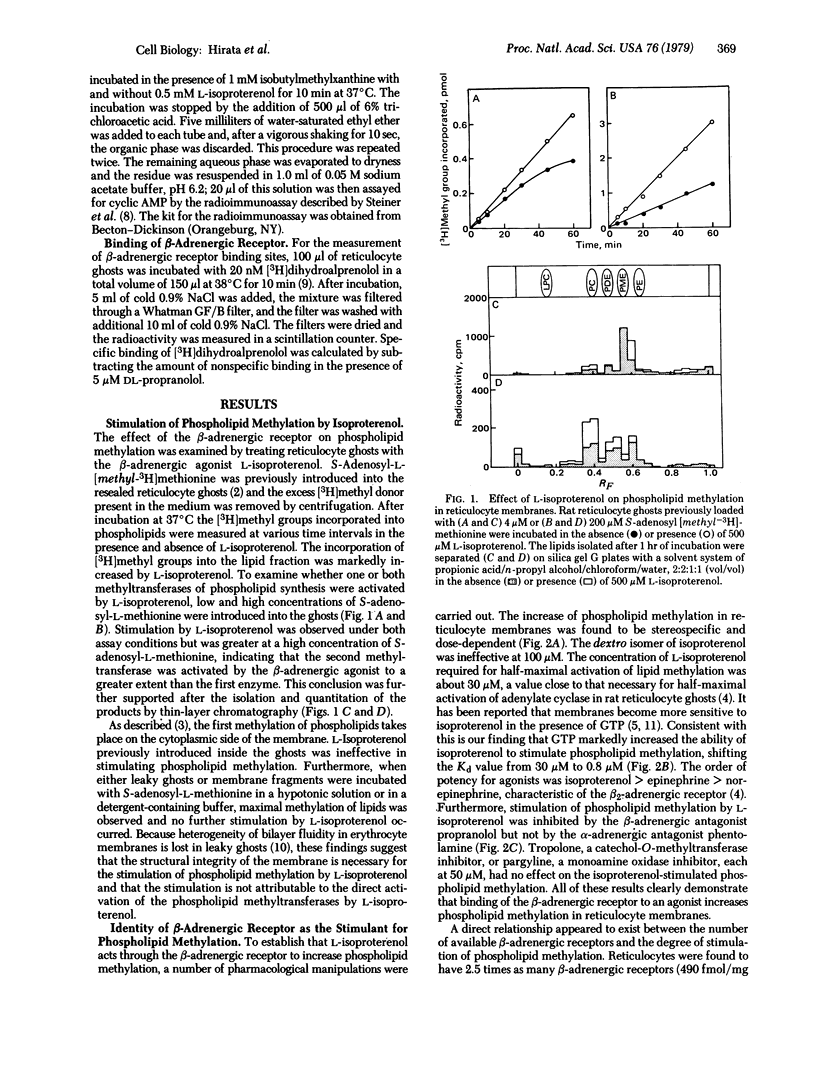
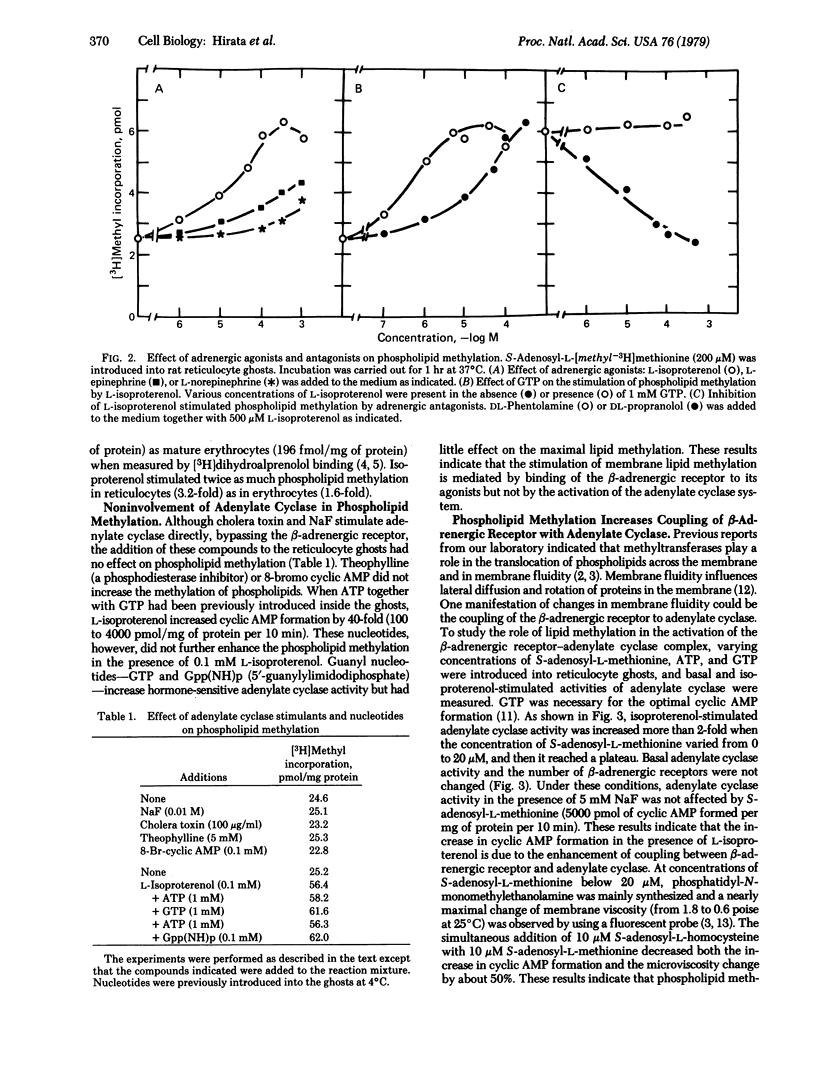
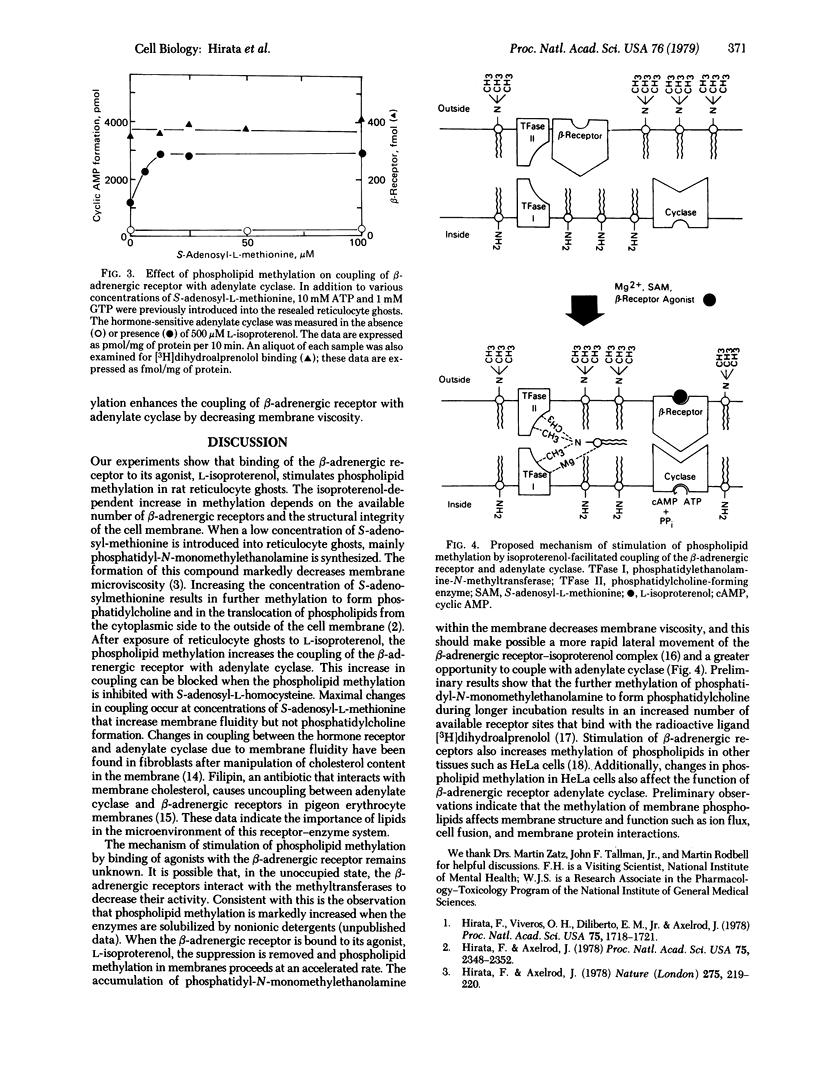
Abstract
The beta-adrenergic agonist L-isoproterenol stimulated the enzymic synthesis of phosphatidyl-N-monomethylethanolamine and phosphatidylcholine in rat reticulocyte ghosts containing the methyl donor S-adenosyl-L-methionine. The stimulation was stereospecific, dose-dependent, and inhibited by the beta-adrenergic agonist propranolol. The addition of GTP inside the resealed ghosts shifted the dose-response of phospholipid methylation by L-isoproterenol to the left by 2 orders of magnitude. Direct stimulation of adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] with sodium fluoride or cholera toxin did not increase the methylation of phospholipids. At a concentration of S-adenosyl-L-methionine that stimulates synthesis of phosphatidyl-N-monomethylethanolamine, the activity of isoproterenol-sensitive adenylate cyclase was increased 2-fold without changes in the basal activity of adenylate cyclase and the number of beta-adrenergic receptors. The increase of phospholipid methylation by L-isoproterenol decreased membrane viscosity and increased translocation of methylated lipids. These findings indicate that enhancement of phospholipid methylation by L-isoproterenol decreases membrane microviscosity and thus increases lateral movement of the beta-adrenergic receptors and coupling with adenylate cyclase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilezikian J. P., Spiegel A. M., Brown E. M., Aurbach G. D. Identification and persistence of beta adrenergic receptors during maturation of the rat reticulocyte. Mol Pharmacol. 1977 Sep;13(5):775–785. [PubMed] [Google Scholar]
- Bilezikian J. P., Spiegel A. M., Gammon D. E., Aurbach G. D. The role of guanyl nucleotides in the expression on of catecholamine-responsive adenylate cyclase during maturation of the rat reticulocyte. Mol Pharmacol. 1977 Sep;13(5):786–795. [PubMed] [Google Scholar]
- Blostein R., Chu L. Sidedness of (sodium, potassium)-adenosine triphosphate of inside-out red cell membrane vesicles. Interactions with potassium. J Biol Chem. 1977 May 10;252(9):3035–3043. [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic methylation of phosphatidylethanolamine increases erythrocyte membrane fluidity. Nature. 1978 Sep 21;275(5677):219–220. doi: 10.1038/275219a0. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic synthesis and rapid translocation of phosphatidylcholine by two methyltransferases in erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 May;75(5):2348–2352. doi: 10.1073/pnas.75.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein I., Moore L., Pastan I. Effect of liposomes containing cholesterol on adenylate cyclase activity of cultured mammalian fibroblasts. Biochim Biophys Acta. 1978 Jan 4;506(1):42–53. doi: 10.1016/0005-2736(78)90433-9. [DOI] [PubMed] [Google Scholar]
- Lyles D. S., Landsberger F. R. Sendai virus-induced hemolysis: reduction in heterogeneity of erythrocyte lipid bilayer fluidity. Proc Natl Acad Sci U S A. 1977 May;74(5):1918–1922. doi: 10.1073/pnas.74.5.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchwein G., Pfeuffer T., Helmreich E. J. Uncoupling of catecholamine activation of pigeon erythrocyte membrane adenylate cyclase by filipin. J Biol Chem. 1974 May 25;249(10):3232–3240. [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Jarett L., Lefkowitz R. J. Adipocyte beta-adrenergic receptors. Identification and subcellular localization by (-)-[3H]dihydroalprenolol binding. J Biol Chem. 1976 May 25;251(10):3096–3104. [PubMed] [Google Scholar]



