Dear Editor
Although cat scratch disease (CSD) is a systemic infectious disease, ocular involvement is often recognizable by its characteristic pattern of stellate neuroretinitis. Less recognizable are other patterns of focal ocular involvement that contribute to visual loss. Here we present an atypical case of Bartonella, complicated by misleading serology, in which four such patterns of focal pathology in the vitreous, macula, neuroretinal rim and retrobulbar optic nerve induced severe visual loss.
The patient was a seemingly healthy 27-year-old fast-food worker from rural central Ohio, who complained of progressive visual loss OD for 2 weeks. Other than her seven outdoor cats, she had an unremarkable medical and social history. Pertinent negatives included no tick bites, cold sores, dermatomal rash, genital ulcers, previous malignancy, cough, hemoptysis, or shortness of breath.
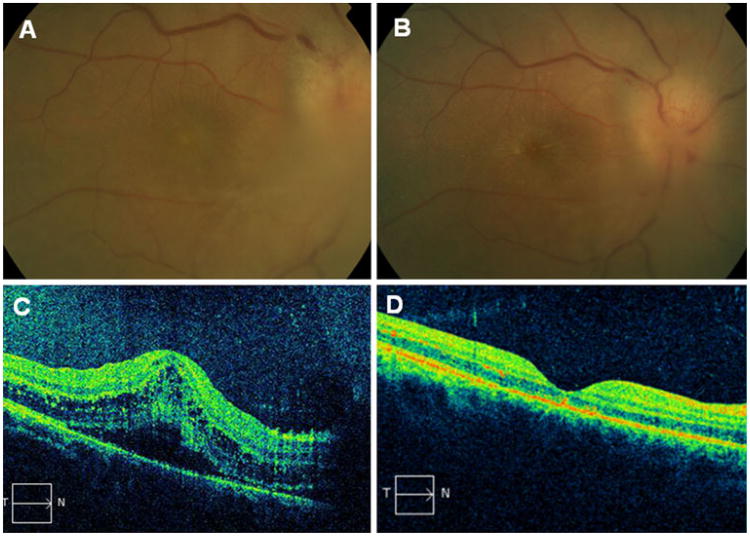
Her pertinent exam findings included CF vision OD and 20/20 OS, with afferent pupillary defect (APD) OD and normal anterior segments OU. Dilated examination showed dense vitreous opacities with snowballs partially obscuring the optic disc and macula OD (Fig. 1a) and clear vitreous OS. Florid disc edema with disc hemorrhages and macular edema OD were visible (Fig. 1a), while there was a clear view of mild disc edema and few macular exudates OS. Fluorescein angiogram demonstrated leakage from the optic disc OD more than OS. Visual fields showed central depressions OU superimposed on a nerve fiber bundle defect OD. The OCT showed a serous macular detachment and macular edema with central thickness of 438 microns OD (Fig. 1c), and an unremarkable macula OS.
Fig. 1.
a Right eye color fundus photograph at presentation shows inflammatory infiltrate over the edematous optic nerve. b Right eye color fundus photograph 1 week later shows optic disc edema with macular star exudates. c OCT scan of the right eye at presentation shows serous macular detachment with intraretinal cystic changes. d OCT scan 1 month later shows complete resolution
Imaging studies showed normal chest X-ray and brain MRI. The abnormal orbital MRI was indicative of retrobulbar optic neuritis with “ … a small area of enhancement at the junction between the optic nerve and globe on the right. There is a localized enhancement at this site over a short segment. The remainder of the right optic nerve has normal signal without enhancement … ”
Laboratory tests were all within normal limits, including Bartonella (B) henselae IgM [1:20] and IgG [1:128], B quintana, Toxoplasma, Toxocara canis, Lyme, serum rapid plasma reagin (RPR), serum herpes simplex virus (HSV) PCR, angiotensin converting enzyme, and lysozyme. Lumbar puncture showed normal pressure (180mmH2O), cells, and chemistries with negative cerebrospinal fluid (CSF) VDRL and RPR. CSF PCR for HSV, varicella zoster virus, and cytomegalovirus were also negative. A diagnostic and therapeutic vitrectomy showed no abnormalities or evidence of bacteria, fungi, or malignancy.
Repeat serology for B henselae a week later was abnormal [IgG 1: 1028] confirming a presumed clinical diagnosis of CSD. She was improving on treatment for presumed CSD with doxycycline 100 twice daily, rifampin 600 daily, and prednisone×3 weeks. Four months later her vision was 20/20 OU, with clear view except for minimal epiretinal membrane and debris OD (Fig. 1b,d).
This case was unusual, and instructive, in that her clinical presentation and lifestyle led to an initial consideration of CSD. Nevertheless the normal serologies, despite such prominent pathology, raised concerns which were cleared the next week by her diagnostic rise in titer to 1:1028. Other reports also suggest the need to reconsider the reliability of negative titers in CSD from labs other than CDC [1].
Of additional interest was this woman's multiplicity of visually impairing lesions from CSD. Central visual depression was caused by a dense intermediate uveitis with snowball vitreous opacities as well as from the abnormal macula. While her OCT characteristics are similar to those reported in CSD neuroretinitis by Habot-Wilner et al. [2], the level of dense vitreous opacities is atypical [3, 4]. A central scotoma and APD resulted from neuroretinitis, and additionally a nerve fiber bundle defect with APD resulted from anterior retrobulbar optic neuritis, localized by her visual field, and shown by MRI scan. This combination of multifocal CSD lesions is unique in our experience. In patients with negative serology but clinical features suggestive of CSD, a repeat titer may be of diagnostic benefit.
Contributor Information
Dominic M. Buzzacco, Email: dominic.buzzacco@osumc.edu, The Havener Eye Institute, The Ohio State University Medical Center, Columbus, OH, USA; Department of Ophthalmology and Vision Science, The Ohio State University Medical Center, 915 Olentangy River Road, Columbus, OH 43212, USA.
Martin Lubow, The Havener Eye Institute, The Ohio State University Medical Center, Columbus, OH, USA.
Frederick H. Davidorf, The Havener Eye Institute, The Ohio State University Medical Center, Columbus, OH, USA
Colleen M. Cebulla, The Havener Eye Institute, The Ohio State University Medical Center, Columbus, OH, USA
References
- 1.Cunningham ET, Koehler JE. Ocular bartonellosis. Am J Ophthalmol. 2000;130(3):340–349. doi: 10.1016/s0002-9394(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 2.Habot-Wilner Z, Zur D, Goldstein M, Shulman S, Kesler A, Giladi M, Neudorfer M. Macular findings on optical coherence tomography in cat scratch disease neuroretinitis. Eye. 2011;25(8):1064–1068. doi: 10.1038/eye.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soheilian M, Markomichelakis N, Foster CS. Intermediate uveitis and retinal vasculitis as manifestations of cat scratch disease. Am J Ophthalmol. 1996;122(4):582–584. doi: 10.1016/s0002-9394(14)72125-7. [DOI] [PubMed] [Google Scholar]
- 4.Kalogeropoulos C, Koumpoulis I, Mentis A, Pappa C, Zafeiropoulos P, Aspiotis M. Bartonella and intraocular inflammation: a series of cases and review of literature. Clin Ophthalmol. 2011;5:817–829. doi: 10.2147/OPTH.S20157. [DOI] [PMC free article] [PubMed] [Google Scholar]