Abstract
Purpose of review
Emerging evidence supports an important role for the heme oxygenase system in the maintenance of a healthy pregnancy. This review attempts to collect these wide-ranging data and summarize the recent progress in the field.
Recent findings
New studies looking at heme oxygenase depletion in a variety of animal models have demonstrated that the heme oxygenase system is an important regulator of placental development, particularly in vascular structure. Furthermore, emerging studies demonstrate a role for heme oxygenase in the maintenance of pregnancy, especially during pathological challenge. Intriguingly, it now appears that the heme oxygenase system can be dramatically altered by pathological disorders of pregnancy, in particular preeclampsia, perhaps functionally in the symptomatic phase of the disorder. Promisingly, however, recent data suggest that induction of the heme oxygenase system, or administration of its bioactive metabolites, could provide a promising novel therapeutic approach to the management of this currently untreatable disease.
Summary
Long considered a molecular housekeeping system, the heme oxygenase system is now known to be an important stress response pathway. New evidence suggests that it is also an important player in pregnancy and preeclampsia. However, the evidence now also suggests that it may provide a therapeutic approach for this common disease with few management options.
Keywords: bilirubin, carbon monoxide, heme oxygenase, preeclampsia
INTRODUCTION
The development of the placenta during pregnancy and the concurrent maternal cardiovascular changes that accompany it are a highly regulated sequence of events. Proper maturation and development of the placental tissue is absolutely necessary to adequately supply the developing fetus with the adequate nutrition to ensure proper fetal development. Additionally, in recent years, it has been appreciated that inadequate delivery of blood to the placenta itself can cause the production of stress-inducible factors, which when released into the maternal circulation can be responsible for maternal disease, as in the case of preeclampsia. Although the temporal development of the gross placental anatomy and function are well characterized, the molecular cues that underpin them are still not fully elucidated. Likewise, the complement of molecular players which maintain normal placental function are still very much an area of active research. One molecular factor that has attracted increased interest as an important regulator of placental development and function is heme oxygenase.
Heme oxygenase consists of two functional isoforms: the inducible form HO-1 and the constitutively active form HO-2. Heme oxygenase’s normally recognized function is to catalyze the rate-limiting step in the conversion of free heme to bilirubin, by converting it to biliverdin, the substrate for the final conversion to bilirubin by biliverdin reductase (Fig. 1). While heme oxygenase has traditionally been considered a housekeeping system, a large body of work now suggests that heme oxygenase has diverse biological activities. Interestingly, the conversion of heme produces three catalytic products that are known to have biological activity: biliverdin/bilirubin, carbon monoxide, and elemental iron. Each of these compounds has demonstrated an important biological activity. Biliverdin and bilirubin are both known to have potent antioxidant effects [1,2]. Carbon monoxide, though toxic at higher levels, is an important endogenous signaling molecule with properties similar to the closely related molecule nitric oxide. Among other functions, carbon monoxide has been implicated in the relaxation of vascular tone and has potent effects on angiogenesis [3,4,5▪,6,7]. Free iron, on its own, is a potent pro-oxidant. However, its continued production leads to an increase in the production of the iron-sequestering protein ferritin, leading to a long-term decrease in free iron and thus oxidative stress [8]. Intriguingly, there is a growing understanding that the activity of heme oxygenase, likely through its bioactive compounds, is an important regulator of placental development. This review seeks to summarize the published literature implicating heme oxygenase as an important regulator of pregnancy and its potential application as a therapeutic in the management of preeclampsia.
FIGURE 1.
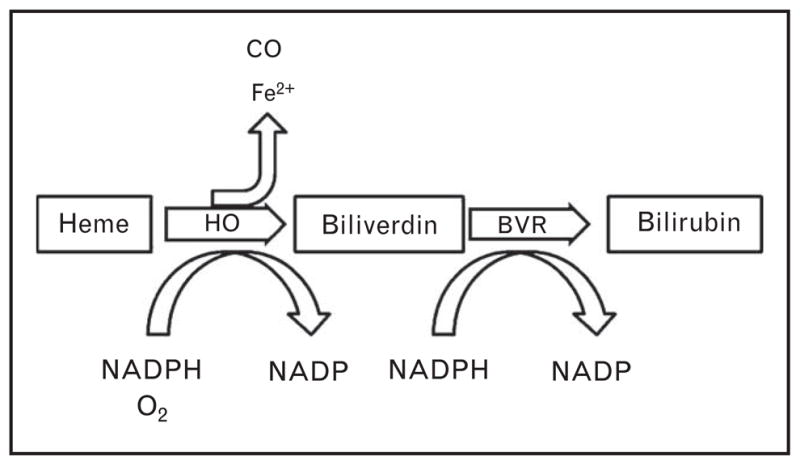
Degradation of heme-containing proteins leads to the release of free heme, a pro-oxidant molecule. In order to clear the potentially damaging molecule, heme oxygenase (HO) converts free heme to biliverdin, releasing free iron and carbon monoxide in the process. The biliverdin is then rapidly converted to bilirubin by biliverdin reductase (BVR). Biliverdin/bilirubin, elemental iron, and carbon monoxide all have important biological functions, making the activity of heme oxygenase an intriguing regulator of cellular and vascular function.
HEME OXYGENASE IN PLACENTAL DEVELOPMENT
Supplying the developing placenta is a complicated process consisting of an intricate temporal developmental program. Although a full explanation of placental and vascular development during placentation is beyond the scope of this article (for an excellent recent review, see [9▪]), a brief overview is in order. To provide adequate blood flow from the maternal spiral arteries, extravillous trophoblasts from the placenta invade the maternal arteries, displace the vascular endothelial lining, and replace the vascular smooth muscle and lamina [9▪]. The invaded segments of these vessels become dramatically distended, offering low resistance, which ensures uninterrupted blood flow to the placenta. Despite this, the oxygen tension in the healthy placenta in late gestation is relatively low, with a pO2 of approximately 55 mmHg at its peak [10]. As blood from the spiral arteries enters the placenta, it passes through the intervillous space where it directly interacts with the placental chorionic villi. These fetally derived villous columns are the major site for nutrient and waste exchange between the maternal and fetal circulations.
Several reports have demonstrated the expression of both HO-1 and HO-2 in the placentas of both humans and animal models [11–13]. Interestingly, in healthy pregnancies, there appears to be greater change in the ‘constitutive’ HO-2 than the ‘inducible’ HO-1 in term versus first-trimester pregnancies, and the two isoforms appear to have distinct temporal–spatial expression patterns in the placenta, suggesting possible functional regulation of the separate isoforms [13,14]. In mice, for instance, HO-1 is expressed in the fetus and placenta at an early gestational stage, increasing steadily through mid-gestation, before gradually decreasing at term [15]. Several lines of evidence support the notion that heme oxygenase activity is important for normal gestational development. A recent in-vitro report showed that human trophoblast invasion could be inhibited by a general heme oxygenase inhibitor or by HO-2-specific antibodies, but that HO-1 antibodies had no significant effect [16]. Even more intriguingly, a second report found decreased HO-1 expression in invasive versus noninvasive trophoblasts. Using in-vitro invasion assays with gain and loss of function experiments, this group also demonstrated a causal inverse relationship between HO-1 expression and trophoblast invasiveness. Finally, this group demonstrated that the effect of HO-1 to suppress invasion was because of its positive regulation of PPARγ expression, as blockade PPARγ essentially eliminated HO-1’s inhibitory effects [17].
Two recent studies utilizing transgenic knockout mice have also demonstrated an important role for HO-1 in placental vascular development and gross morphology. The first and most direct effect of heme oxygenase depletion was the extremely low (~2.4%) survival of homozygous HO-1 knockout mice. Indeed, crosses between HO-1+/− mice resulted in significant decreases in both overall fetal weight and litter size. Analysis of the fetal HO-1+/− placentas revealed changes in morphology, specifically thinning of the junctional zone. Perhaps most intriguing, fetal HO-1+/− placentas were accompanied by a concomitant increase in HO-2 expression which led to very modest and largely insignificant changes in overall heme oxygenase activity, again suggesting a functional heterogeneity in the two isoforms [18].
In the second study, an analysis of the placental vascular structure of the HO-1+/− transgenic line indicated a deficiency in vascular remodeling, malformation of the maternal/fetal interface, and changes in uterine natural killer cell proliferation and maturation, which the authors attributed, in part, to the changes in the angiogenic profile in the maternal HO-1+/− animals. In this case, the phenotype appeared to be independent of fetal genotype, but instead followed the maternal heme oxygenase levels, suggesting that maternal heme oxygenase may have an indirect role on placental development [19▪ ▪]. More recently, these studies have been confirmed in a separate mouse line, which also found massive fetal loss and intrauterine growth restriction. Intriguingly, inhalation of low-dose carbon monoxide was found to cause significant improvement in both fetal weight and fetal survival, suggesting that carbon monoxide is at least partially responsible for the protective effects of HO-1 during pregnancy [20]. Together, the weight of these recent studies suggests a very important role for the heme oxygenase system in the normal course of placental development.
HEME OXYGENASE IN THE MAINTENANCE OF PREGNANCY
There are also interesting indications in the literature that heme oxygenase has an important role on vascular function in the mother and fetus, including the placental vasculature. In one early report, using ex-vivo vascular preparations from pregnant human myometrial vessels, heme oxygenase induction by the porphyrin derivative hemin led to decreased uterine vessel spontaneous contractility and contractions in response to oxytocin, suggesting a possible role in maintaining uterine vessel quiescence during pregnancy [21]. In another study utilizing ex-vivo human placental perfusion preparations, it was shown that carbon monoxide added to the perfusate significantly decreased the perfusion pressure of the fetal circulation. Furthermore, inhibition or activation of soluble guanylyl cyclase (sGC), the secondary messenger for nitric oxide/carbon monoxide, had a direct effect on the ability of carbon monoxide to modulate perfusion pressure. This again suggests that carbon monoxide can act as an important regulator of vascular function in the placenta during gestation [22].
There is also an emerging link between heme oxygenase and protection from miscarriage. Studies from the Zenclussen laboratory have demonstrated that induction of HO-1 by either chemical induction with cobalt protoporphyrin (CoPP) or viral expression is protective in a well established abortion prone mouse model. Further, these studies found that the protective effect was associated with increased levels of the cytoprotective protein Bag-1 and neuropilin-1, a T-regulatory cell marker, at the maternal–fetal junction [23,24]. The authors concluded that activation of T cells might be a major mechanism in heme oxygenase’s protective effect by improving fetal tolerance. Support for this idea came with a subsequent study which demonstrated that inhibition of heme oxygenase significantly attenuated the protective effects of adoptive T-reg transfer in the same abortion prone model [25▪ ▪]. A separate group has recently demonstrated the beneficial effects of HO-1 induction in the Brucella abortus and Listeria monocytogenes-induced abortion mouse models. Specifically, they found the infections themselves downregulated HO-1 expression in the placenta, and that induction of HO-1 by CoPP dramatically increased fetal survival in infected animals, coincident with decreased cell death in the placenta [26▪,27]. Finally, there is an intriguing report that found that polymorphisms within the heme oxygenase-1 gene are associated with incidence of idiopathic recurrent miscarriage in Caucasian women [28]. Together, these data suggest that heme oxygenase plays an important role in the maintenance of a healthy pregnancy under physiological and pathological conditions. Further examination of genetic variation of the heme oxygenase genes in patients presenting with gestational disorders and further mechanistic studies examining the molecular mechanism affected by heme oxygenase in pregnancy should prove enlightening.
HEME OXYGENASE IN PREECLAMPSIA
Preeclampsia is one of the most prevalent gestational disorders, affecting approximately 8% of pregnancies in the United States. It is also a major risk factor for perinatal mortality [29]. The major symptomatic manifestations of preeclampsia include new-onset hypertension and proteinuria, although it has been suggested that a more global view of renal function might be more indicative than absolute protein excretion [30]. The underlying mechanisms that initiate preeclampsia are still unknown, but there is a growing consensus that the symptomatic phase of the disorder stems from inadequate remodeling of the maternal spiral arteries to provide adequate blood flow to the placenta. In response, the placenta becomes extremely hypoxic and begins to produce soluble factors, which when released into the maternal bloodstream are responsible for the maternal symptoms [31,32▪,33,34]. With the mounting evidence for the importance of heme oxygenase in the development and maintenance of placental structure and function, it is logical to suspect that alterations in the heme oxygenase system could be an important factor in the development and symptoms of preeclampsia.
Given the continuing interest in the placental response to hypoxia, two separate laboratories have examined the effects of hypoxia and hypoxia–reoxygenation on placental tissue ex vivo. In the first, villous explants from human term placentas were subjected to anoxia–reoxygenation in either 5 or 20% oxygen-treated media. In none of the performed experiments was any change seen in either HO-1 or HO-2, suggesting that anoxia–reoxygenation does not influence heme oxygenase expression [35]. In stark contrast, when rodent placental villous explants were exposed to chronic hypoxia (1%) for 48 h, they exhibited a marked decreased in HO-1 expression when compared to their 6% ‘normoxic’ controls [5▪]. The contradictory nature of these results has not been resolved and more work remains to clarify the role of hypoxia in driving HO-1 expression in the placental vasculature.
Several groups have examined the expression of HO-1 in the circulation and placenta in preeclamptic patients. Intriguingly, several studies have indicated changes in the ‘constitutively’ expressed isoform, HO-2. Early studies looking at HO-1 and HO-2 expression in placental tissue in preeclamptic patients revealed little change in most cell subtypes, but demonstrated a significant reduction in placental endothelial cells in preeclamptic women when compared with healthy pregnant controls [12]. Subsequent studies not only confirmed the earlier results, but also indicated that there was a significant reduction in the syncytiotrophoblast expression of HO-2 [36]. In a recent report, decreases in HO-2 were observed not only in syncytiotrophoblasts, but also in invasive trophoblasts [37].
Changes in HO-1 expression have also been noted by numerous investigators. Early investigations were suggestive, though not definitive. In a broad examination of placental tissue from several pregnancy disorders, including preeclampsia, a trend for decreased HO-1 expression was noted, though this failed to meet statistical significance [37]. Interestingly, subsequent studies looking at the decidua basalis of the placenta have suggested significantly increased levels of HO-1 expression in preeclamptic placentas. Even more, studies looking at the HO-1 protein levels in the serum of preeclamptic women also found an increase, suggesting a possible biomarker for the diagnosis of preeclampsia [38]. Most recently, examination of HO-1 levels in serum from preeclamptic women prior to parturition demonstrated markedly increased protein levels. Interestingly, elevated serum HO-1 persisted in the antenatal period whereas in healthy controls HO-1 decreased significantly [39▪]. Although the functional implications of changes during heme oxygenase expression during preeclampsia remain to be elucidated, it appears likely that changes in heme oxygenase could be an important factor in the origins and the physiological response to placental ischemia during preeclampsia.
THE HEME OXYGENASE SYSTEM: POSSIBLE THERAPIES FOR PREECLAMPSIA?
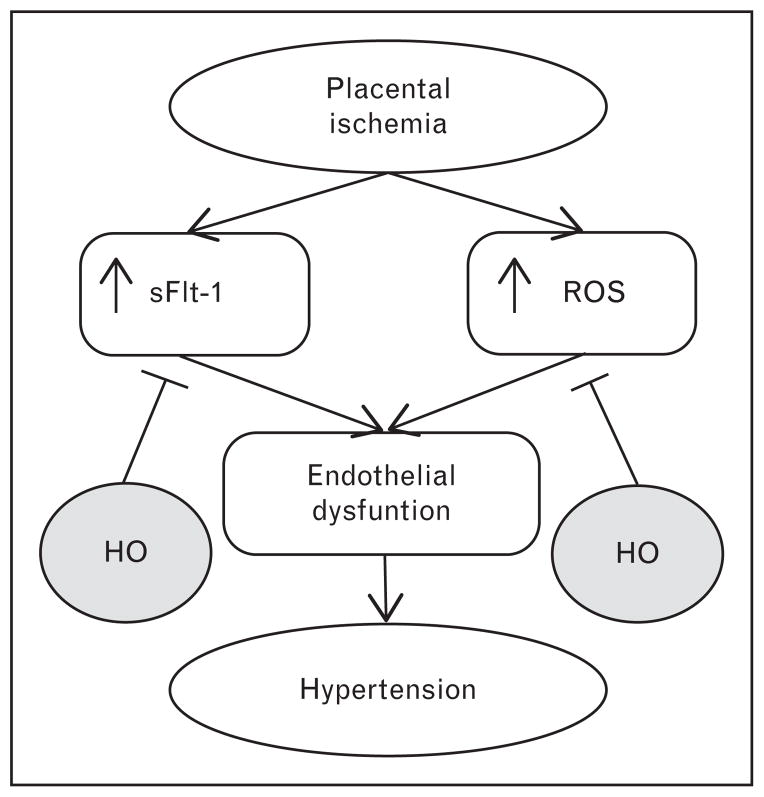
While the role of heme oxygenase in the development of preeclampsia is very much an open question, there has been a great deal of speculation about the utilization of the heme oxygenase system as a therapeutic approach to the management of preeclamptic symptoms [40,41]. Two of the major pathways involved in the manifestation of the symptomatic phase of the disorder are increased release of the vascular endothelial growth factor (VEGF) antagonist soluble fms-like tyrosine kinase-1 (sFlt-1) from the placenta and increased production of reactive oxygen species in the hypoxic placenta [42]. The bioactive metabolites of heme degradation have been postulated to attenuate the effects of both of these pathways (Fig. 2) [40,42]. Additionally, a growing body of experimental evidence both in vitro and in vivo has demonstrated that there may be utility in the heme oxygenase system in the management of preeclampsia.
FIGURE 2.
At the root of preeclampsia is placental insufficiency leading to placental hypoxia/ischemia. In response, the placenta releases factors leading to the maternal symptoms. Two important pathways activated in response to placental ischemia are the production of the vascular endothelial growth factor (VEGF) antagonist sFlt-1 and increased production of reactive oxygen species (ROS), both of which are important players in the development of maternal hypertension. Heme oxygenase (HO), through production of both carbon monoxide and bilirubin, has the ability to block both of these pathways and could be a novel therapeutic agent in the management of preeclampsia.
sFlt-1 is one of the major pathogenic agents produced by the preeclamptic placenta. An intriguing report from Cudmore et al. [4] first demonstrated that induction of HO-1 could dramatically negatively regulate sFlt-1 release from placental villous explants in response to either VEGF or interferon-γ administration. Specifically, administration of carbon monoxide by carbon-monoxide-releasing molecules could recapitulate the effect, suggesting its importance in the downregulation of sFlt-1. Subsequent studies from our laboratory have demonstrated similar results when sFlt-1 is induced by oxygen concentrations mimicking the preeclamptic placenta. Interestingly, both carbon monoxide and bilirubin significantly decreased the hypoxia-induced increase in sFlt-1, suggesting a possible dual role for heme oxygenase in the suppression of sFlt-1 in hypoxic conditions [5▪]. One recent report also indicates that resveratrol, which upregulates HO-1 production, significantly decreases sFlt-1 release from placental tissues and cell types from the placenta in response to a variety of stimuli, again suggesting that HO-1 induction could significantly blunt the hypertensive activity of sFlt-1 in preeclampsia [43▪]. Additionally, oxidative stress is also a pivotal factor in the manifestation of preeclampsia, and the potent antioxidant properties of bilirubin could conceivably be a potent therapeutic in reducing the oxidative stress in the ischemic placenta. Indeed, in-vitro data from our laboratory indicate that HO-1 induction significantly reduces hypoxia-induced oxidative stress from rodent placental villous explants, which is also recapitulated by exogenous bilirubin administration [5▪].
In addition, we have recently demonstrated the in-vivo efficacy of HO-1 induction in the attenuation of hypertension in two established models of gestational hypertension. In the first, we utilized the reduced uterine perfusion pressure (RUPP) rodent model, which consists of a mechanical constriction of the arteries feeding the uterus. This results in chronic placental ischemia and mimics many of the symptoms of human preeclampsia [44]. RUPP animals, which typically exhibit approximately 30 mmHg increase in mean arterial pressure, demonstrated a significant attenuation of hypertension when treated with the HO-1 inducer CoPP. This protective effect on blood pressure was accompanied by a proangiogenic shift in the VEGF/sFlt-1 balance in the placenta, which translated into an increase in the level of bioavailable VEGF in the maternal circulation. Finally, a significant decrease in RUPP-induced oxidative stress was also seen, presumably because of increased bilirubin production [45▪ ▪].
Interestingly, HO-1 induction also decreased sFlt-1 induced hypertension in pregnant rats. In this model, sFlt-1 levels are clamped at an elevated level, with no compensatory reduction in the production of sFlt-1 from the placenta. Still, an increase in bioavailable VEGF was seen, suggesting that HO-1 might be able to induce VEGF expression in these animals independently of its ability to suppress sFlt-1. Most recently, we have demonstrated that HO-1 induction during RUPP treatment leads to a shift in signal transduction pathways in the placenta from a proinjury to a prosurvival phenotype, again suggesting a potential protective role in preeclampsia [46▪].
What practical therapies could be derived from the heme oxygenase system to treat the preeclamptic patient? In the absence of suitable small molecule heme oxygenase inducers, there is an intriguing possibility that carbon monoxide could be used as a therapeutic agent. Several epidemiological studies have linked increased environmental carbon monoxide exposure to decreased risk of preeclampsia [47▪ ▪,48]. In a suggestive recent study, El-Mousleh et al. [49▪] demonstrated that low-dose carbon monoxide administration during pregnancy could attenuate changes in the fetal weight in a mouse fetal growth restriction model and could significantly increase the production of angiogenic factors. The possibility of using carbon monoxide inhalation as a new therapy for preeclampsia is an interesting area of investigation. Further investigations into the in-vitro effects on placental tissue and its physiological effects in the relevant models of preeclampsia are of keen interest.
CONCLUSION
Recent evidence suggests that the heme oxygenase system is important in the normal course of gestation. The heme oxygenase system appears to have a major role in placental development and the maintenance of pregnancy under normal and challenged conditions. Importantly, changes in heme oxygenase expression may be important in the development of preeclampsia. Finally, manipulation of heme oxygenase or its metabolites might be an intriguing new therapy in the treatment of preeclampsia.
KEY POINTS.
Proper development of the placenta during gestation is regulated in part by the heme oxygenase system.
Changes in heme oxygenase levels in the uteroplacental unit are altered during preeclampsia, though whether as a cause or a consequence remains to be elucidated.
Heme oxygenase and its metabolites carbon monoxide and bilirubin may be effective new therapeutics for the management of preeclampsia.
Acknowledgments
This work was supported by the NIH grants HL105324-01, HL51971, HL108618-01 1T32HL105324-01 (J.P. Granger), and American Heart Association Fellowship 11POST7840039 (E.M. George).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪ ▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an anti-oxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 2.McGeary RP, Szyczew AJ, Toth I. Biological properties and therapeutic potential of bilirubin. Mini Rev Med Chem. 2003;3:253–256. doi: 10.2174/1389557033488213. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 4.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 5▪.George EM, Colson D, Dixon J, et al. Heme oxygenase-1 attenuates hypoxia-induced sFlt-1 and oxidative stress in placental villi through its metabolic products CO and bilirubin. Int J Hypertens. 2012;2012:486053. doi: 10.1155/2012/486053. This study demonstrates that HO-1 and its metabolites can block hypoxia-induced sFlt-1 and ROS from placental tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulak J, Jozkowicz A, Foresti R, et al. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid Redox Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 7.Jozkowicz A, Huk I, Nigisch A, et al. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein RS, Garcia-Mayol D, Pettingell W, Munro HN. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci USA. 1991;88:688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res & Clin Obstet Gynaecol. 2011;25:273–285. doi: 10.1016/j.bpobgyn.2010.10.009. This comprehensive review lays out the importance of vascular remodeling during the gestational process. [DOI] [PubMed] [Google Scholar]
- 10.Jauniaux E, Watson AL, Hempstock J, et al. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odrcich MJ, Graham CH, Kimura KA, et al. Heme oxygenase and nitric oxide synthase in the placenta of the guinea-pig during gestation. Placenta. 1998;19:509–516. doi: 10.1016/s0143-4004(98)91044-x. [DOI] [PubMed] [Google Scholar]
- 12.Barber A, Robson SC, Myatt L, et al. Heme oxygenase expression in human placenta and placental bed: reduced expression of placenta endothelial HO-2 in preeclampsia and fetal growth restriction. FASEB J. 2001;15:1158–1168. doi: 10.1096/fj.00-0376com. [DOI] [PubMed] [Google Scholar]
- 13.Lyall F, Barber A, Myatt L, et al. Heme oxygenase expression in human placenta and placental bed implies a role in regulation of trophoblast invasion and placental function. FASEB J. 2000;14:208–219. doi: 10.1096/fasebj.14.1.208. [DOI] [PubMed] [Google Scholar]
- 14.Yoshiki N, Kubota T, Aso T. Expression and localization of heme oxygenase in human placental villi. Biochem Biophys Res Commun. 2000;276:1136–1142. doi: 10.1006/bbrc.2000.3551. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe S, Akagi R, Mori M, et al. Marked developmental changes in heme oxygenase-1 (HO-1) expression in the mouse placenta: correlation between HO-1 expression and placental development. Placenta. 2004;25:387–395. doi: 10.1016/j.placenta.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 16.McCaig D, Lyall F. Inhibitors of heme oxygenase reduce invasion of human primary cytotrophoblast cells in vitro. Placenta. 2009;30:536–538. doi: 10.1016/j.placenta.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Bilban M, Haslinger P, Prast J, et al. Identification of novel trophoblast invasion-related genes: heme oxygenase-1 controls motility via peroxisome proliferator-activated receptor gamma. Endocrinology. 2009;150:1000–1013. doi: 10.1210/en.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Wong RJ, Kalish FS, et al. Effect of heme oxygenase-1 deficiency on placental development. Placenta. 2009;30:861–868. doi: 10.1016/j.placenta.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪ ▪.Zhao H, Azuma J, Kalish F, et al. Maternal heme oxygenase 1 regulates placental vasculature development via angiogenic factors in mice. Biol Reprod. 2011;85:1005–1012. doi: 10.1095/biolreprod.111.093039. This study implicates heme oxygenase as an important regulator of placental structural development in HO-1-deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zenclussen ML, Casalis PA, El-Mousleh T, et al. Haem oxygenase-1 dictates intrauterine fetal survival in mice via carbon monoxide. J Pathol. 2011;225:293–304. doi: 10.1002/path.2946. [DOI] [PubMed] [Google Scholar]
- 21.Acevedo CH, Ahmed A. Hemeoxygenase-1 inhibits human myometrial contractility via carbon monoxide and is upregulated by progesterone during pregnancy. J Clin Invest. 1998;101:949–955. doi: 10.1172/JCI927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bainbridge SA, Farley AE, McLaughlin BE, et al. Carbon monoxide decreases perfusion pressure in isolated human placenta. Placenta. 2002;23:563–569. doi: 10.1053/plac.2002.0845. [DOI] [PubMed] [Google Scholar]
- 23.Sollwedel A, Bertoja AZ, Zenclussen ML, et al. Protection from abortion by heme oxygenase-1 up-regulation is associated with increased levels of Bag-1 and neuropilin-1 at the fetal–maternal interface. J Immunol. 2005;175:4875–4885. doi: 10.4049/jimmunol.175.8.4875. [DOI] [PubMed] [Google Scholar]
- 24.Zenclussen ML, Anegon I, Bertoja AZ, et al. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol. 2006;69:35–52. doi: 10.1016/j.jri.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25▪ ▪.Schumacher A, Wafula PO, Teles A, et al. Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS One. 2012;7:e42301. doi: 10.1371/journal.pone.0042301. This study looks at the molecular role of HO-1 in the maturation of dendritic cells and in the beneficial effects of T cells during preganncy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Tachibana M, Hashino M, Nishida T, et al. Protective role of heme oxygenase-1 in Listeria monocytogenes-induced abortion. PLoS One. 2011;6:e25046. doi: 10.1371/journal.pone.0025046. This study is one of several recent reports suggesting heme oxygenase can have a protective effect during challenge-induced abortion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tachibana M, Watanabe K, Yamasaki Y, et al. Expression of heme oxygenase-1 is associated with abortion caused by Brucella abortus infection in pregnant mice. Microb Pathog. 2008;45:105–109. doi: 10.1016/j.micpath.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Denschlag D, Marculescu R, Unfried G, et al. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol Hum Reprod. 2004;10:211–214. doi: 10.1093/molehr/gah024. [DOI] [PubMed] [Google Scholar]
- 29.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 30.Lindheimer MD, Kanter D. Interpreting abnormal proteinuria in pregnancy: the need for a more pathophysiological approach. Obstet Gynecol. 2010;115 (2 Pt 1):365–375. doi: 10.1097/AOG.0b013e3181cb9644. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert JS, Ryan MJ, LaMarca BB, et al. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 32▪.Khong Y, Brosens I. Defective deep placentation. Best Pract Res Clin Obstet & Gynaecol. 2011;25:301–311. doi: 10.1016/j.bpobgyn.2010.10.012. This study is a comprehensive review which clearly lays out the case for inadequate maternal vascular remodeling as a cause of preeclampsia. [DOI] [PubMed] [Google Scholar]
- 33.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The ‘Great Obstetrical Syndromes’ are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 35.McCaig D, Lyall F. Heme oxygenase expression in human placental villous tissue in response to exposure to in vitro ischemia–reperfusion injury. Hypertens Pregnancy. 2009;28:256–272. doi: 10.1080/10641950802132811. [DOI] [PubMed] [Google Scholar]
- 36.Shrestha Dangol D, Chen HP. Role of hemeoxygenase-2 in pregnancy-induced hypertension. Int J Gynaecol Obstet. 2004;85:44–46. doi: 10.1016/S0020-7292(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 37.Zenclussen AC, Lim E, Knoeller S, et al. Heme oxygenases in pregnancy. II: HO-2 is downregulated in human pathologic pregnancies. Am J Reprod Immunol. 2003;50:66–76. doi: 10.1034/j.1600-0897.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 38.Eide IP, Isaksen CV, Salvesen KA, et al. Decidual expression and maternal serum levels of heme oxygenase 1 are increased in preeclampsia. Acta Obstet Gynecol Scand. 2008;87:272–279. doi: 10.1080/00016340701763015. [DOI] [PubMed] [Google Scholar]
- 39▪.Vitoratos N, Papakonstantinou K, Deliveliotou A, et al. Antepartum and postpartum serum heme oxygenase-1 levels in preeclamptic and normotensive pregnant women. In Vivo. 2011;25:445–450. This study found that preeclamptic patients exhibit significant elevation in circulating HO-1. [PubMed] [Google Scholar]
- 40.Ahmed A, Cudmore MJ. Can the biology of VEGF and haem oxygenases help solve preeclampsia? Biochem Soc Trans. 2009;37 (Pt 6):1237–1242. doi: 10.1042/BST0371237. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed A, Rahman M, Zhang X, et al. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol Med. 2000;6:391–409. [PMC free article] [PubMed] [Google Scholar]
- 42.George EM, Granger JP. Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep. 2011;13:269–275. doi: 10.1007/s11906-011-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Cudmore MJ, Ramma W, Cai M, et al. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012;206:253.e10–253.e15. doi: 10.1016/j.ajog.2011.11.010. This intriguing report suggests that HO-1 induction by resveratrol could help block sFlt-1 production from placental tissue. [DOI] [PubMed] [Google Scholar]
- 44.Alexander BT, Kassab SE, Miller MT, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 45▪ ▪.George EM, Cockrell K, Aranay M, et al. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57:941–948. doi: 10.1161/HYPERTENSIONAHA.111.169755. This study demonstrates that HO-1 induction has antihypertensive effects in a rodent model of preeeclampsia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.George EM, Arany I. Induction of heme oxygenase-1 shifts the balance from proinjury to prosurvival in the placentas of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Regul Integr Comp Physiol. 2012;302:R620–R626. doi: 10.1152/ajpregu.00617.2011. This study shows that HO-1 induction shifts molecular signaling to a prosurvial profile in the ischemic placenta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪ ▪.Zhai D, Guo Y, Smith G, et al. Maternal exposure to moderate ambient carbon monoxide is associated with decreased risk of preeclampsia. Am J Obstet Gynecol. 2012;207:57.e1–57.e9. doi: 10.1016/j.ajog.2012.03.022. This intriguing study finds an inverse association between maternal ambient carbon monoxide exposure and incidence of preeclampsia. [DOI] [PubMed] [Google Scholar]
- 48.Wikstrom AK, Stephansson O, Cnattingius S. Tobacco use during pregnancy and preeclampsia risk: effects of cigarette smoking and snuff. Hypertension. 2010;55:1254–1259. doi: 10.1161/HYPERTENSIONAHA.109.147082. [DOI] [PubMed] [Google Scholar]
- 49▪.El-Mousleh T, Casalis PA, Wollenberg I, et al. Exploring the potential of low doses carbon monoxide as therapy in pregnancy complications. Med Gas Res. 2012;2:4. doi: 10.1186/2045-9912-2-4. This interesting study demonstrated improvements in fetal weight in a model of fetal growth restriction animals administered low-dose carbon monoxide. [DOI] [PMC free article] [PubMed] [Google Scholar]