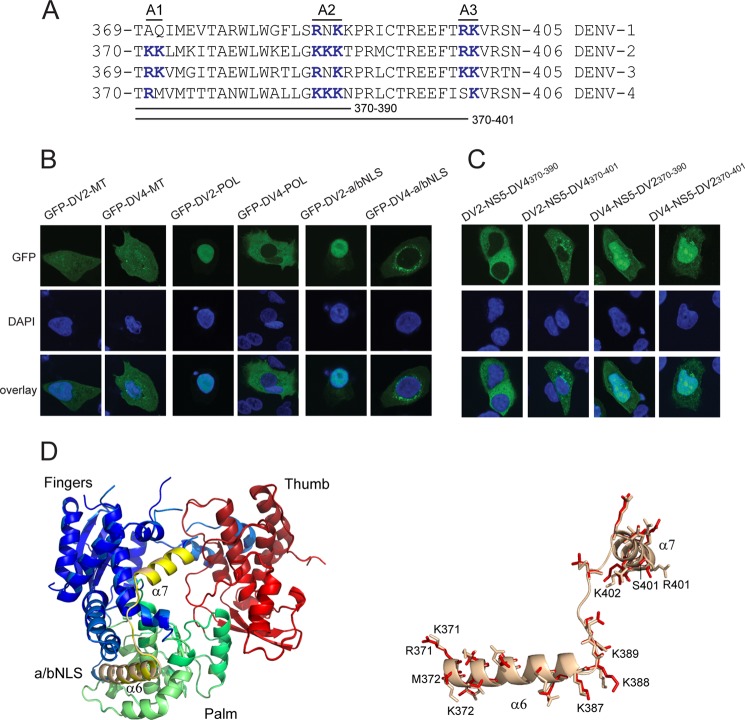
DENV-4 NS5 Does Not Have a Functional a/bNLS
Comparison of the amino acid sequence of the DENV-2 NS5 a/bNLS with the corresponding sequences of the DENV-1, -3, and -4 NS5 proteins shows that the basic clusters of amino acids essential for nuclear localization of DENV-2 NS5 (28–30) are not conserved (Fig. 4A). We therefore examined whether the failure of DENV-4 NS5 to accumulate in the nucleus was due to the amino acid changes in the a/bNLS region or, rather, inhibitory regions in the remainder of the protein. Plasmids encoding GFP N-terminally fused to the DENV-2 and -4 MTase (amino acids 1–273) and POL (amino acids 274–900) domains were transiently expressed in A549 cells, and the localization of the corresponding proteins was analyzed (Fig. 4B). As observed in previous studies, the DENV-2 GFP-MTase and GFP-POL were predominantly localized throughout the cytoplasm and nucleus and the nucleus, respectively (30, 31). By contrast, although the DENV-4 GFP-MTase protein was localized similarly to the DENV-2 GFP-MTase protein, the DENV-4 GFP-POL protein was confined to the cytoplasm, showing that the MTase domain did not inhibit DENV-4 NS5 nuclear localization. Sequences encoding the DENV-2 a/bNLS (amino acids 369–405) and the corresponding region of DENV-4 NS5 were then C-terminally fused to a tandem GFP gene construct, encoding a protein >55 kDa in molecular mass that could only be transported to the nucleus in an active process. Transient expression of the genes encoding the 2×GFP-a/bNLS proteins in A549 cells demonstrated that the DENV-2 NS5 a/bNLS was capable of mediating the nuclear transport of a tandem GFP protein, whereas the corresponding region of the DENV-4 NS5 protein did not. Taken together, the results suggested that DENV-4 NS5 amino acids 369–405 do not function as an NLS.
FIGURE 4.
DENV-4 NS5 does not have a functional a/bNLS. A, alignment of the DENV-1, -2, -3, and 4 NS5 amino acid sequences corresponding to the DENV-2 NS5 a/bNLS. Three basic amino acid clusters found in the DENV-2 a/bNLS (termed A1, A2, and A3) and conserved basic amino acid sequences in the other sequences are shown in boldface type and colored blue. The amino acid sequences interchanged between DENV-2 and -4 NS5 are shown. B, CLSM images of A549 cells transfected with pcGFP-DV2-MT (GFP-DV2-MT) and pcGFP-DV4-MT (GFP-DV4-MT) (left panels), pcGFP-DV2-POL (GFP-DV2-POL) and pcGFP-DV4-POL (GFP-DV4-POL) (middle panels), and pc2xGFP-DV2-NLS (GFP-DV2-a/bNLS) and pc2xGFP-DV4-NLS (GFP-DV4-a/bNLS) (right panels). C, CLSM images of A549 cells transfected with pcGFP-DV2-NS5-DV4(370–390) (DV2-NS5-DV4370–390), pcGFP-DV2-NS5-DV4(370–401) (DV2-NS5-DV4370–401), pcGFP-DV4-NS5-DV2(370–390) (DV4-NS5-DV2370–390), and pcGFP-DV4-NS5(370–401) (DV4-NS5-DV2370–401). The transfected cells were analyzed for GFP fluorescence at 24 h post-transfection. Nuclear DNA was stained with DAPI in all experiments. D, superimposition of models of the POL structures of DENV-2 NS5 and DENV-2 NS5 containing DENV-4 amino acids 370–401. The DENV-2 POL sequences were used to generate hidden Markov models (72), which were then compared against a database of hidden Markov models with solved atomic structures. The MODELLER software suite (73) was used to generate the three-dimensional models based upon the x-ray structure of the DENV-3 NS5 POL domain (60). PyMOL (74) was the used to align the structures and for molecular graphics. The aligned POL structures are shown on the left, with the fingers, thumb, and palm subdomains and the a/bNLS (containing α helices 6 and 7) shaded in blue/light blue, red/salmon, green/pale green, and yellow/sand for the DENV-2 and DENV-2/4 POLs, respectively. On the right, an expanded view of the a/bNLS alignment is shown in schematic form. Amino acids that differ in side chain residue or orientation are shown in stick form. The amino acids in the A1, A2, and A3 clusters are numbered for the DENV-2 and -4 sequences.
To further test this finding, we exchanged the DENV-2 sequences encompassing the a/bNLS or the “minimal” a/bNLS (amino acids 369–389) with the corresponding sequences of DENV-4 NS5 and vice versa in the context of plasmids encoding the GFP-NS5F proteins. Transient expression of the plasmids in A549 cells revealed that the introduction of the DENV-4 sequences into the GFP-DV2-NS5F protein abolished nuclear localization (Fig. 4C). The introduction of the DENV-2 a/bNLS and minimal a/bNLS amino acid sequences into the GFP-DV4-NS5F protein increased the nuclear localization of DENV-4 NS5. However, compared with the nuclear localization of DENV-2 NS5, the modified DENV-4 NS5 proteins were substantially less nucleus-localized (Fig. 4C). These results suggested that the DENV-2 NS5 a/bNLS sequences were sufficient to localize the chimeric GFP-DV4-NS5 protein to the nucleus but that other regions of the DENV-4 NS5 protein also determined the efficiency of nuclear localization.
To confirm these results in the context of virus infection, sequences encoding the DENV-4 NS5 amino acids 370–401 and 370–390 were introduced into a DENV-2 infectious cDNA clone. In vitro RNA transcripts produced from the clones were used to transfect mammalian BHK-21 and insect C6/36 cells. However, despite several attempts and repeated passaging of culture supernatants from the initially transfected cells to encourage reversion, it was not possible to recover DENV-2 viruses containing the DENV-4 sequences (data not shown).
Interaction of Importin-α with NS5
To determine if the defect in DENV-4 NS5 nuclear localization lay in the inability of the protein to interact with importin-α, co-IP experiments were conducted. It is known that there are seven isoforms of human importin-α (KPNA1 to -7), which are classed into three subfamilies (α1, containing KPNA1, -5, and -6; α2, containing KPNA2 and -7; and α3, containing KPNA3 and -4 (50, 51)). DENV-2 NS5 has previously been shown to interact with a precomplexed murine importin-α (mImp58)/importin-β heterodimer by ELISA and a pull-down assay (29, 30). Because mImp58 corresponds with the human importin-α isoform KPNA2, we initially examined the interaction of DENV-2 and -4 NS5F with V5-tagged KPNA2, using proteins exogenously expressed in HEK293 cells. However, using co-IP conditions previously used to examine the interaction of KPNA2 with a FLAG-tagged version of Rac1 (42), neither DENV-2 nor -4 NS5F bound KPNA2-V5, although the interaction with Rac1-F was confirmed (Fig. 5A). The interaction of DENV-2 and -4 NS5F with KPNA2-V5, V5-tagged versions of KPNA1, -3, -4, and -6, and KPNB1 was then examined under varying salt (75–300 mm) and detergent (0.1–1% Triton X-100) conditions. No interactions were observed between DENV-2 and -4 NS5 and KPNA1, -3, or -4 or KPNB1. Furthermore, it was not possible to unequivocally demonstrate an interaction between DENV-2 and -4 NS5 with KPNA2 or KPNA6 that was either specific or correlated with DENV-2 NS5 nuclear localization (data not shown). By comparison, both DENV-2 and -4 NS5 specifically bound to exogenously expressed STAT2, a known binding partner of DENV-2 NS5 (Fig. 5B) (15, 16).
FIGURE 5.
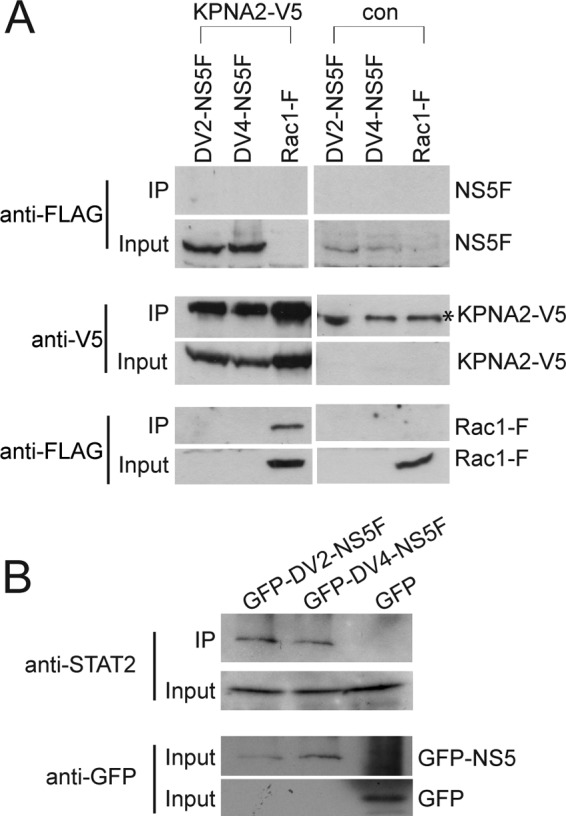
Co-IP analysis of DENV-2 and -4 NS5 interactions. A, HEK293 cells were co-transfected with either a plasmid coding for KPNA2-V5 or no plasmid (con) together with pcDV2-NS5F (DV2-NS5F), pcDV4-NS5F (DV4-NS5F), or pc3xFLAG-Rac1 (Rac1-F). Co-IP analysis was done using anti-V5 beads. The immunoprecipitates (IP) and total cell lysates (Input) were analyzed by SDS-PAGE and Western blotting using an anti-FLAG antibody to detect NS5F and Rac1-F and an anti-V5 antibody to detect KPNA2-V5. *, nonspecific band recognized by the anti-V5 antibody. B, HEK293 cells were co-transfected with either pGFP-DV2-NS5F (GFP-DV2-NS5F), pGFP-DV4-NS5F (GFP-DV4-NS5F), or pEGFP (GFP) together with a plasmid coding for human STAT2. Co-IP analysis was conducted using GFP-Trap beads and TBS containing 150 mm NaCl and 1% Triton X-100 for cell lysis and washing. The immunoprecipitates (IP) and total cell lysates (Input) were analyzed by SDS-PAGE and Western blotting using anti-STAT2 and anti-GFP antibodies to detect STAT2 and GFP-NS5/GFP, respectively. All co-IP assays were done 24 h post-transfection.
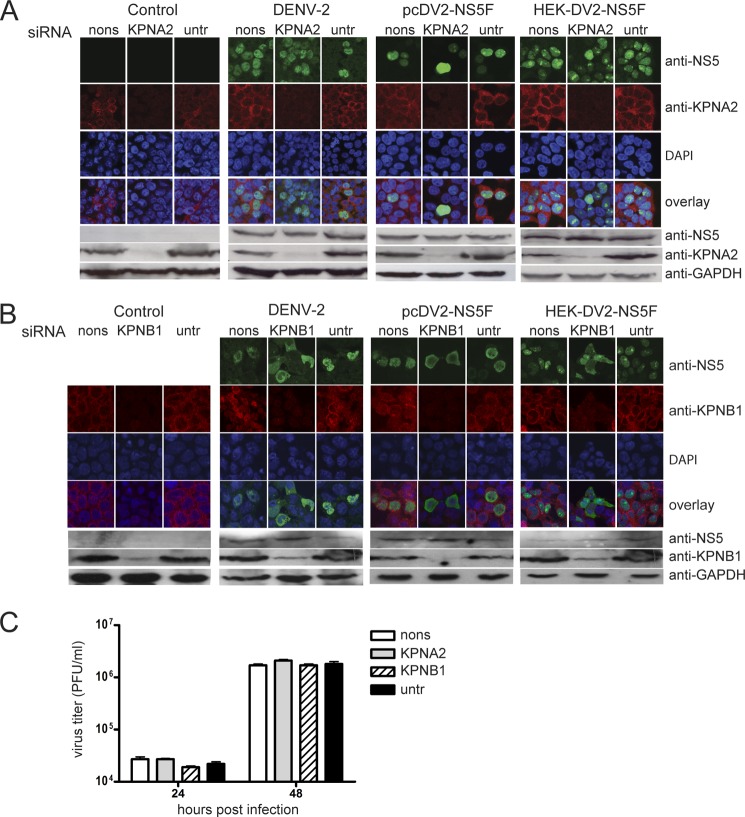
Knockdown of KPNB1 but Not KPNA2 Affects DENV-2 NS5 Nuclear Localization
Following the results of the co-IP experiments, siRNA knockdown experiments were done to examine the requirement of KPNA2 and KPNB1 for NS5 nuclear localization. KPNA2 was knocked down in HEK293 cells before DENV-2 infection or either transient or stably induced expression of DENV-2 NS5. Only minimal amounts of KPNA2 could be detected in KPNA2 siRNA-treated cells by Western blot analysis compared with non-sense siRNA-treated or -untreated cells (Fig. 6A). Surprisingly, there was little or no decrease in NS5 nuclear localization between cells transfected with the KPNA2 siRNAs, non-silencing siRNAs, and untransfected cells. Following the same experimental procedure, the effect of KPNB1 knockdown on DENV-2 NS5 nuclear localization was examined. In this case, although KPNB1 knockdown was not as complete as for KPNA2, there was a major decrease in the amount of nucleus-localized NS5 in KPNB1 siRNA-treated cells, either during DENV-2 infection or when NS5 alone was transiently or stably expressed in cells (Fig. 6B). The knockdown of either KPNA2 or KPNB1 did not effect DENV-2 replication as determined by the amounts of NS5 protein in the cell lysates or the titer of virus in the culture supernatants (Fig. 6, A–C).
FIGURE 6.
Effects of siRNA knockdown of KPNA2 and KPNB1 on NS5 nuclear localization. A, HEK293 cells or HEK293 cells stably expressing DENV-2 NS5 (HEK-DV2-NS5F), in response to doxycycline induction, were transfected with either a non-sense (nons) or a KPNA2 siRNA pool or left untreated (untr). 72 h post-transfection, HEK293 cells were either not treated (Control), infected with DENV-2, or transfected with pcDV2-NS5F. The HEK-DV2-NS5F cells were treated with doxycycline to induce NS5 production. 24 h later, the localization and amounts of NS5 and KPNA2 in the respective cells were analyzed by IFA and SDS-PAGE and Western blotting. The top panels show CLSM images of the cells immunostained for the presence of NS5 (shown in green) using anti-NS5 (for DENV infection) or anti-FLAG antibodies and the presence of KPNA2 (shown in red) using an anti-KPNA2 antibody. Nuclear DNA was stained with DAPI in all experiments. The bottom panels show an analysis of KPNA2 knockdown efficiency by SDS-PAGE and Western blot analysis of extracts from the cells using the same antibodies and an antibody against GAPDH as a loading control. B, the experiment described in A was repeated except that the siRNA pool against KPNA2 was replaced with an siRNA pool against KPNB1. The efficiency of KPNB1 knockdown was assessed by IFA and Western blot analysis using antibodies against KPNB1. The immunostaining of DENV-infected cells using anti-NS5 and anti-KPB1 antibodies was done on duplicate samples because both antibodies were raised in rabbits. In this case, only the CLSM images of the anti-NS5- and DAPI-stained cells were overlaid. C, the titers of virus in the culture supernatants of DENV-2-infected HEK293 cells transfected with either a non-sense (nons), a KPNA2, or a KPNB1 siRNA pool or left untreated (untr) 48 h prior to infection were determined by a plaque assay. The titers were determined using two independent DENV-2-infected cell samples at 24 and 48 h postinfection. Error bars, S.D.
DENV NS5-induced IL-8 Gene Expression Does Not Correlate with Nuclear Localization
We previously showed that decreased NS5 nuclear localization, either during DENV-2 infection or when transiently expressed, increased IL-8 secretion (30). It was therefore of interest to determine if the observed serotypic differences in NS5 nuclear localization correlated with differences in IL-8 gene expression. An IL-8 luciferase gene reporter assay was used to measure IL-8 gene expression in HEK293 cells transiently expressing plasmids encoding DENV-1, -2, -3, and -4 NS5F or DENV-2, -3, and -4 Ub-NS5F (Fig. 7A). Expression of DV3-NS5F or Ub-DV3-NS5F resulted in the highest induction of luciferase expression from the IL-8 promoter, followed by DV1-NS5F and DV2-NS5F/Ub-DV2-NS5F. Luciferase gene expression driven by the IL-8 promoter was lowest in cells expressing DV4-NS5F or Ub-DV4-NS5F. This pattern was supported by qRT-PCR analysis of IL-8 mRNA amounts in HEK293 cells expressing DENV-1, -2, -3, and -4 NS5F (Fig. 7B). The results showed that although there were serotypic differences in the ability of NS5 to induce IL-8 expression, these differences did not strictly correlate with the serotypic differences in NS5 nuclear localization.
FIGURE 7.
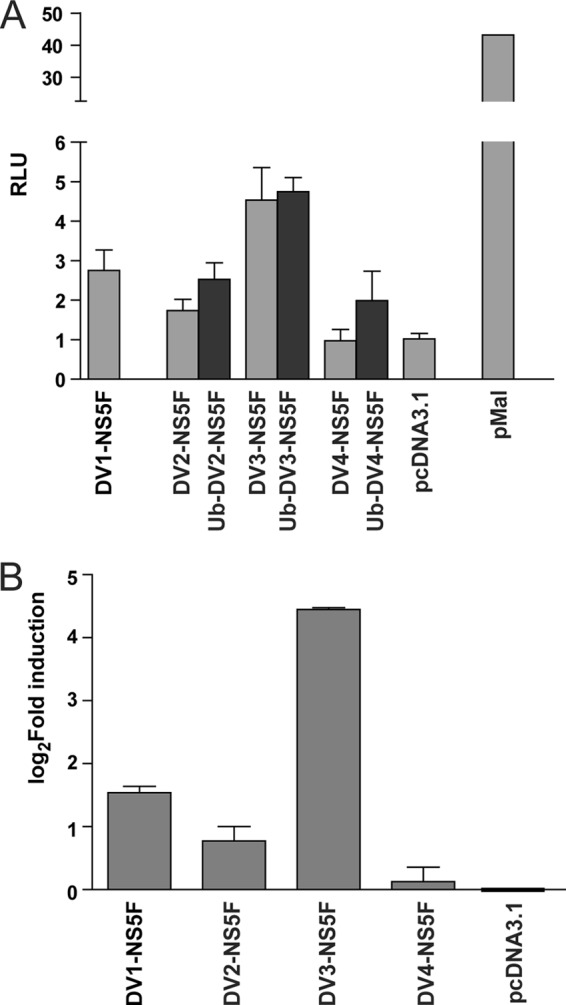
The effect of DENV1–4 NS5 on IL-8 gene expression. A, HEK293 cells were transfected with either pcDV1-NS5F (DV1-NS5F), pcDV2-NS5F (DV2-NS5F), pcUb-DV2-NS5F (Ub-DV2-NS5F), pcDV3-NS5F (DV3-NS5F), pcUb-DV3-NS5F (Ub-DV3-NS5F), pcDV4-NS5F (DV4-NS5F), and pcUb-DV4-NS5F (Ub-DV4-NS5F), pMal as a positive control, or pcDNA3.1 as a negative control together with pRL-TK, expressing Renilla luciferase and an IL-8 promoter-driven firefly luciferase reporter plasmid (43). At 24 h post-transfection, the cells were harvested, and luciferase activities were measured. Each value represents the average of three independent experiments and is shown in relative light units (RLU). B, qRT-PCR was done using RNA extracted from duplicate samples described in A. IL-8 and neomycin resistance (NeoR) gene transcripts were quantified. The neomycin resistance gene transcripts were quantified to control for differences in the levels of transfection efficiency, and IL-8 mRNA levels were normalized against NeoR expression. Each bar represents the average of three independent experiments. The values are expressed as the -fold change in IL-8 transcript amount compared with that induced in cells transfected with pcDNA3.1 and are depicted using a logarithmic scale.
DISCUSSION
The nuclear localization of DENV NS5 has generally been assumed to be a common feature of all DENV serotypes. However, the results of this study demonstrate for the first time that there are serotypic differences in NS5 nuclear localization. Whereas the DENV-2 and -3 proteins accumulate in the nucleus, DENV-1 and -4 NS5 are predominantly if not exclusively localized to the cytoplasm. These findings were shown both by transient expression of GFP- or FLAG-tagged NS5 or by examination of NS5 produced during virus infection of both mammalian and insect cells. The failure of DENV-4 NS5 to accumulate in the nucleus was shown not to be due to rapid nuclear export of the protein, but rather to the failure of amino acids 369–405 to function as an NLS.
Structural and peptide binding studies have defined six classes of monopartite NLS consensus sequences (52) and a consensus bipartite NLS as K/R(K/R)X10–12(K/R)3/5 (where (K/R)3/5 is defined as three of either K or R in five consecutive amino acids (26, 53)). Comparison of the DENV-2 a/bNLS amino acid sequence with the corresponding sequences in the DENV-1, -3, and -4 NS5 proteins (Fig. 4A) shows that only the DENV-2 sequence contains the three clusters of basic amino acids originally used to identify a bipartite NLS (28). These clusters are conserved to varying extents in the NS5 of other serotypes. Mutagenesis studies on DENV-2 NS5 have shown the importance of the first two clusters of basic amino acids (Lys-371/Lys-372 and Lys-387/Lys-388/Lys-389, termed A1 and A2, respectively) for NS5 nuclear localization, both in vitro and during the virus life cycle (29, 30). In the case of DENV-4 NS5, there is only one cluster of basic amino acids (Lys-387/Lys-388/Lys-389) in the region corresponding to the DENV-2 a/bNLS, which does not fit the consensus sequence for a mono- or bipartite NLS, supporting the finding that DENV-4 NS5 amino acids 369–405 do not function as a NLS. For DENV-1 and -3 NS5, the situation is more complex. The dibasic Lys-371/Lys-372 cluster in DENV-2 is present as AQ and RK in DENV-1 and -3, respectively, whereas the A2 cluster Lys-387/Lys-388/Lys-389 is substituted with the sequence RNK in DENV-1 and -3 NS5. Structural analysis of the interaction between importin-α and bipartite NLS sequences has shown that a Lys is strongly preferred to Arg at the residue corresponding to DENV-2 Lys-387, and a basic residue is preferred at the following position (26, 53, 54), suggesting that DENV-1 and -3 NS5 also do not contain a bipartite NLS. However, it has previously been suggested that the sequence 363PKAKRG in DENV-1 NS5 could function as a monopartite NLS (29), and the sequences 388KKPR and 388KRPR in DENV-1 and -3 NS5 could function as class II monopartite NLSs (52). In addition to the basic clusters that mediate importin-α binding, the linker sequence in the bipartite NLS and flanking sequences can influence importin-α binding (52, 55, 56), which may contribute to the different patterns of nuclear localization observed between the DENV-1 and -3 proteins. The introduction of the DENV-2 a/bNLS into DENV-4 NS5 did not fully transfer the properties of nuclear localization to the DENV-4 protein. Previous studies have shown that both the NLS and the protein context determine the efficiency of nuclear transport (57), suggesting that other regions in DENV-4 NS5 may also contribute to its cytoplasmic localization.
Analysis of the conservation of NS5 amino acids 369–405 using the Dengue Virus Variation Database (58) revealed that the sequences of the DENV serotypes used in this study represented the consensus sequence for each serotype and are highly conserved between laboratory-adapted and clinical strains but diverge among the different serotypes. For example, there are currently 948 DENV-2 NS5 sequences in the database, and the A1 and A2 clusters are 100 and >95% conserved, respectively. Of the currently available 101 DENV-4 sequences, none have mutations that lead to a monopartite or bipartite NLS consensus sequence (in the a/bNLS region), although this could be achieved by a single amino acid change. This strong sequence conservation between serotypes suggests that nuclear localization may be important in a serotype-specific manner.
Although there is ∼80% similarity between the DENV-2 and -4 NS5 amino acid sequences 369–405, it was not possible to rescue DENV-2 viruses with the NS5 a/bNLS or minimal a/bNLS replaced by the corresponding regions of DENV-4. Analysis of the x-ray structure of the DENV-3 and West Nile virus POL domains revealed that the a/bNLS region is composed of two helical regions (α6 and α7) linked by a loop and connects the “palm” and the “fingers” subdomains of the POL structure (45, 59). Superimposition of structural models of the DENV-2 POL and the DENV-2 POL containing DENV-4 NS5 amino acids 370–390 or 370–401 (produced from the DENV-3 POL x-ray structure) (60) revealed no overall change to the a/bNLS structure. However, there were differences in the identity or orientation of specific amino acid side chains in the a/bNLS region (Fig. 4D). The substitution of specific DENV-2 amino acids with those of DENV-4 NS5 may therefore have had an overall effect on the stability and/or enzymatic function of the POL domain in addition to their effects on NS5 localization. Previous reverse genetic analysis studies have shown that the mutation of specific amino acids in the a/bNLS region can have a major effect on virus replication. The mutation K387A/K388A, in DENV-4 (strain 814669) NS5 caused a temperature-sensitive phenotype in cell culture and virus attenuation in a mouse neurovirulence model (61). Mutation of the DENV-2 NS5 A1 and A2 clusters to Ala individually decreased virus replication, whereas mutation of both clusters abolished virus replication (30). The mutation of the A2 cluster to Ala was found to delay NS5 nuclear localization. Studies investigating the effects of mutations made to DENV-2 NS5 a/bNLS on virus replication and POL activity, using bacterially expressed NS5, have shown that only the mutation R401A/K401A (Ser-401/Lys-402 in DENV-4) abolished virus replication and POL activity (46, 62), whereas other mutations could reduce viral replication without affecting POL activity (46), highlighting the pleiotropic effects of mutations in the a/bNLS region.
Previous studies using ELISA-based and column pull-down assays showed that DENV-2 NS5 and/or the NS5 a/bNLS interacted with the murine equivalent of human KPNA2 complexed to importin-β (28–30). However, it was not determined whether DENV-2 NS5 could bind to other importin-α isoforms. In addition, by analogy to the species-specific difference in the binding of NS5 to murine and human STAT2 (17), there may have been differences in the binding of NS5 to murine and human importin-α. Therefore, we analyzed the interaction of DENV-2 and -4 NS5 with 5 human importin-α isoforms, representative of the three importin-α subfamilies (51), after exogenous expression in mammalian cells. However, using a range of binding conditions, we could neither detect binding of DENV-2 or -4 NS5 to importin-α isoforms 1, 3, and 4 nor unequivocally detect an interaction with KPNA2 or -6 that was specific or correlated with DENV-2 NS5 nuclear localization. Previous studies using yeast two-hybrid analysis have not detected a direct interaction between any of the importin-α isoforms and NS5 of DENV-2 (32, 63), DENV-1, or other flaviviruses (64), suggesting that interaction studies require specific conditions that allow the formation of a stable complex between NS5 and the importin-α/importin-β heterodimer, perhaps reflecting the transient nature of the interaction.
As an alternative to interaction studies, we examined the localization of DENV-2 NS5 in cells in which KPNA2 and KPNB1 had been knocked down. Although we observed strong knockdown of KPNA2, there was little to no effect on DENV-2 NS5 nuclear localization in infected cells or cells either transiently or stably expressing DENV-2 NS5. By contrast, the knockdown of KPNB1 severely reduced or abolished the nuclear localization of NS5. There are a number of possible explanations for these results. First, KPNA2 is reported to be the most abundant importin isoform in many eukaryotic cells (65, 66), and there may have still been sufficient KPNA2 after knockdown to mediate DENV-2 NS5 nuclear transport. Second, there may be redundancy in the binding of DENV-2 NS5 to importin-α isoforms, and we did not test binding to KPNA5 and -7, although other members of these subfamilies were tested. Finally, previous studies have shown that KPNB1 can bind to a region of DENV-2 NS5 known as the bNLS (amino acids 320–368) (29, 32). However, binding only occurred when the a/bNLS was absent. In the absence of KPNA2, KPNB1 may be able to function directly to transport NS5 to the nucleus, which could not occur when KPNB1 was knocked down.
In many cases, the nuclear import of viral proteins is required for virus replication; however, viruses also actively target specific karyopherins to inhibit the import of transcription factors that activate antiviral responses (67). For example, the Ebola virus VP24 protein binds to human KPNA1, -5, and -6, preventing the binding and subsequent nuclear translocation of phosphorylated STAT1, inhibiting IFN signaling (68, 69), whereas the Hantaan virus N protein binds to KPNA1, -2, and -4, inhibiting the nuclear translocation and activity of NF-κB (70). The results of our work and a recent study (46) suggest that nuclear localization of DENV NS5 is not strictly essential for DENV replication. However, previously, we found that mutations in DENV-2 NS5 that delay nuclear localization lead to decreased virus replication (30). The nuclear localization of NS5 or its interaction with the nuclear import/export machinery may therefore play an auxiliary role in enhancing virus production, for at least the DENV-2 serotype. It has previously been shown that the cellular localization of DENV-2 NS5 affects IL-8 production (30, 31, 43). In this study, we found that serotypic differences in NS5 nuclear localization did not correlate with IL-8 expression. By contrast, we found that both DENV-2 and -4 NS5 bound to STAT2, extending the previous finding that DENV-2 and -1 bind STAT2 (15–17) which is likely to be a common feature of DENV NS5. Therefore, there appear to be commonalities and differences in the way that NS5 of different DENV serotypes interacts with the host cell. It is known that different flavivirus NS5 proteins use distinct mechanisms to perturb type I IFN signaling (11–16). It remains to be investigated whether the effects of the serotypic differences in DENV-2 NS5 nuclear localization reported in this study are compensated by other mechanisms or play a specific role in pathogenesis. It has recently been shown that inhibition of nuclear import pathways has potential as an antiviral strategy against DENV-2 (71). However, this study demonstrates that, when investigating antiviral strategies against DENV, serotypic differences need to be taken into account.
Acknowledgments
We thank the individuals mentioned throughout for the supply of plasmids. Imaging was performed at the Wolfson Bioimaging Facility, University of Bristol.
This work was supported by Medical Research Council Grant G0801973 (to A. D. D.).

This article contains supplemental Table 1.
H.-C. Chiu, H. Hannemann, J. Bird, and A. D. Davidson, manuscript in preparation.
- DENV
- dengue virus
- CLSM
- confocal laser-scanning microscopy
- co-IP
- co-immunoprecipitation
- FRAP
- fluorescence recovery after photobleaching
- IFA
- immunofluorescence assay
- KPNA
- karyopherin-α
- KPNB
- karyopherin-β
- LMB
- leptomycin B
- MTase
- methyltransferase
- NLS
- nuclear localization sequence
- OL-PCR
- overlap PCR
- qRT-PCR
- quantitative RT-PCR
- POL
- RNA-dependent RNA polymerase
- Ub
- ubiquitin
- RFU
- relative fluorescence unit(s).
REFERENCES
- 1. Guzman M. G., Halstead S. B., Artsob H., Buchy P., Farrar J., Gubler D. J., Hunsperger E., Kroeger A., Margolis H. S., Martínez E., Nathan M. B., Pelegrino J. L., Simmons C., Yoksan S., Peeling R. W. (2010) Dengue. A continuing global threat. Nat. Rev. Microbiol. 8, S7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (2009) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. World Health Organization, Geneva: [PubMed] [Google Scholar]
- 3. Lindenbach B. D., Thiel H. J., Rice C. M. (2007) Flaviviridae. The viruses and their replication. in Fields Virology, 5th Ed (Knipe D. M., Howley P. M., eds) pp. 1101–1152, Lippincott-Raven Publishers, Philadelphia [Google Scholar]
- 4. Egloff M. P., Benarroch D., Selisko B., Romette J. L., Canard B. (2002) An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5. Crystal structure and functional characterization. EMBO J. 21, 2757–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ray D., Shah A., Tilgner M., Guo Y., Zhao Y., Dong H., Deas T. S., Zhou Y., Li H., Shi P. Y. (2006) West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. J. Virol. 80, 8362–8370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Issur M., Geiss B. J., Bougie I., Picard-Jean F., Despins S., Mayette J., Hobdey S. E., Bisaillon M. (2009) The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA 15, 2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ackermann M., Padmanabhan R. (2001) De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J. Biol. Chem. 276, 39926–39937 [DOI] [PubMed] [Google Scholar]
- 8. Tan B. H., Fu J., Sugrue R. J., Yap E. H., Chan Y. C., Tan Y. H. (1996) Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology 216, 317–325 [DOI] [PubMed] [Google Scholar]
- 9. Dong H., Zhang B., Shi P. Y. (2008) Flavivirus methyltransferase. A novel antiviral target. Antiviral Res. 80, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malet H., Massé N., Selisko B., Romette J. L., Alvarez K., Guillemot J. C., Tolou H., Yap T. L., Vasudevan S., Lescar J., Canard B. (2008) The flavivirus polymerase as a target for drug discovery. Antiviral Res. 80, 23–35 [DOI] [PubMed] [Google Scholar]
- 11. Best S. M., Morris K. L., Shannon J. G., Robertson S. J., Mitzel D. N., Park G. S., Boer E., Wolfinbarger J. B., Bloom M. E. (2005) Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 79, 12828–12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laurent-Rolle M., Boer E. F., Lubick K. J., Wolfinbarger J. B., Carmody A. B., Rockx B., Liu W., Ashour J., Shupert W. L., Holbrook M. R., Barrett A. D., Mason P. W., Bloom M. E., García-Sastre A., Khromykh A. A., Best S. M. (2010) The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J. Virol. 84, 3503–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin R. J., Chang B. L., Yu H. P., Liao C. L., Lin Y. L. (2006) Blocking of interferon-induced Jak-Stat signaling by Japanese encephalitis virus NS5 through a protein tyrosine phosphatase-mediated mechanism. J. Virol. 80, 5908–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werme K., Wigerius M., Johansson M. (2008) Tick-borne encephalitis virus NS5 associates with membrane protein scribble and impairs interferon-stimulated JAK-STAT signalling. Cell Microbiol. 10, 696–712 [DOI] [PubMed] [Google Scholar]
- 15. Ashour J., Laurent-Rolle M., Shi P. Y., García-Sastre A. (2009) NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 83, 5408–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mazzon M., Jones M., Davidson A., Chain B., Jacobs M. (2009) Dengue virus NS5 inhibits interferon-α signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J. Infect. Dis. 200, 1261–1270 [DOI] [PubMed] [Google Scholar]
- 17. Ashour J., Morrison J., Laurent-Rolle M., Belicha-Villanueva A., Plumlee C. R., Bernal-Rubio D., Williams K. L., Harris E., Fernandez-Sesma A., Schindler C., García-Sastre A. (2010) Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe 8, 410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daffis S., Szretter K. J., Schriewer J., Li J., Youn S., Errett J., Lin T. Y., Schneller S., Zust R., Dong H., Thiel V., Sen G. C., Fensterl V., Klimstra W. B., Pierson T. C., Buller R. M., Gale M., Jr., Shi P. Y., Diamond M. S. (2010) 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 468, 452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor R. T., Lubick K. J., Robertson S. J., Broughton J. P., Bloom M. E., Bresnahan W. A., Best S. M. (2011) TRIM79α, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 10, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C. K., Walther P., Fuller S. D., Antony C., Krijnse-Locker J., Bartenschlager R. (2009) Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davidson A. D. (2009) Chapter 2. New insights into flavivirus nonstructural protein 5. Adv. Virus Res. 74, 41–101 [DOI] [PubMed] [Google Scholar]
- 22. Chook Y. M., Süel K. E. (2011) Nuclear import by karyopherin-βs. Recognition and inhibition. Biochim. Biophys. Acta 1813, 1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stewart M. (2007) Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell Biol. 8, 195–208 [DOI] [PubMed] [Google Scholar]
- 24. Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. (2004) Importin α. A multipurpose nuclear-transport receptor. Trends Cell Biol. 14, 505–514 [DOI] [PubMed] [Google Scholar]
- 25. Lange A., Mills R. E., Lange C. J., Stewart M., Devine S. E., Corbett A. H. (2007) Classical nuclear localization signals. Definition, function, and interaction with importin α. J. Biol. Chem. 282, 5101–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marfori M., Mynott A., Ellis J. J., Mehdi A. M., Saunders N. F., Curmi P. M., Forwood J. K., Bodén M., Kobe B. (2011) Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim. Biophys. Acta 1813, 1562–1577 [DOI] [PubMed] [Google Scholar]
- 27. Kapoor M., Zhang L., Ramachandra M., Kusukawa J., Ebner K. E., Padmanabhan R. (1995) Association between NS3 and NS5 proteins of dengue virus type 2 in the putative RNA replicase is linked to differential phosphorylation of NS5. J. Biol. Chem. 270, 19100–19106 [DOI] [PubMed] [Google Scholar]
- 28. Forwood J. K., Brooks A., Briggs L. J., Xiao C. Y., Jans D. A., Vasudevan S. G. (1999) The 37-amino-acid interdomain of dengue virus NS5 protein contains a functional NLS and inhibitory CK2 site. Biochem. Biophys. Res. Commun. 257, 731–737 [DOI] [PubMed] [Google Scholar]
- 29. Brooks A. J., Johansson M., John A. V., Xu Y., Jans D. A., Vasudevan S. G. (2002) The interdomain region of dengue NS5 protein that binds to the viral helicase NS3 contains independently functional importin β1 and importin α/β-recognized nuclear localization signals. J. Biol. Chem. 277, 36399–36407 [DOI] [PubMed] [Google Scholar]
- 30. Pryor M. J., Rawlinson S. M., Butcher R. E., Barton C. L., Waterhouse T. A., Vasudevan S. G., Bardin P. G., Wright P. J., Jans D. A., Davidson A. D. (2007) Nuclear localization of dengue virus nonstructural protein 5 through its importin α/β-recognized nuclear localization sequences is integral to viral infection. Traffic 8, 795–807 [DOI] [PubMed] [Google Scholar]
- 31. Rawlinson S. M., Pryor M. J., Wright P. J., Jans D. A. (2009) CRM1-mediated nuclear export of dengue virus RNA polymerase NS5 modulates interleukin-8 induction and virus production. J. Biol. Chem. 284, 15589–15597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johansson M., Brooks A. J., Jans D. A., Vasudevan S. G. (2001) A small region of the dengue virus-encoded RNA-dependent RNA polymerase, NS5, confers interaction with both the nuclear transport receptor importin-β and the viral helicase, NS3. J. Gen. Virol. 82, 735–745 [DOI] [PubMed] [Google Scholar]
- 33. Gualano R. C., Pryor M. J., Cauchi M. R., Wright P. J., Davidson A. D. (1998) Identification of a major determinant of mouse neurovirulence of dengue virus type 2 using stably cloned genomic-length cDNA. J. Gen. Virol. 79, 437–446 [DOI] [PubMed] [Google Scholar]
- 34. Pryor M. J., Carr J. M., Hocking H., Davidson A. D., Li P., Wright P. J. (2001) Replication of dengue virus type 2 in human monocyte-derived macrophages. Comparisons of isolates and recombinant viruses with substitutions at amino acid 390 in the envelope glycoprotein. Am. J. Trop. Med. Hyg. 65, 427–434 [DOI] [PubMed] [Google Scholar]
- 35. Kroschewski H., Lim S. P., Butcher R. E., Yap T. L., Lescar J., Wright P. J., Vasudevan S. G., Davidson A. D. (2008) Mutagenesis of the dengue virus type 2 NS5 methyltransferase domain. J. Biol. Chem. 283, 19410–19421 [DOI] [PubMed] [Google Scholar]
- 36. Depping R., Steinhoff A., Schindler S. G., Friedrich B., Fagerlund R., Metzen E., Hartmann E., Köhler M. (2008) Nuclear translocation of hypoxia-inducible factors (HIFs). Involvement of the classical importin α/β pathway. Biochim. Biophys. Acta 1783, 394–404 [DOI] [PubMed] [Google Scholar]
- 37. Melen K., Fagerlund R., Franke J., Kohler M., Kinnunen L., Julkunen I. (2003) Importin α nuclear localization signal binding sites for STAT1, STAT2, and influenza A virus nucleoprotein. J. Biol. Chem. 278, 28193–28200 [DOI] [PubMed] [Google Scholar]
- 38. Didcock L., Young D. F., Goodbourn S., Randall R. E. (1999) The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73, 9928–9933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chu P. W., Westaway E. G. (1992) Molecular and ultrastructural analysis of heavy membrane fractions associated with the replication of Kunjin virus RNA. Arch. Virol. 125, 177–191 [DOI] [PubMed] [Google Scholar]
- 40. Miller S., Sparacio S., Bartenschlager R. (2006) Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J. Biol. Chem. 281, 8854–8863 [DOI] [PubMed] [Google Scholar]
- 41. Williams B. J., Boyne J. R., Goodwin D. J., Roaden L., Hautbergue G. M., Wilson S. A., Whitehouse A. (2005) The prototype γ-2 herpesvirus nucleocytoplasmic shuttling protein, ORF 57, transports viral RNA through the cellular mRNA export pathway. Biochem. J. 387, 295–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandrock K., Bielek H., Schradi K., Schmidt G., Klugbauer N. (2010) The nuclear import of the small GTPase Rac1 is mediated by the direct interaction with karyopherin α2. Traffic 11, 198–209 [DOI] [PubMed] [Google Scholar]
- 43. Medin C. L., Fitzgerald K. A., Rothman A. L. (2005) Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J. Virol. 79, 11053–11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mackenzie J. M., Kenney M. T., Westaway E. G. (2007) West Nile virus strain Kunjin NS5 polymerase is a phosphoprotein localized at the cytoplasmic site of viral RNA synthesis. J. Gen. Virol. 88, 1163–1168 [DOI] [PubMed] [Google Scholar]
- 45. Malet H., Egloff M. P., Selisko B., Butcher R. E., Wright P. J., Roberts M., Gruez A., Sulzenbacher G., Vonrhein C., Bricogne G., Mackenzie J. M., Khromykh A. A., Davidson A. D., Canard B. (2007) Crystal structure of the RNA polymerase domain of the West Nile virus non-structural protein 5. J. Biol. Chem. 282, 10678–10689 [DOI] [PubMed] [Google Scholar]
- 46. Kumar A., Bühler S., Selisko B., Davidson A., Mulder K., Canard B., Miller S., Bartenschlager R. (2013) Nuclear localization of dengue virus nonstructural protein 5 does not strictly correlate with efficient viral RNA replication and inhibition of type I interferon signaling. J. Virol. 87, 4545–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seibel N. M., Eljouni J., Nalaskowski M. M., Hampe W. (2007) Nuclear localization of enhanced green fluorescent protein homomultimers. Anal. Biochem. 368, 95–99 [DOI] [PubMed] [Google Scholar]
- 48. Uchil P. D., Satchidanandam V. (2003) Architecture of the flaviviral replication complex. Protease, nuclease, and detergents reveal encasement within double-layered membrane compartments. J. Biol. Chem. 278, 24388–24398 [DOI] [PubMed] [Google Scholar]
- 49. Sinnecker D., Voigt P., Hellwig N., Schaefer M. (2005) Reversible photobleaching of enhanced green fluorescent proteins. Biochemistry 44, 7085–7094 [DOI] [PubMed] [Google Scholar]
- 50. Kelley J. B., Talley A. M., Spencer A., Gioeli D., Paschal B. M. (2010) Karyopherin alpha7 (KPNA7), a divergent member of the importin α family of nuclear import receptors. BMC Cell Biol. 11, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mason D. A., Stage D. E., Goldfarb D. S. (2009) Evolution of the metazoan-specific importin α gene family. J. Mol. Evol. 68, 351–365 [DOI] [PubMed] [Google Scholar]
- 52. Kosugi S., Hasebe M., Matsumura N., Takashima H., Miyamoto-Sato E., Tomita M., Yanagawa H. (2009) Six classes of nuclear localization signals specific to different binding grooves of importin α. J. Biol. Chem. 284, 478–485 [DOI] [PubMed] [Google Scholar]
- 53. Fontes M. R., Teh T., Jans D., Brinkworth R. I., Kobe B. (2003) Structural basis for the specificity of bipartite nuclear localization sequence binding by importin-α. J. Biol. Chem. 278, 27981–27987 [DOI] [PubMed] [Google Scholar]
- 54. Yang S. N., Takeda A. A., Fontes M. R., Harris J. M., Jans D. A., Kobe B. (2010) Probing the specificity of binding to the major nuclear localization sequence-binding site of importin-α using oriented peptide library screening. J. Biol. Chem. 285, 19935–19946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fontes M. R., Teh T., Toth G., John A., Pavo I., Jans D. A., Kobe B. (2003) Role of flanking sequences and phosphorylation in the recognition of the simian-virus-40 large T-antigen nuclear localization sequences by importin-α. Biochem. J. 375, 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marfori M., Lonhienne T. G., Forwood J. K., Kobe B. (2012) Structural basis of high-affinity nuclear localization signal interactions with importin-α. Traffic 13, 532–548 [DOI] [PubMed] [Google Scholar]
- 57. Friedrich B., Quensel C., Sommer T., Hartmann E., Köhler M. (2006) Nuclear localization signal and protein context both mediate importin α specificity of nuclear import substrates. Mol. Cell Biol. 26, 8697–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Resch W., Zaslavsky L., Kiryutin B., Rozanov M., Bao Y., Tatusova T. A. (2009) Virus variation resources at the National Center for Biotechnology Information. Dengue virus. BMC Microbiol. 9, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yap L. J., Luo D., Chung K. Y., Lim S. P., Bodenreider C., Noble C., Shi P. Y., Lescar J. (2010) Crystal structure of the dengue virus methyltransferase bound to a 5′-capped octameric RNA. PLoS One 5, e12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yap T. L., Xu T., Chen Y. L., Malet H., Egloff M. P., Canard B., Vasudevan S. G., Lescar J. (2007) Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J. Virol. 81, 4753–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanley K. A., Lee J. J., Blaney J. E., Jr., Murphy B. R., Whitehead S. S. (2002) Paired charge-to-alanine mutagenesis of dengue virus type 4 NS5 generates mutants with temperature-sensitive, host range, and mouse attenuation phenotypes. J. Virol. 76, 525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iglesias N. G., Filomatori C. V., Gamarnik A. V. (2011) The F1 motif of dengue virus polymerase NS5 is involved in promoter-dependent RNA synthesis. J. Virol. 85, 5745–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Khadka S., Vangeloff A. D., Zhang C., Siddavatam P., Heaton N. S., Wang L., Sengupta R., Sahasrabudhe S., Randall G., Gribskov M., Kuhn R. J., Perera R., Lacount D. J. (2011) A physical interaction network of dengue virus and human proteins. Mol. Cell Proteomics 10, M111.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Le Breton M., Meyniel-Schicklin L., Deloire A., Coutard B., Canard B., de Lamballerie X., Andre P., Rabourdin-Combe C., Lotteau V., Davoust N. (2011) Flavivirus NS3 and NS5 proteins interaction network. A high-throughput yeast two-hybrid screen. BMC Microbiol. 11, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Köhler M., Fiebeler A., Hartwig M., Thiel S., Prehn S., Kettritz R., Luft F. C., Hartmann E. (2002) Differential expression of classical nuclear transport factors during cellular proliferation and differentiation. Cell Physiol. Biochem. 12, 335–344 [DOI] [PubMed] [Google Scholar]
- 66. Quensel C., Friedrich B., Sommer T., Hartmann E., Kohler M. (2004) In vivo analysis of importin α proteins reveals cellular proliferation inhibition and substrate specificity. Mol. Cell Biol. 24, 10246–10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fulcher A. J., Jans D. A. (2011) Regulation of nucleocytoplasmic trafficking of viral proteins. An integral role in pathogenesis? Biochim. Biophys. Acta 1813, 2176–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reid S. P., Leung L. W., Hartman A. L., Martinez O., Shaw M. L., Carbonnelle C., Volchkov V. E., Nichol S. T., Basler C. F. (2006) Ebola virus VP24 binds karyopherin α1 and blocks STAT1 nuclear accumulation. J. Virol. 80, 5156–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reid S. P., Valmas C., Martinez O., Sanchez F. M., Basler C. F. (2007) Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin α proteins with activated STAT1. J. Virol. 81, 13469–13477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Taylor S. L., Frias-Staheli N., García-Sastre A., Schmaljohn C. S. (2009) Hantaan virus nucleocapsid protein binds to importin α proteins and inhibits tumor necrosis factor α-induced activation of nuclear factor κB. J. Virol. 83, 1271–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wagstaff K. M., Sivakumaran H., Heaton S. M., Harrich D., Jans D. A. (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 443, 851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Söding J., Biegert A., Lupas A. N. (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sali A., Blundell T. L. (1993) Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 74. DeLano W. L. (2010) The PyMOL Molecular Graphics System, version 1.3r1, Schrodinger, LLC, New York [Google Scholar]