1. INTRODUCTION
Until the advent of neuroimaging techniques which could be applied to study structure, function and molecular aspects of brain activity in living healthy humans and in patient populations, research in complex, symptom based disorders (e.g. in the absence of detectable peripheral causes of symptoms) in psychiatry, psychology, pain and gastroenterology had to rely exclusively on subjective reports by study subjects and on behavioral responses, such as autonomic and neuroendocrine outcome measures. This may be one of the major reasons why, in contrast to other, pathology-based disorders (“organic disorders”), progress in understanding the mechanisms underlying symptom based syndromes, and in the development of effective medications has been slow.
An attempt to correlate observations in putative animal models of such uniquely human disorders as functional GI disorders (FGIDs) with subjective patient reports has traditionally been based on (usually erroneous) assumptions that the animal models reflected in some way the dysregulation of the brain gut axis underlying symptoms in patients. However, during the last decade, an explosive growth of publications has occurred in the above mentioned areas, and this has lead to dramatic breakthroughs in our understanding of central aspects of pain modulation in humans. In particular, it has led to a better understanding of the role of attentional and other cognitive processes, as well as the role of emotions in the modulation of the human pain experience in healthy subjects and in patients with chronic pain disorders, such as fibromyalgia1 and chronic back pain.2 Possibly even greater progress has been made in our understanding of brain circuits, their underlying neurotransmitter systems and the association of these systems with gene polymorphisms in several psychiatric disorders, including schizophrenia, Post Traumatic Stress Disorder (PTSD) and depression,3 and many of these conceptual breakthroughs had a cross fertilizing effect on the field of neuroimaging in FGIDs. However, in comparison to these fields of medicine, progress in the understanding of central mechanisms contributing to the pathophysiology of FGIDs has been more modest. Only a small number of groups in the world have applied brain imaging techniques to gastroenterological disease (including functional pain disorders and appetite regulation), and the quality of published studies has generally not matched the level reached in other fields of neuroscience.
In this article, we will provide the interested reader with a brief review of technical information required to understand the basics of the most commonly used techniques (a more comprehensive version of this section is available as an on-line supplement at http://dx.doi.org/10.1111/j.1365-2982.2009.01304.x), and review published results of imaging studies in healthy control subjects and of different patient populations. Even though insights from brain imaging are relevant for a better understanding of both afferent and motor aspects of brain gut interactions, this review will deal primarily with imaging of brain responses to visceral afferent stimulation from the esophagus, stomach and distal colon. It will summarize areas in need of improvement in patient selection, study design and analysis, even though it is emphasized that the field of brain imaging is too dynamic and in evolution at the moment to propose strict guidelines for any of these areas. It is anticipated that from the information provided in the review readers naïve to this field will be able to critique existing literature and receive guidance about the best principles for undertaking functional brain imaging studies in the future.
Why do we need brain imaging in the study of complex symptom based disorders such as IBS, functional dyspepsia and functional heartburn and non-cardiac chest pain? Despite tremendous efforts aimed at identifying and characterizing peripheral aspects of the brain gut axis which might be responsible for characteristic symptoms in functional GI disorders, such as pain and discomfort, results have been inconsistent, and therapeutic strategies disappointing. In order to understand and treat the complex human pain experience, it has to be realized that there is no linear relationship between the information that is encoded by primary afferents in the gut and the conscious perception of such information. Multiple factors including cognitive, emotional and reward processes, as well as memories of past experiences are integrated in specific brain circuits (including the anterior insula [antINS] and dorsal anterior cingulate cortex [dACC]) which ultimately determine the subjective experience. The only currently available technique to dissect the various components of this subjective human experience is functional brain imaging. It allows not only the quantification of the viscerosensory input reaching the brain (otherwise only possible in animals, using electrophysiological or immunohistochemical techniques), but also an understanding of how psychological factors and psychiatric comorbidity contribute to the overall phenotype. Functional brain imaging is the only way to identify human brain circuits which correlate with various phenotypic and behavioral manifestations of FGIDs, such as psychological states and traits, symptoms, and dysregulations of the hypothalamic pituitary adrenal (HPA) axis and the autonomic nervous system (ANS).
2. FUNCTIONAL NEUROANATOMY OF THE BRAIN RELEVANT TO THIS REVIEW
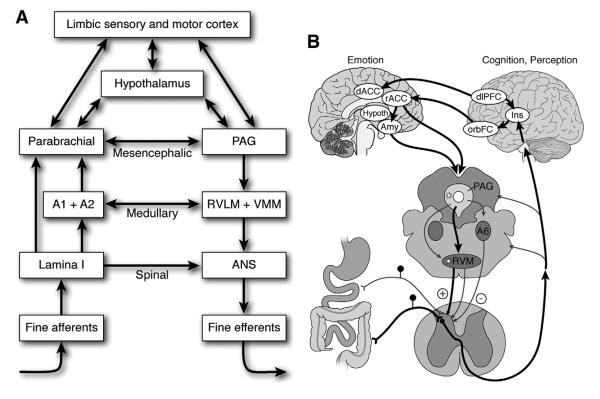
In this section we briefly consider some of the key brain structures mediating pain and emotion and their points of interaction in the brain (Fig. 1). Craig has suggested that all feelings from the body, including the mechanical, thermal, chemical, metabolic and hormonal status of skin, muscle, joints, teeth and viscera are mediated by a phylogenetically new system involved in interoception which only exists in humans and higher primates.4, 5 Small diameter primary afferent fibers that innervate all tissues of the body terminate monosynaptically in lamina I of the spinal and trigeminal dorsal horns. These lamina I fibers project to autonomic cell columns in the spinal cord as well as to pre-autonomic sites in the brainstem. Lamina I fibers as well as fibers from the nucleus of the solitary tract in the medulla project to the parabrachial nucleus (PB) in the brainstem, which is the main integration site for all homeostatic afferent activity. The PB in turn projects to periaqueductal gray (PAG), another brainstem nucleus, which coordinates the physiological and behavioral responses to threat, and to the hypothalamus, which guides goal-directed autonomic, neuroendocrine and behavioral activity. Afferent information from the entire body, including the viscera and including pain-related information is routed to the thalamus (the mediodorsal, basal ventral medial and posterior ventral medial nuclei), from where two parallel streams of information reach the insula (INS) and (ACC) respectively, which together play a key role in the conscious experience (feeling), represented in the antINS, and in the motivation (autonomic, somatomotor response), represented in the dorsal anterior cingulated cortex (dACC), characteristic for any human emotion, including pain. Both of these brain regions are part of a homeostatic afferent brain network, and figure prominently in imaging studies of brain gut interactions and of the FGIDs (see following section on brain imaging studies in healthy controls and patients), and a detailed discussion of the relevance of the homeostatic afferent network in brain gut interactions can be found elsewhere.6
Figure 1. Schematic illustration of functional organization of central neuroaxis in processing and modulation of visceral afferent signals.
A. Hierarchial organization of reflex responses to visceral afferent stimuli
B. Modulation of visceral afferent input by cognitive and emotional factors within the central neuroaxis (Need to get copyright permission from Gastroenterology/ Elsevier)
Legend: PAG Periaqueductal grey; RVLM Rostroventrolateral medulla; VMM ventromedial medulla; ANS autonomic nervous system; hypoth hypothalamus; Amy amygdala; orbFC orbitofrontal cortex
The amygdala is a prototypical emotion-related structure that has typically been associated with responses to fear and aversive learning.7, 8 However, recent findings of a meta-analysis suggest amygdala activity is not specific to negative emotions but to emotional stimuli in general. Nevertheless, the probability of activity occurring in the amygdala is increased when negative emotional stimuli such as fear, disgust and aversive conditioning are processed, suggesting a preferential role in processing negative stimuli.9 The amygdala participates in orchestrating somatomotor, visceral and cognitive responses to threats by virtue of its connections with other brain structures, in particular, the hippocampal network. The nucleus accumbens and ventral striatum participate in reward responses and positive emotional states. Other structures that are involved in generating both positive and negative emotional responses include the thalamus, hypothalamus, basal ganglia, and ventromedial prefrontal cortex (vmPFC).
The posterior insular cortex (postINS) is the primary projection area for visceral afferent information, while the mid and anterior insula subregions, particularly on the right side, are considered higher association area for these bodily signals. Following multiple representations of the interoceptive signal, activity in the right antINS is considered essential for the conscious experience of bodily feelings.
The ACC, by contrast, is a multifunctional structure situated in the medial frontal lobe that is highly interconnected with the anterior insula, as well as prefrontal, limbic and other subcortical structures. The ACC has several divisions. In humans, the subgenual (or infragenual) cingulate cortex is the principal site of autonomic (primarily vagal) regulation in the frontal lobe and has important bidirectional connections with the dorsal vagal complex, the amygdala and with the rostral or supragenual ACC. This brain region has strong bidirectional connections with the PAG, nucleus accumbens, hypothalamus, antINS, and PFC regions. It is activated in a variety of emotional states and seems to mediate prefrontal influences related to emotion modulation and corticolimbic inhibition. In close interactions with prefrontal regions (dorsolateral and ventrolateral PFC), it is involved in cognitive operations, such as thinking about feelings, reflecting upon feelings and resolving emotional conflicts. The mid-cingulate cortex (MCC), which is part of the dACC, is a multifunctional region involved in the executive control of attention, as well as in motivational and motor aspects (sympathetic, somatomotor) of emotions. It receives its input from the mediodorsal aspect of the thalamus (MDvc), as well as from prefrontal and parietal cortices, and is connected to pre and supplementary motor areas. Similarly to the INS, the dACC is an interface for cognition, affect and interoceptive input and is a higher-level brain area where the physiological adjustments are generated that are needed to support cognitive and affective responses.
The dorsolateral PFC is involved in attentional processes and in the mediation of working memory and setting goals for behavioral responses. It is densely connected to the motor cortex and the hippocampus and plays a key role in integrating behavior with existing circumstances in the external environment, including regulation of emotional behavior. By contrast, the medial PFC is involved in representing states of the self and monitoring and regulating the internal milieu.
3. TECHNICAL AND PRACTICAL ASPECTS OF FUNCTIONAL BRAIN IMAGING
(For a more detailed version of this section, we refer to the online supplementary information at http://dx.doi.org/10.1111/j.1365-2982.2009.01304.x). Human brain activity can be measured and imaged using several techniques and two basic classes of mapping technique have evolved: those that map or localize the underlying electrical activity of the brain; and those that map local physiological or metabolic consequences of altered brain electrical activity. Among the former are the non-invasive neural electromagnetic techniques of electroencephalography (EEG) and magnetoencephalography (MEG). These methods allow exquisite temporal resolution of neural processes (typically over a 10–100 ms time scale), but suffer from poor spatial resolution (between 1 and several centimeters). Positron Emission Tomography (PET) and Functional MRI (fMRI) methods are in the second category and the latter can be made sensitive to the changes in regional blood perfusion, blood volume (for example, using injected magnetic resonance contrast agents), or blood oxygenation that accompany neuronal activity. Blood oxygenation level dependent (BOLD) fMRI, which is sensitive primarily to the last of these variables, allows an image spatial resolution that is of the order of a few millimeters, with a temporal resolution of a few seconds (limited by the haemodynamic response itself).
fMRI
The potential of fMRI is vast because it is uniquely non-invasive, has good sensitivity, and gives relatively high spatial and temporal resolution. It has replaced [15O]-PET in many research areas where localization of function is of primary interest. As fMRI does not rely on the use of radiolabelled compounds, there is great potential for longitudinal studies in large numbers of patients as well as in pharmacological studies.
The main limitations of fMRI arise from the vascular origin of the signal changes that are correlated with neuronal activity. This imposes physiological constraints on temporal and spatial resolution. The haemodynamic response takes place over several seconds, and it varies somewhat across brain tissue. Normally, a resolution of no better than a few seconds can be expected, much greater than the temporal response of neurons that are in the millisecond range.
PET
As a positron emitting radioligand is given intravenously to measure regional cerebral blood perfusion, there are limitations in the subject cohorts that can be investigated and the number of times one subject can be exposed to the radioactivity. This therefore puts limitations on the experimental protocol. Spatial and temporal resolution are generally poorer compared to fMRI. Another limitation stems from the fact that there are no more than a dozen probes which work in vivo.
4. FUNCTIONAL BRAIN IMAGING: BRAIN RESPONSES TO VISCERAL STIMULATION
4.1 General considerations
Functional imaging of the human brain during stimulation of the healthy GI tract and in patients with FGIDs has included many of the sections of the upper and lower gut using several different methods of stimulation and various imaging modalities. As is the case with any relatively new scientific endeavor, there is considerable divergence in findings but also some consistent results that provide important information on the pathways and modulation of visceral sensation in health and disease (see Tables 1 and 2).
Table 1.
Brain Imaging Literature on Healthy Control Subjects using Visceral Stimuli (all studies used volume/pressure controlled distension)
| Author | Year | Reference | Site | Imaging Modality |
N tot |
M | F | postIns | antIns | ACC | S1 | PFC | POC | Tha | BA6 | Cereb | PCC | MCC | M1 | S2 | PAG | TP | OFC | SM | NC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hamaguchi | 2004 | 109 | Cdist | PET | 15 | 15 | 0 | B+ | B+ | L+ | R+ | B+ | B+ | B+ | B+ | L+ | R+ | R+ | L+ | ||||||
| Aziz Q | 1997 | 23 | E | PET | 8 | 7 | 1 | B+ | B+ | B+ | B+ | B+ | B+ | ||||||||||||
| Binkofsky F | 1998 | 21 | E | fMRI | 5 | 5 | 0 | L+ | B+ | B+ | R+ | B+ | |||||||||||||
| Aziz Q | 2000 | 24 | Edist | fMRI | 6 | 6 | 0 | R+ | R+ | R+ | B+ | L+ | B+ | L+ | L+ | R+ | R+ | L+ | |||||||
| Aziz Q | 2000 | 24 | Epost | fMRI | 6 | 6 | 0 | R+ | R+ | L+ | R+ | L+ | R+ | R+ | L+ | ||||||||||
| Strigo | 2002 | 38 | E | fMRI | 7 | 4 | 3 | L+ | R+ | L+ | B+ | B+ | L+ | B+ | B+ | B+ | B+ | ||||||||
| Phillips ML | 2003 | 48 | E | fMRI | 8 | 8 | 0 | B+ | B+ | R+ | B+ | B+ | B+ | B+ | |||||||||||
| Lawal | 2008 | 110 | Edist | fMRI | 20 | 4 | 16 | B+ | B+ | B+ | B+ | ||||||||||||||
| Paine | 2008 | 111 | Edist | fMRI | 7 | 4 | 3 | R+ | R+ | R+ | R+ | ||||||||||||||
| Ladabaum | 2001 | 29 | Gdist | PET | 15 | 6 | 9 | B+ | B+ | R+ | B+ | B+ | B+ | B+ | B+ | ||||||||||
| Stephan | 2003 | 112 | Gprox | PET | 18 | 0 | 18 | B+ | R+ | B+ | |||||||||||||||
| Lu | 2004 | 113 | Gdist | fMRI | 10 | 8 | 2 | B+ | B+ | B+ | B+ | B+ | |||||||||||||
| Vandenbergh J |
2005 | 114 | Gpost | PET | 11 | 5 | 6 | R+ | R+ | R+ | B+ | L+ | R+ | B+ | L+ | ||||||||||
| Ladabaum | 2007 | 115 | Gprox | fMRI | 18 | 8 | 10 | B+ | B+ | L+ | R+ | B+ | B+ | B+ | L+ | R+ | |||||||||
| Wang | 2007 | 116 | Gprox | fMRI | 18 | 15 | 3 | B+ | B+ | L+ | L+ | ||||||||||||||
| Van Oudenhove |
2008 | 117 | Gpost | PET | 11 | 5 | 6 | B+ | L+ | R+ | R+ | B+ | B+ | ||||||||||||
| Kern | 1998 | 22 | R | fMRI | 10 | 4 | 6 | B+ | B+ | B+ | B+ | B+ | B+ | ||||||||||||
| Bouras | 1999 | 118 | R | SPECT | 10 | 3 | 7 | B+ | B+ | ||||||||||||||||
| Hobday | 2001 | 30 | R | fMRI | 8 | 8 | 0 | B+ | B+ | B+ | B+ | B+ | R+ | B+ | B+ | B+ | |||||||||
| Lotze | 2001 | 33 | R | fMRI | 8 | 4 | 4 | B+ | B+ | B+ | B+ | B+ | R+ | L+ | B+ | L+ | B+ | B+ | R+ | B+ | |||||
| Verne | 2002 | 119 | R | fMRI | 9 | 3 | 6 | L+ | L+ | R+ | R+ | L+ | L+ | ||||||||||||
| Dunkley | 2005 | 43 | R | fMRI | 10 | 5 | 5 | B+ | B+ | B+ | B+ | ||||||||||||||
| Lawal | 2005 | 120 | R | fMRI | 18 | 8 | 10 | B+ | B+ | ||||||||||||||||
| Bittorf | 2006 | 121 | R | fMRI | 13 | 11 | 2 | B+ | B+ | B+ | B+ | B+ | B+ | ||||||||||||
| Song | 2006 | 122 | R | fMRI | 12 | 0 | 12 | B+ | B+ | B+ | B+ | B+ | L+ | B+ | |||||||||||
| Berman | 2008 | 83 | R | fMRI | 12 | 0 | 12 | B+ | B+ | ||||||||||||||||
| Eickhoff | 2008 | 123 | R | fMRI | 8 | 4 | 4 | L+ | L+ | L+ | L+ | ||||||||||||||
| Kwan | 2005 | 99 | R | fMRI | 11 | na | na | R+ | R+ | ||||||||||||||||
| Verne | 2003 | 119 | R | fMRI | 9 | 3 | 6 | R+ | L+ | ||||||||||||||||
| Andresen | 2005 | 124 | R | fMRI | 8 | 5 | 3 | B+ | B+ | B+ | B+ | B+ | |||||||||||||
| Naliboff | 2001 | 28 | R | PET | 12 | 10 | 2 | B+ | B+ | B+ | R+ | B+ |
Legend: Abbreviations used
ACC Anterior cingulate cortex
BA Brodmann area
B−/B+ Bilateral deactivation/activation
Cereb Cerebellum
Cdist Distal colon distension
Edist Distal esophagus distension
Eprox Proximal esophagus distension
F Frontal area
fMRI Functional Magnetic Resonance Imaging
G Gastric Distention
antIns Anterior insular cortex
postIns Posterior insular cortex
L+/− Left activation/ deactivation
M Males
M1 Primary motor cortex
MCC Middle cingulate cortex
NC Nucleus caudatus
NA Number unknown
OFC Orbitofrontal cortex
PAG Periaqueductal grey
PET Positron emission tomography
PFC Prefrontal cortex
POC Parieto occipital cortex
R Rectal distension
R+/− Right activation/ deactivation
SM Supramarginal gyrus (BA39/40)
S1 Primary sensorial area
S2 Secondary sensorial area
SPECT Single positron emission tomography
Tha Thalamus
Tot total
UC Ulcerative colitis
Table 2.
Brain Imaging Literature on Patient Populations using Visceral Stimuli (all studies were performed using controlled pressure/volume distension)
| Author | Year | Reference | Site | Stim | Met | tot | H | Di | M | F | ACC | InsA | InsP | PF/Fa | T | S1 | S2 | BA6 | Cereb | OFC | PCG | pag | M1 | MCG | TP | SM | POC | NC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Van Oudenhove |
2008 | 117 | Gprox | Bst | fMRI | 13 | 0 | 13 | dyspepsia | na | na | L+ | L+ | B+ | ||||||||||||||
| Silverman | 1997 | 25 | R | Bst | PET | 12 | 6 | 6IBS | na | na | B+ | |||||||||||||||||
| Mertz | 2000 | 27 | R | Bst | fMRI | 34 | 16 | 18IBS | 4 | 30 | R+ | L+ | L+ | R+ | R+ | |||||||||||||
| Naliboff | 2001 | 28 | R | Bst | PET | 24 | 12 | 12IBS | 20 | 4 | B+ | L+ | B+ | B+ | L+ | B+ | B+ | L+ | L+ | |||||||||
| Bernstein | 2002 | 34 | R | Bst | fMRI | 18 | 6 | 4Cr,2UC,6IBS | 10 | 8 | B+ | B+ | B+ | B+ | ||||||||||||||
| Bonaz | 2002 | 125 | R | Bst | fMRI | 12 | 0 | 12 | IBS | 1 | 11 | R- | ||||||||||||||||
| Verne | 2003 | 119 | R | Bst | fMRI | 9 | 0 | 9 | IBS | 3 | 6 | L+ | L+ | R+ | R+ | L+ | L+ | L+ | ||||||||||
| Ringel | 2003 | 126 | R | Bst | PET | 12 | 6 | 6IBS | 0 | 12 | L+ | |||||||||||||||||
| Wilder | 2004 | 100 | R | Bst | fMRI | 20 | 10 | 10IBS | 20 | 0 | B+ | B+ | B+ | B+ | B+ | B+ | B+ | B+ | ||||||||||
| Andresen | 2005 | 124 | R | Bst | fMRI | 16 | 8 | 8 IBS | 8 | 8 | B+ | B + | B + | B + | B + |
B + |
B + |
B + |
||||||||||
| Kwan | 2005 | 99 | R | Bst | fMRI | 20 | 11 | 9 | IBS | na | na | R+ | R+ | B+ | B+ | |||||||||||||
| Mayer | 2005 | 84 | R | Bst | PET | 22 | 7 | 8UC,7IBS | 22 | 0 | R+ | B- | B+ | |||||||||||||||
| Naliboff | 2006 | 93 | R | Bst | PET | 20 | 0 | 20 | IBS | 6 | 14 | B+ | R+ | B+ | B+ | |||||||||||||
| Song | 2006 | 122 | R | Bst | fMRI | 12 | 0 | 12 | IBS | 0 | 12 | B+ | B+ | L+ | L+ | L+ | R+ | |||||||||||
| Price | 2007 | 127 | R | Bst | fMRI | 9 | 0 | 9 | IBS | na | na | B+ | L+ | L+ | R+ | L+ | B+ | |||||||||||
| Berman | 2008 | 83 | R | Bst | fMRI | 14 | 0 | 14 | IBS | 0 | 14 | B+ | B+ | B+ | ||||||||||||||
| Ringel | 2008 | 128 | R | Bst | fMRI | 20 | 10 | 10 | IBS | 20 | L+ | |||||||||||||||||
| Wietek | 2008 | 129 | R | Bst | fMRI | 11 | 0 | 11 | paraplegic | 10 | 1 | B+ | B+ | B+ | B+ | L+ | R+ | B+ | B+ | B+ |
Legend: Abbreviations used
A Anus
ACG anterior cingulate gyrus
BA Brodmann area
B−/B+ bilateral deactivation/activation
Bst Barostat
Cereb Cerebellum
Cr Crohn’s Disease
D Disease
DC distal colon
ED distal esophagus
EP proximal esophagus
Fa Frontal area
fMRI Functional Magnetic Resonance Imaging
Gprox Proximal gastric distension
H Healthy
IBS Irritable Bowel Syndrome
InsA Anterior insular cortex
InsP Posterior insular cortex
L+/− Left activation/ deactivation
M Males
Man manual
Met Method
M1 Primary motor cortex
MCG Middle cingulate cortex
NC Nucleus caudatus
NA Number unknown
OFC Orbitofrontal cortex
PAG Periaqueductal grey
PCG Posterior cingulate gyrus
PET Positron emission tomography
PFC Prefrontal cortex
POC Parieto occipital cortex
R Rectal distension
R+/− Right activation/ deactivation
SD Stomach distal
SM Supramarginal gyrus (BA39/40)
SP Stomach proximal
SPECT Single positron emission tomography
Stim stimulus
S1 Primary sensorial area
S2 Secondary sensorial area
T Thalamus
Tot Total
TP Temporal area
UC Ulcerative colitis
4.2 Sources of variability across studies
A wide range of variables can significantly impact the results of brain imaging studies and probably account for the large inter-study variability. These include:
Method of Stimulation
a variety of methods including electrical, mechanical and chemical stimulation have been used to stimulate the upper and lower gut. These stimuli differ in the type and quantity of peripheral encoding mechanisms and afferent fibers that are activated (e.g. A delta, C), the time course of activation (from milliseconds to seconds) and often the location (upper or lower esophagus, bladder, rectum, sigmoid colon).
Study procedures
Investigators have used stimulus intensities from subliminal to painful as well as examining conditioned and sham responses associated with visceral stimulation. Although some of the brain areas and networks activated by these different stimuli overlap, it is not surprising that differences, especially in cortical and/or limbic activation occur depending on stimulus salience, intensity, unpleasantness, time course and familiarity. In addition, only a few studies have used a familiarization session to the scanner or the stimuli in order to decrease novelty effects and increase response consistency.
Number of subjects
Although it is not clear the precise number of subjects needed to adequately power functional brain imaging studies, it is likely that many of the published studies are underpowered. A simple fixed effects analysis has shown ‘significant’ brain activations from as few as 5 subjects but these results must be seen as highly exploratory. Most healthy volunteer studies have greater than 10 subjects with the typical range around 12 to 18. A random effects analysis usually requires more than 12 subjects, which is more robust and allows for generalizability of the results. Certainly due to the smaller mean differences of subtle pathology relative to healthy volunteers, greater numbers are needed to demonstrate significant differences.
Study Populations
Studies of even ‘healthy’ subjects have included a very heterogeneous set of samples from the standpoint of age, sex, ethnicity, and experience with pain. Since most studies have used too small a sample size it is therefore not possible to examine how these differences in subject characteristics might have influenced the outcome.
Image processing and analysis
As discussed further in the online technical supplement, there is little consistency in the processing and analysis methods across studies and widely different approaches to statistical decision making have been employed.
In summary, the examination of a large range of stimuli and study procedures is critical for understanding brain responses to visceral stimulation; however, few studies directly and systematically compare these different methods. In addition, variability (in subject numbers, characteristics and analysis methods) makes direct comparison of studies difficult and limits the use of meta-analysis techniques to make more general conclusions.
5. BRAIN RESPONSES TO VISCERAL STIMULI IN HEALTHY SUBJECTS
A review of neuroimaging studies using visceral stimulation published by Derbyshire in 200210 included data from 15 relevant articles.11-35 For the current review, we identified a total of 31 relevant studies reporting findings in healthy control subjects (Table 1; esophageal, gastric and rectal) and 18 relevant studies in patient populations (Table 2; primarily IBS) reported during the past 10 years (1997 to 2008). In the studies looking at healthy control subjects, 8 were performed with esophageal distension, 7 with gastric distension, 1 with colonic distension and 15 with rectal distension. Similar to the findings of the 2002 review, and similar to studies using somatic pain stimuli, overall, the most consistently activated brain regions in all reports were the insular cortex (both anterior and posterior subregions) and the ACC, followed (in order of reported frequency) by primary sensory cortex (S1), PFC regions, posterior parietal cortex (PPC) and thalamus. The consistent activation of insular and anterior cingulate cortices, despite very different study paradigms and analysis methods is remarkable, given that these two regions are regularly reported in somatic pain studies provides support for the concept of the homeostatic afferent network. This brain network, which can be engaged by the spinal lamina I afferent input to the brain, signals to the CNS a potential threat to the organism’s homeostasis.6 Even though a formal meta analysis of reported brain regions has not been reported, differences between brain activation patterns between esophageal, gastric and rectal stimuli are likely to be present.6 While INS and ACC were the most commonly reported regions for all 3 distension sites, S1/S2 and M1 were more often reported from studies in the upper GI tract.
5.1 Comparison of brain activation to visceral and somatic pain stimuli
Although there are many similarities in brain activation to visceral and somatic pain (in particular in the activation of homeostatic afferent regions), it is also clear that perceptual, autonomic and behavioral responses to noxious stimulation of somatic structures differ from those of the viscera.36, 37 These differences have been explained based on the functional neuroanatomic differences between visceral and somatic pain processing. Experimentally-induced aversive visceral sensations in humans are generally described as more unpleasant and diffuse than somatic sensations.38, 39 In a series of studies comparing a visceral mechanical stimulus to cutaneous thermal pain of similar intensity it has been shown that secondary somatosensory and parietal cortices, thalamus, basal ganglia and cerebellum are activated by both stimuli. However, cutaneous heat pain evoked greater activation in the bilateral antINS and ventrolateral PFC while visceral mechanical pain evoked in the same dermatome was associated with activation of bilateral inferior primary somatosensory cortex, bilateral primary motor cortex and a more rostral region within the dACC.38, 40, 41 Visceral stimulation of the esophagus resulted in the activation of a more lateral region in the parasylvian cortex than cutaneous stimulation of the trunk elicited. Hobday et al. found similar brain activation to visceral (rectal) and somatic (anal) distension, even though a greater activation of motor cortex by the somatic stimulus was observed.30 Dunckley et al.20, 42 compared cutaneous heat to rectal distension using matched stimulus unpleasantness and also found that the relative unpleasantness of the subjective experience of the visceral mechanical stimuli was higher than that of the somatic thermal stimuli. In a follow up study43 of moderately painful, electrical stimuli to either the midline lower abdomen or the rectum, significant activation associated with both stimuli was observed in several brainstem regions including the PAG, the parabrachial nucleus (PBN), the locus coeruleus complex (LCC) and the nucleus cuneiformis (NCF). A significantly greater activation of a region identified as the NCF and a significant correlation of the right PAG with anxiety ratings was observed with the visceral stimulus, suggesting that the observed differences may represent a greater nociceptive response and a greater emotive salience of visceral pain. While the above mentioned studies provide some insight to differences and similarities in the behavioral responses and brain processing of visceral and somatic pain, it is worth noting that these studies are confounded by the fact that the stimuli differed in the modality used (mechanical vs. thermal) to stimulate the gut and somatic tissues.
5.2 Sex-related differences in brain activation
Only a few studies have examined sex-related differences in brain responses to visceral pain in humans. Kern et al. studied healthy subjects and demonstrated that the volume of cortical activity during rectal distension was significantly greater in females than in males.44 Male subjects showed localized clusters of activity primarily in the sensory motor cortex and posterior parietal regions, whereas female subjects additionally showed activity in the dACC, PFC regions and in the INS. Berman et al. studied brain responses to rectal distension at uncomfortable and mildly painful levels as well as to an expected, but undelivered, rectal distension.31 The painful distension significantly activated the INS and ACC in both sexes. Although all activations appeared more extensive in men, no sex-related differences attained significance perhaps due to the small sample sizes. An exploratory voxel by voxel analysis suggested greater activations in the INS in men compared to women and greater deactivations in women in the amygdala and MCC. Overall, these studies suggest some sex-related differences, but further study is clearly needed to make firm conclusions.
5.3 Studies of psychological factors modulating the brain processing of visceral sensation
Pain is often described in purely physiological terms reflecting the assumption that perceived intensity of stimulation correlates well with level of noxious stimulation. However, the role of psychological factors in the modulation of pain processing has received much attention and the impact of factors such as stress, anxiety, mood and personality on an individual’s pain experience is well recognized.45-49
Cognitive Factors
Phenomena such as learning, anticipation, attention/distraction and the placebo effect are known to influence somatic pain experience.50-57 Functional brain imaging studies have shown that a number of regions involved in somatic pain processing can be modulated by attention, including the ACC, SI, INS and PFC.51, 58-61 The MCC is considered integral to the attentional modulation of pain,51, 59-64 with evidence of increased activity while attention is focused on pain and a decreased activity during distraction.59, 60, 62 (For further information on the role of the different cortical areas and their interconnections, see section above, FUNCTIONAL NEUROANATOMY OF THE BRAIN RELEVANT TO THIS REVIEW.)
Functional brain imaging studies involving visceral sensation and its attentional modulation are few and far between. Using a selective/divided attention task, Gregory et al. presented healthy volunteers with visual and visceral non-painful oesophageal stimulation.56 Selectively attending to visceral stimulation activated the visceral neuro-matrix including SI/S2 and ACC, while selectively focusing attention towards visual stimulation resulted in activation of the visual cortex. However, when attention was divided between the visual and visceral stimulation, more neural resources were devoted to process visceral stimuli. Moreover, selectively attending to visual stimulus attenuated regions involved in visceral processing, in particular the ACC, highlighted the importance of attention on the brain activity following visceral stimulation.
In a more recent study of the role of distraction on the brain processing of esophageal sensation, healthy subjects experienced stimuli ranging from non-painful to painful while they performed a memory task. During distraction, progressively increasing esophageal sensation was associated with a linear increase in the intensity of activation in the primary somatosensory cortex (SI) (bilateral) and left mid ACC. However, distraction reduced pain ratings and was accompanied by reduction in brain activity in the right ACC and right PFC with no effect on SI activity.65 This suggests that the SI is involved in processing sensory-discriminative aspects of visceral sensation while MCC is multifunctional, being involved in sensory and cognitive appraisal of visceral pain; the right PFC seems to be involved in only cognitive responses to pain. Furthermore, the fact the right PFC is modulated by cognitive manipulation resulting in reduced pain scores supports studies involving somatic pain, suggesting a role for this region in analgesia via a well defined opiate sensitive descending pathway.65
Using a model of Pavlovian conditioning, Yaguez et al. studied the role of anticipation in the brain processing of oesophageal pain and demonstrated that the regions such as the ACC and the PFC are not only involved in the perception of pain but are also involved when pain is predicted by a visual cue without it actually being delivered (anticipation).57 This suggests that it is possible for associative learning to occur so that certain environmental cues may predict the occurrence of a painful experience and hence even normal sensory experience may be exaggerated.
Emotional Factors
Emotional factors such as positive or negative affect, unpleasantness, emotional context, emotional state and trait contribute to the affective motivational component of the pain experience. This affective component can influence the pain experience and alter aspects such as perception of pain intensity as well as cognitive factors including how much attention is directed toward a painful event. Examples of how the affective motivational component of the pain can influence the pain experience can be seen in examples such as stress induced analgesia, sadness induced increase in pain perception66-69 and positive emotional state induced increase in pain tolerance.66, 69-72 Through several brain imaging studies, an affective-motivational pain network within the brain has been identified which includes regions such as the amygdala, anterior insula and the perigenual to mid cingulate cortex.
Studies on the brain processing of pain and emotion have classically implicated both the INS and the ACC in processing the affective dimension of pain.52, 73-78 Studies by Tolle et al.74 and Rainville et al.52 have shown that activation in the ACC is modulated by pain unpleasantness rather than intensity. Similarly, the INS has been implicated in many studies in somatic pain particularly when accompanied by a strong emotional response.79, 80
Knowledge of the emotional modulation of brain processing of visceral sensation is sparse. Phillips et al. used fMRI to study brain responses in healthy volunteers watching fearful (negative emotional context) or neutral facial expressions while non-painful oesophageal sensation was delivered.48 Increased activation in the ACC and right INS as well as increased ratings of anxiety and discomfort were demonstrated when esophageal sensation was experienced during the negative emotional context in comparison to the neutral context providing further support for the role of negative emotions modulating visceral sensation.48
It is clear that the importance of cognitive and emotional factors in the brain processing of visceral sensation cannot be underestimated and the above studies also highlight the importance of controlling for these psychological factors when planning functional brain imaging studies involving sensory perception.
6. BRAIN RESPONSES TO VISCERAL STIMULI IN PATIENTS
Of the total of 18 patient population studies, 16 were performed in IBS patients using rectal distension, 1 in functional dyspepsia using gastric distension, and 1 paraplegic using rectal distension. The large majority of studies using visceral stimuli in patients have been in IBS patients and these studies are summarized in Table 2. Similar to the findings in healthy subjects, the INS and ACC were the most commonly reported regions. The majority of studies are descriptive, not hypothesis driven, and did not control for various important factors such as expectation, response requirements, previous exposure to the stimulus, affective comorbidity, symptom-related anxiety, or sex of the subjects, therefore leaving cognitive and affective processing uncontrolled. Several of the earlier studies examined the differences between patients with IBS and healthy controls during visceral stimulation11, 17, 18 and anticipation of visceral stimulation.18 The findings suggested that patients showed similar areas of activation to controls81 but evidenced greater activation in some regions, including the ACC and INS, in addition to limbic areas including the hypothalamus, infragenual cingulate cortex, and amygdala.18 Decreased activation in the dorsal pons (in the region of the periaqueductal gray [PAG]) was also reported in IBS patients,18 and these results gave rise to an initial hypothesis that patients might have increased affective and attentional responses to actual or anticipated visceral stimuli (hypervigilance), as well as potentially decreased descending pain inhibition.18 More recent studies have confirmed some of these concepts.82-84 In the following, we will highlight some of the studies performed in IBS patients.
6.1 Differences in central processing of somatic and visceral experimental stimuli
Verne et al. reported that both somatic and visceral nociceptive stimuli evoked greater neural activity in brain regions of IBS patients compared to healthy controls.85 These regions included both those related to homeostatic afferent processing (thalamus, somatosensory and insular cortices) as well as those more related to cognitive and emotional modulation (anterior and posterior cingulate and prefrontal cortices). Chang et al. studied female IBS patients with and without a comorbid diagnosis of fibromyalgia.86 Group differences in regional brain activation were only observed within the dACC, where IBS patients showed a greater response to visceral stimuli and IBS+fibromyalgia patients showed a greater response to somatic stimuli. The authors concluded from their findings that chronic stimulus-specific enhancement of dACC responses to sensory stimuli in both syndromes may be associated with cognitive enhancement of either visceral (IBS) or somatic (IBS+fibromyalgia) sensory input. The fact that no group differences were observed in primary sensory areas (thalamus, somatosensory cortex, insula) is consistent with the concept that afferent input that reaches the brain is not different between the two patient populations, while arousal and attentional mechanisms may differ.
6.2 Sex-related differences in brain activation in IBS patients
There is now substantial evidence that sex-related differences in IBS prevalence and symptom presentation exist in both clinic samples and in the large group of IBS sufferers outside of the medical system.87 Several pieces of evidence suggest that female sex is associated with a higher prevalence overall of chronic pain disorders and that both female experimental animals and healthy women may be more sensitive to experimental pain than their male counterparts.13, 15, 88, 89
A series of brain imaging studies have addressed sex differences in the brain’s response to somatic and to visceral pain stimuli.89 Berman et al. reported the first study of brain responses to rectal distension in male and female IBS patients.31 In males, but not females, rectal distension was associated with activation of antINS and dACC. Naliboff et al. conducted a larger PET study of male and female IBS patients and found greater activation for female patients in limbic (amygdala) and paralimbic regions (ventromedial PFC, iACC and dACC), whereas male patients showed greater activation of the midposterior INS, dorsolateral PFC and dorsal pons.12 Similar sex-related differences were observed during the expectation condition. This study replicated the finding from the earlier study showing greater activation by male patients of the insular cortex.31 The findings also suggested that female patients in response to a pelvic aversive stimulus show greater responses of limbic and paralimbic regions, while male patients show greater activation of regions belonging to a corticolimbic pain inhibition system. In a follow up study, using connectivity modeling of the same data set, female patients differed primarily during the expectation condition, where they showed evidence for greater activation of an emotional arousal circuit.90 It needs to be emphasized that the published literature on sex difference in brain activation by visceral stimuli is still sparse and somewhat contradictory. Studies with different results are difficult to compare in terms of methodology, study population (controls vs. patients) and data analysis. Future studies will need to establish group differences in brain activation to standardized stimuli between healthy males and females, and between female and male patients with IBS.
6.3 Studies of psychological factors modulating the brain processing of visceral sensation
Several lines of evidence indicate that patients with IBS and other functional disorders have hypervigilance for symptom relevant sensations91 and that symptom related worries may play an important role in symptom severity.92 In a longitudinal study of IBS patients exposed to 6 sessions of rectal inflations over a 1-year period, Naliboff et al. examined regional cerebral blood flow to the inflations and anticipation of inflations using PET at the first and last session.93 Subjective ratings of the rectal inflations normalized over the 12 months of the study indicating decreased vigilance towards the experimental stimulus. In response to rectal distension, stable activation of homeostatic afferent network (including thalamus and insula) was observed over the 12-month period, while activity in limbic, paralimbic and pontine regions decreased. During the anticipation condition, there were significant decreases in amygdala, dACC and dorsal brainstem activation at 12 months. An analysis examining the covariation of these brain regions supported the hypothesis of changes in an emotional arousal network including limbic, pontine and cortical areas underlying the decreased perception seen over the multiple stimulation studies. Berman et al. studied brain responses to anticipated and delivered mild and moderate rectal distention in 14 female IBS patients and 12 healthy controls.83 During cued anticipation of distention, activity decreased in the INS, sACC, amygdala, and dorsal brainstem (including the LCC) of controls, while patients showed less anticipatory inactivation. Self-rated measures of negative affect during scanning were higher in patients than controls and the anticipatory brain response decreases in dorsal pons were inversely correlated with these ratings. During subsequent distention, both groups showed activity increases in INS, dACC, and dorsal pons and decreases in the iACC. The increases were more extensive in patients, producing significant group differences in dACC and dorsal pons. The amplitude of the anticipatory decrease in the pontine portion of DBS was associated with greater activation during distention in right ventrolateral PFC and bilateral sACC. Both regions have been associated previously with corticolimbic inhibition and cognitive coping. Based on these findings, the authors suggested that deficits in the preparatory inhibition of the LCC and PB may interfere with descending corticolimbic inhibition and contribute to enhanced brain responsiveness and perceptual sensitivity to visceral stimuli in IBS.
In order to minimize cognitive/emotional modulation of visceral afferent signals, Kern et al. used a technique of “subliminal” visceral stimulation, whereby a rectal balloon is inflated to pressures below conscious perception and associated brain responses are recorded with fMRI.94 They reported that IBS patients showed a larger response to all three distension pressures than the control group. The authors interpreted their findings as evidence for an increased sensitivity of visceral afferent pathways, regardless of stimulus-related cognitive processes.95 However, it may be assumed that other than in fully anesthetized subjects, cognitive and emotional modulation of the afferent signal will always occur and influence the subjective experience of the stimulus. Such modulation is likely to occur at the level of the brain, for example via locally released opioids,96 or by activation of descending pain inhibitory and/or facilitatory pathways modulating excitability of the spinal cord.97, 98
6.4 Differences in perception-related brain activation
Kwan et al. studied brain responses associated with either stimulus intensity (as in most reported studies), or with the time series of continuous subjective rating of the stimulus (percept-related brain responses).99 Percept-related activations were more extensive than stimulus-related activations in control subjects. IBS patients, but not controls, showed urge-related activation in primary somatosensory cortex and pain-related activations in medial thalamus and hippocampus, while controls, but not IBS patients, showed pronounced urge- and pain-related activations in homeostatic afferent brain regions (right antINS and right ACC). The authors interpreted their findings as consistent with IBS visceral hypersensitivity (increased activation in primary sensory cortex), but with possible deficits in interoceptive processing (lack of anterior insular activation) and decreased attentional engagement in IBS patients. The design, findings and conclusions of this study are clearly different from those reported by other investigators.17, 18, 27, 28, 85
6.5 Evidence for alterations in descending pain modulation systems in IBS
Two studies are suggestive of compromised engagement of brain regions involved in endogenous pain modulation in IBS patients. In a H 152O-PET study, Mayer et al. found that IBS patients compared to both the ulcerative colitis patients and control subjects showed consistently greater activation of limbic/paralimbic brain regions. In contrast, colitis patients and control subjects, but not IBS patients, showed activations in the lateral frontal regions and a brain region including the PAG. A connectivity analysis using structural equation modeling supported these regions acting as part of a pain inhibition network that involves lateral and medial frontal influences on the PAG.84 Wilder-Smith and coworkers performed an fMRI study to test the hypothesis that IBS patients show abnormal activation of diffuse noxious inhibitory control (DNICs) systems in response to a noxious stimulus.100 DNIC activation can be quantified by the perceptual modulation of a painful stimulus (in this case, noxious rectal balloon distension) by a secondary heterotypically applied nociceptive stimulus (in this case, ice water immersion of the foot). The investigators found that subjective pain ratings of rectal volume distension by the heterotypic cold pain stimulus was reduced in healthy controls but not in the IBS patient group, suggesting an inadequate activation of DNICs in the patients. Following the heterotypic cold stimulus, a complex set of differences in brain activation response to rectal pain were found among the controls and the two IBS sub-groups (constipation and diarrhea). These included a decreased insular, thalamus, and PAG activation in the controls (perhaps reflecting the DNIC process) that was absent in the IBS subjects. Additional hypothesis-driven studies with validated paradigms to engage endogenous pain modulation systems are clearly needed to confirm these preliminary findings.
In summary, functional brain imaging studies in IBS have shown abnormal CNS activation patterns in response to visceral stimuli and during expectation of such stimuli, mainly in the affective-motivational pain systems of the brain. The exact implications of these findings for FGID pathophysiology remain unclear, as these abnormalities can be caused by abnormal afferent input as well as central modulation leading to altered brain processing of the afferent input (increased selective attention to visceral stimuli associated with central pain amplification, abnormal cognitive or affective processing of normal afferent input and compromised endogenous pain inhibition systems).
7. BRAIN IMAGING TECHNIQUES IN EVALUATION OF TREATMENT RESPONSES IN IBS PATIENTS
Despite the lack of consensus regarding brain responses to visceral stimuli in healthy controls and group differences between IBS patients and control subjects, functional brain imaging has been used to identify changes in cerebral activation associated with various treatment modalities, including pharmacological treatments101-104 and non-pharmacological treatments.105, 106 Only a few of the reported studies were of sufficient quality (statistical power, blinding, homogeneous study populations) to allow any conclusions from the results. The information gained from these limited studies has to be considered as preliminary. The finding of selective effects of alosetron treatment on limbic, but not primary pain regions seen during non-distension conditions, and the correlation of these limbic effects with IBS symptom ratings,83 demonstrates the potential strength of this technique to understanding the action of new IBS treatments. Well designed treatment studies, with adequate sample size, homogeneous study populations, and reproducible study paradigms are needed to confirm the validity of this approach to monitor treatment effects, and predict possible clinical outcomes.
8. CRITICAL ASSESMENT, RECOMMENDATIONS AND FUTURE DIRECTIONS
In summary, the literature in the area of brain imaging of visceral perception published since 2002 clearly indicates significant progress in study design, methodology and analysis techniques. While consensus is evolving in some areas (including the cognitive and emotional modulation of pain perception), considerable differences in reported results remain in other areas, from the comparison of brain responses to somatic and visceral pain stimuli to differences between control subjects and IBS patients to sex-related differences in brain activation. However, given the rapid advances that are being made in such diverse fields as somatic pain modulation, emotion regulation and imaging genetics, it is likely that the application of neuroimaging techniques to the study of brain-gut interactions in health and disease will lead to breakthroughs in the understanding of pathophysiology of chronic visceral pain conditions, including functional GI disorders and in the prediction of treatment responses in the near future.
The vast majority of studies described in this review have involved detecting and determining the extent of regional brain activation across levels of an independent variable such as stimulus intensity or group. This is essentially a univariate analysis in that each brain volume or a-priori chosen region is examined separately and the statistical threshold is adjusted for the large number of individual comparisons made. While important for generating hypotheses about what parts of the brain might be involved in visceral sensation and the response to these sensations, this descriptive approach to imaging clearly does not capture the critical interrelationships among structures that form the foundation of brain function. The brain operates as functional networks and activations in specific brain areas may have very different interpretations based on the co-activation of other regions that are connected via a network of inputs and projections.107 The first step towards understanding networks involves detection of regional pairwise associations. This bivariate correlational technique can be labeled as a functional connectivity analysis and can show important relationships between separate regions, but does not allow for directly testing the nature of these associations over time or across conditions, provides no information on how these associations may come about, and permits only rudimentary inferences regarding the characterization of neural networks.
Much more sophisticated connectivity analyses that include a much larger set of highly specific brain regions are now becoming possible and they go hand-in-hand with other advances in brain imaging, including increased spatial and temporal scanner resolution,43 use of radioligand tracers specific to molecules of interest,96 and the application of genetic analyses related to the development of specific brain circuitry.108 These new analytical tools should yield important breakthroughs in our understanding of central processes related to the pathophysiology of visceral pain disorders.
It is clear from the discussion above that in order to perform meaningful functional brain imaging studies investigators must be mindful of the numerous factors that are likely to influence results. As a starting point, it is important to pay particular attention to subject selection. Comparison of two groups for instance where age, sex, previous imaging experience or experience of stimulation techniques used in the study, or where psychological state and trait measures including personality and psychiatric co-morbidity are not controlled for will mean that differences in brain areas activated may well reflect the differences in the variables described above rather than a reflection of the disease state or condition being studied. Particular care must be taken when studying patients especially those with FGIDs which are inherently heterogenous. Every effort must be made to ensure that patients are as psycho-physiolgoically homogenous as possible. An important exception to this rule of studying homogenous populations is of course when the variability in certain psychophysiological parameters between subjects is itself under study. These measures can then be used as covariates of the imaging findings. One might predict, for example, that opposite findings in a given area of the ACC may be observed as a function of whether the individuals studied score high or low in anxiety state.
It is desirable that each new laboratory should undertake studies to develop normal values and reproducibility in healthy volunteers for the stimulation modality and parameters, sex and age before embarking upon patient studies. This will allow investigators to gain experience and establish normal values for their laboratory for variables that are important for their studies. It must be remembered that all studies must be powered to detect differences in the variables under study, e.g. sex of the subject. In some cases, for example, it may be more appropriate to study one parameter alone (e.g. female subjects only) if the study cannot be powered well enough to comment on multiple parameters.
Adequate behavioral measures must be used during studies involving experimental stimulation of the gastrointestinal tract. For instance, when studying pain it is desirable to control not only for intensity but also for unpleasantness when comparing two study populations. This could be achieved by titrating groups to a chosen level of either intensity or unpleasantness on a well characterized visual analogue scale. Visual analogue scales and verbal descriptors of sensory experience and psychological state such as anxiety should be used in a controlled manner throughout the study so that acquisition of these does not interfere with data analysis and does not confound the results. Independent physiological measures of stress responsiveness during the study of visceral sensation such as autonomic nervous system measures and cortisol levels may also be helpful in subsequent data interpretation. Correlation of the psycho-physiological state with the imaging results can provide important information which would be missed if the above measures are ignored.
The experimental environment within the brain imaging suite and the mental state of the subject must be adequately controlled. For instance, different brain areas will be activated if subjects attend to bodily sensations vs. emotional feelings, etc. This is particularly important during rest or baseline conditions when volunteers quite often have no control task to perform. The extent to which psychological factors are controlled and the extent to which behavioral measures are obtained during imaging to verify that the instructions were followed (for example, measuring reactions times during an attention task) are essential for the conduct of good quality studies. In some of the previously published studies, no specific instructions had been given and this may well be the reason why there are sometimes “contradictory” findings across studies.
In summary, meticulous attention to controlling for age, sex, stimulus modality, psycho-phsyiological factors, the imaging environment and the task will undoubtedly lead to a considerable improvement in the quality of studies performed and will lead to a significant advance in our understanding of the brain processing of visceral sensation in health and disease.
Acknowledgements
This review is a working team report that was commissioned and sponsored by the Rome Foundation. The sequence of the authors reflects their role in the working team (working team leader and senior author listed as first and second author).
REFERENCES
- 1.Clauw DJ. Fibromyalgia: update on mechanisms and management. J Clin Rheumatol. 2007;13:102–9. [PubMed] [Google Scholar]
- 2.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. Journal of Neuroscience. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 4.Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 5.Lane R. Brain-Gut Interactions and Interoceptive Awareness of Emotion. 2007.
- 6.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: From basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Phillips ML, Young AW, Scott SK, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings Biological Sciences. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 9.Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. Journal of Pain. 2002;3:401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- 11.Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 12.Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: Central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 13.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 14.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2001;280:G629–G639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 15.Sarlani E, Greenspan JD. Gender differences in temporal summation of mechanically evoked pain. Pain. 2002;97:163–169. doi: 10.1016/s0304-3959(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 16.Teff KL, Alavi A, Chen J, Pourdehnad M, Townsend RR. Muscarinic blockade inhibits gastric emptying of mixed-nutrient meal: effects of weight and gender. American Journal of Physiology. 1999;276:R707–R714. doi: 10.1152/ajpregu.1999.276.3.R707. [DOI] [PubMed] [Google Scholar]
- 17.Tillisch K, Labus JS, Naliboff BD, et al. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. American Journal of Gastroenterology. 2005;100:896–904. doi: 10.1111/j.1572-0241.2005.41211.x. [DOI] [PubMed] [Google Scholar]
- 18.Tillisch K, Mayer EA, Labus JS, et al. Sex-specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viramontes BE, Camilleri M, McKinzie S, et al. Gender-related differences in slowing colonic transit by a 5-HT 3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. American Journal of Gastroenterology. 2001;96:2671–2679. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 20.Hobday DI, Aziz Q, Thacker N, et al. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124:361–8. doi: 10.1093/brain/124.2.361. [DOI] [PubMed] [Google Scholar]
- 21.Binkofski F, Schnitzler A, Enck P, et al. Somatic and limbic cortex activation in esophageal distension: A functional magnetic resonance imaging study. Annals of Neurology. 1998;44:811–815. doi: 10.1002/ana.410440516. [DOI] [PubMed] [Google Scholar]
- 22.Kern MK, Birn RM, Jaradeh S, et al. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distension. Gastroenterology. 1998;115:1353–1362. doi: 10.1016/s0016-5085(98)70013-7. [DOI] [PubMed] [Google Scholar]
- 23.Aziz Q, Andersson JL, Valind S, et al. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113:50–59. doi: 10.1016/s0016-5085(97)70079-9. [DOI] [PubMed] [Google Scholar]
- 24.Aziz Q, Thompson DG, Ng VWK, et al. Cortical processing of human somatic and visceral sensation. Journal of Neuroscience. 2000;20:2657–2663. doi: 10.1523/JNEUROSCI.20-07-02657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman DH, Munakata JA, Ennes H, et al. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 26.Rosen SD, Paulesu E, Frith CD, et al. Central nervous pathways mediating angina pectoris. Lancet. 1994;344:147–150. doi: 10.1016/s0140-6736(94)92755-3. [DOI] [PubMed] [Google Scholar]
- 27.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distension. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 28.Naliboff BD, Derbyshire SWG, Munakata J, et al. Cerebral activation in irritable bowel syndrome patients and control subjects during rectosigmoid stimulation. Psychosomatic Medicine. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Ladabaum U, Minoshima S, Hasler WL, et al. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120:369–376. doi: 10.1053/gast.2001.21201. [DOI] [PubMed] [Google Scholar]
- 30.Hobday DI, Aziz Q, Thacker N, et al. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124:361–368. doi: 10.1093/brain/124.2.361. [DOI] [PubMed] [Google Scholar]
- 31.Berman S, Munakata J, Naliboff B, et al. Gender differences in regional brain response to visceral pressure in IBS patients. European Journal of Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 32.Baciu MV, Bonaz BL, Papillon E, et al. Central processing of rectal pain: A functional MR imaging study. American Journal of Neuroradiology. 1999;20:1920–1924. [PMC free article] [PubMed] [Google Scholar]
- 33.Lotze M, Wietek B, Birbaumer N, et al. Cerebral activation during anal and rectal stimulation. Neuroimage. 2001;14:1027–1034. doi: 10.1006/nimg.2001.0901. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein CN, Frankenstein UN, Rawsthorne P, et al. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. American Journal of Gastroenterology. 2002;97:319–327. doi: 10.1111/j.1572-0241.2002.05464.x. [DOI] [PubMed] [Google Scholar]
- 35.Kern MK, Jaradeh S, Arndorfer RC, et al. Gender differences in cortical representation of rectal distension in healthy humans. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2001;281:G1512–G1523. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- 36.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 37.Mayer EA, Raybould HE. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology. 1990;99:1688–1704. doi: 10.1016/0016-5085(90)90475-g. [DOI] [PubMed] [Google Scholar]
- 38.Strigo IA, Bushnell MC, Boivin M, Duncan GH. Psychosocial analysis of visceral and cutaneous pain in human subjects. Pain. 2002;97:235–246. doi: 10.1016/S0304-3959(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 39.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 40.Strigo IA, Duncan GH, Boivin M, Bushnell MC. Differentiation of visceral and cutaneous pain in the human brain. Journal of Neurophysiology. 2003;89:3294–3303. doi: 10.1152/jn.01048.2002. [DOI] [PubMed] [Google Scholar]
- 41.Strigo IA, Albanese MC, Bushnell MC, Duncan GH. Visceral and cutaneous pain representation in parasylvian cortex. Neuroscience Letters. 2005;384:54–59. doi: 10.1016/j.neulet.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 42.Dunckley P, Aziz Q, Wise RG, et al. Attentional modulation of visceral and somatic pain. Neurogastroenterol Motil. 2007;19:569–77. doi: 10.1111/j.1365-2982.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- 43.Dunckley P, Wise RG, Fairhurst M, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kern MK, Jaradeh S, Arndorfer RC, et al. Gender differences in cortical representation of rectal distension in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1512–23. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- 45.James JE, Hardardottir D. Influence of attention focus and trait anxiety on tolerance of acute pain. Br J Health Psychol. 2002;7:149–62. doi: 10.1348/135910702169411. [DOI] [PubMed] [Google Scholar]
- 46.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 47.Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips ML, Gregory LJ, Cullen S, et al. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126:669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- 49.Whitehead WE, Diamant N, Meyer K, et al. Pain thresholds measured by the barostat predict the severity of clinical pain in patients with irritable bowel syndrome. Gastroenterology. 1998;114:859. [Google Scholar]
- 50.Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415–8. doi: 10.1007/BF00228914. [DOI] [PubMed] [Google Scholar]
- 51.Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85:19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 52.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 53.Buffington AL, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain. 2005;113:172–84. doi: 10.1016/j.pain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112:48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 55.Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. American Journal of Gastroenterology. 2003;98:12–20. doi: 10.1111/j.1572-0241.2003.07168.x. [DOI] [PubMed] [Google Scholar]
- 56.Gregory LJ, Yaguez L, Williams SC, et al. Cognitive modulation of the cerebral processing of human oesophageal sensation using functional magnetic resonance imaging. Gut. 2003;52:1671–1677. doi: 10.1136/gut.52.12.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yaguez L, Coen S, Gregory LJ, et al. Brain response to visceral aversive conditioning: A functional magnetic resonance imaging study. Gastroenterology. 2005;128:1819–1829. doi: 10.1053/j.gastro.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 58.Bushnell MC, Duncan GH, Hofbauer RK, et al. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705–9. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longe SE, Wise R, Bantick S, et al. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. NeuroReport. 2001;12:2021–2025. doi: 10.1097/00001756-200107030-00047. [DOI] [PubMed] [Google Scholar]
- 60.Bantick SJ, Wise RG, Ploghaus A, et al. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 61.Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- 62.Peyron R, Garcia-Larrea L, Gregoire MC, et al. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(Pt 9):1765–80. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 63.Vogt BA, Derbyshire SWG, Jones AKP. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. European Journal of Neuroscience. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 64.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 65.Coen SJ, Aziz Q, Yaguez L, et al. Effects of Attention on Visceral Stimulus Intensity Encoding in the Male Human Brain. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Zelman DC, Howland EW, Nichols SN, Cleeland CS. The effects of induced mood on laboratory pain. Pain. 1991;46:105–11. doi: 10.1016/0304-3959(91)90040-5. [DOI] [PubMed] [Google Scholar]
- 67.Weisenberg M. Cognitive aspects of pain and pain control. Int J Clin Exp Hypn. 1998;46:44–61. doi: 10.1080/00207149808409989. [DOI] [PubMed] [Google Scholar]
- 68.Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: effects of affective picture modulation. Psychosom Med. 2001;63:79–90. doi: 10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Whipple B, Glynn NJ. Quantification of the effects of listening to music as a noninvasive method of pain control. Sch Inq Nurs Pract. 1992;6:43–58. discussion 59-62. [PubMed] [Google Scholar]
- 70.Zillmann D, de Wied M, King-Jablonski C, Jenzowsky S. Drama-induced affect and pain sensitivity. Psychosom Med. 1996;58:333–41. doi: 10.1097/00006842-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Roy EA, Hollins M, Maixner W. Reduction of TMD pain by high-frequency vibration: a spatial and temporal analysis. Pain. 2003;101:267–74. doi: 10.1016/S0304-3959(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 72.Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106:101–8. doi: 10.1016/s0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 73.Craig AD. A new view of pain as a homeostatic emotion. Trends in Neurosciences. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 74.Tolle TR, Kaufmann T, Siessmeier T, et al. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann Neurol. 1999;45:40–7. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 75.Kulkarni B, Bentley DE, Elliott R, et al. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci. 2005;21:3133–42. doi: 10.1111/j.1460-9568.2005.04098.x. [DOI] [PubMed] [Google Scholar]
- 76.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osaka N, Osaka M, Morishita M, Kondo H, Fukuyama H. A word expressing affective pain activates the anterior cingulate cortex in the human brain: an fMRI study. Behav Brain Res. 2004;153:123–7. doi: 10.1016/j.bbr.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 78.Craig AD. The functional anatomy of lamina I and its role in post-stroke central pain. Prog Brain Res. 2000;129:137–51. doi: 10.1016/S0079-6123(00)29010-9. [DOI] [PubMed] [Google Scholar]
- 79.Rainville P, Bushnell MC, Duncan GH. Representation of acute and persistent pain in the human CNS: potential implications for chemical intolerance. Ann N Y Acad Sci. 2001;933:130–41. doi: 10.1111/j.1749-6632.2001.tb05820.x. [DOI] [PubMed] [Google Scholar]
- 80.Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- 81.Chang L, Mayer EA, Labus JS, et al. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R277–84. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 82.Wilder-Smith CH, Song G, Yeoh KG, Ho KY. Activating endogenous visceral pain modulation: A comparison of heterotopic stimulation methods in healthy controls. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 85.Verne GN, Himes NC, Robinson ME, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 86.Chang L, Berman S, Mayer EA, et al. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. American Journal of Gastroenterology. 2003;98:1354–1361. doi: 10.1111/j.1572-0241.2003.07478.x. [DOI] [PubMed] [Google Scholar]
- 87.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 88.Berkley KJ. Female vulnerability to pain and the strength to deal with it. Behavioral and Brain Sciences. 1997;20:473–479. [Google Scholar]
- 89.Mayer EA, Labus JS, Berkley K. In: Sex on the Brain: From Genes to Behavior. J.B.e., editor. Oxford University Press; 2007. pp. 371–396. [Google Scholar]
- 90.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–43. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayer EA, Craske MG, Naliboff BD. Depression, anxiety and the gastrointestinal system. Journal of Clinical Psychiatry. 2001;62:28–36. [PubMed] [Google Scholar]
- 92.Labus J, Bolus R, Chang L, et al. The visceral sensitivity index: Development and validation of a gastrointestinal symptom-specific anxiety scale. Alimentary Pharmacology and Therapeutics. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 93.Naliboff BD, Berman S, Suyenobu B, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Kern MK, Shaker R. Cerebral cortical registration of subliminal visceral stimulation. Gastroenterology. 2002;122:290–298. doi: 10.1053/gast.2002.30989. [DOI] [PubMed] [Google Scholar]
- 95.Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology. 2006;130:26–33. doi: 10.1053/j.gastro.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 96.Zubieta JK, Smith YR, Bueller JA, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. Journal of Neuroscience. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tracey I. Nociceptive processing in the human brain. Current Opinion in Neurobiology. 2005;15:478–487. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 98.Tracey I. Functional connectivity and pain: How effectively connected is your brain? Pain. 2005;116:173–174. doi: 10.1016/j.pain.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 99.Kwan CL, Diamant NE, Pope G, et al. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65:1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- 100.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kilpatrick L, Berman SM, Labus JS, et al. Tegaserod (TEG) reduces brain responses to rectal distension in IBS-C patients: a functional magnetic resonance imaging (FMRI) study. Gastroenterology. 2008;134:A–157. [Google Scholar]
- 102.Tillisch K, Bueller JA, Naliboff BD, et al. The effect of neurokinin-1 receptor antagonism on central responses to visceral pain in irritable bowel syndrome (IBS): a pilot study. Gastroenterology. 2008;134:A–545. [Google Scholar]
- 103.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berman SM, Chang L, Suyenobu B, et al. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist alosetron. Gastroenterology. 2002;123:969–977. doi: 10.1053/gast.2002.35990. [DOI] [PubMed] [Google Scholar]
- 105.Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology. 2004;72:1100–1113. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 106.Lieberman MD, Jarcho JM, Berman S, et al. The neural correlates of placebo effects: A disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 107.McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- 108.Hariri AR, Weinberger DR. Imaging genomics. British Medical Bulletin. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 109.Hamaguchi T, Kano M, Rikimaru H, et al. Brain activity during distention of the descending colon in humans. Neurogastroenterol Motil. 2004;16:299–309. doi: 10.1111/j.1365-2982.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 110.Lawal A, Kern M, Sanjeevi A, et al. Neurocognitive processing of esophageal central sensitization in the insula and cingulate gyrus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G787–94. doi: 10.1152/ajpgi.00421.2007. [DOI] [PubMed] [Google Scholar]
- 111.Paine PA, Hamdy S, Chitnis X, et al. Modulation of activity in swallowing motor cortex following esophageal acidification: a functional magnetic resonance imaging study. Dysphagia. 2008;23:146–54. doi: 10.1007/s00455-007-9114-3. [DOI] [PubMed] [Google Scholar]
- 112.Stephan E, Pardo JV, Faris PL, et al. Functional neuroimaging of gastric distention. J Gastrointest Surg. 2003;7:740–9. doi: 10.1016/s1091-255x(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 113.Lu CL, Wu YT, Yeh TC, et al. Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterol Motil. 2004;16:575–87. doi: 10.1111/j.1365-2982.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 114.Vandenbergh J, Dupont P, Fischler B, et al. Regional brain activation during proximal stomach distention in humans: A positron emission tomography study. Gastroenterology. 2005;128:564–573. doi: 10.1053/j.gastro.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 115.Ladabaum U, Roberts TP, McGonigle DJ. Gastric fundic distension activates fronto-limbic structures but not primary somatosensory cortex: a functional magnetic resonance imaging study. Neuroimage. 2007;34:724–32. doi: 10.1016/j.neuroimage.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 116.Wang GJ, Tomasi D, Backus W, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–31. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 117.van Oudenhove L, Vandenberghe J, Dupont P, et al. Cortical deactivations during gastric fundus distension in health: visceral pain-specific response or attenuation of ‘default mode’ brain function? A H(2)(15)O-PET study. Neurogastroenterol Motil. 2008 doi: 10.1111/j.1365-2982.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- 118.Bouras EP, Camilleri M, Burton DD, McKinzie S. Selective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humans. Gut. 1999;44:682–686. doi: 10.1136/gut.44.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Current Rheumatology Reports. 2002;4:322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- 120.Lawal A, Kern M, Sanjeevi A, Hofmann C, Shaker R. Cingulate cortex: a closer look at its gut-related functional topography. Am.J Physiol Gastrointest.Liver Physiol. 2005;289:G722–G730. doi: 10.1152/ajpgi.00016.2005. [DOI] [PubMed] [Google Scholar]
- 121.Bittorf B, Ringler R, Forster C, Hohenberger W, Matzel KE. Cerebral representation of the anorectum using functional magnetic resonance imaging. Br J Surg. 2006;93:1251–7. doi: 10.1002/bjs.5421. [DOI] [PubMed] [Google Scholar]
- 122.Song GH, Venkatraman V, Ho KY, et al. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006 doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 123.Eickhoff SB, Lotze M, Wietek B, et al. Segregation of visceral and somatosensory afferents: an fMRI and cytoarchitectonic mapping study. Neuroimage. 2006;31:1004–14. doi: 10.1016/j.neuroimage.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 124.Andresen V, Bach DR, Poellinger A, et al. Brain activation responses to subliminal or supraliminal rectal stimuli and to auditory stimuli in irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:827–37. doi: 10.1111/j.1365-2982.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 125.Bonaz B, Baciu M, Papillon E, et al. Central processing of rectal pain in patients with irritable bowel syndrome: An fMRI study. American Journal of Gastroenterology. 2002;97:654–661. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- 126.Ringel Y, Drossman DA, Turkington TG, et al. Regional brain activation in response to rectal distension in patients with irritable bowel syndrome. Digestive Diseases and Sciences. 2003;48:1774–1781. doi: 10.1023/a:1025455330704. [DOI] [PubMed] [Google Scholar]
- 127.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 128.Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 129.Wietek BM, Baron CH, Erb M, et al. Cortical processing of residual ano-rectal sensation in patients with spinal cord injury: an fMRI study. Neurogastroenterol Motil. 2008;20:488–97. doi: 10.1111/j.1365-2982.2007.01063.x. [DOI] [PubMed] [Google Scholar]