Abstract
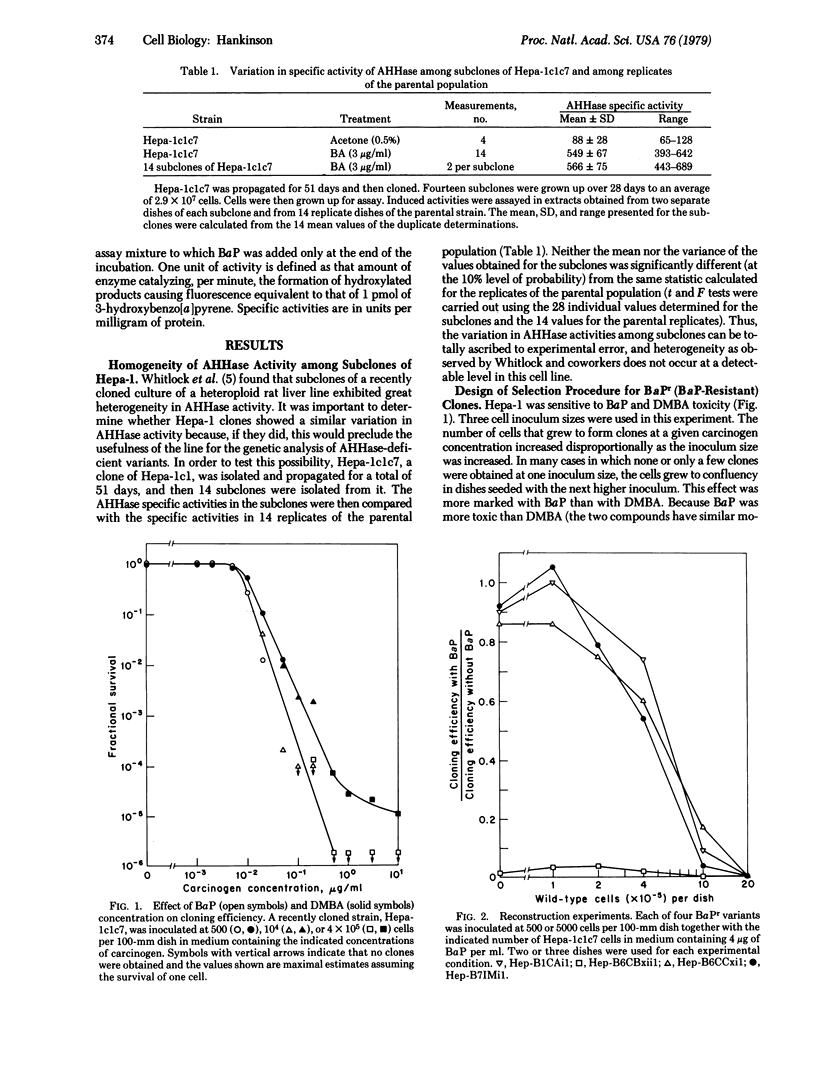
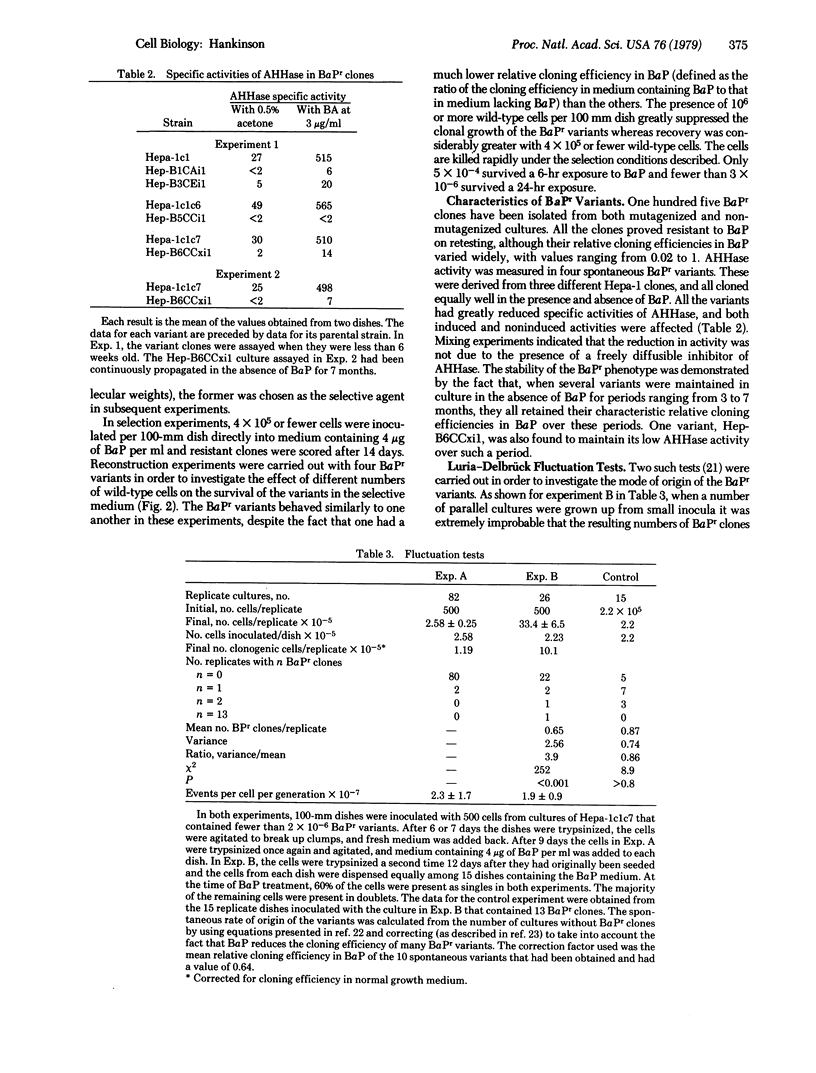
The mouse hepatoma line Hepa-1 has high and inducible aryl hydrocarbon (benzo[a]pyrene) hydroxylase[benzo[a]pyrene, reduced-flavoprotein:oxygen oxidoreductase (3-hydroxylating), EC 1.14.14.2] activity. Fourteen subclones of a clonal derivative of Hepa-1 were isolated and shown not to display heterogeneity in aryl hydrocarbon hydroxylase activity beyond what could be ascribed to experimental error. Hepa-1 was found to be very sensitive to benzo[a]pyrene (BaP) toxicity and a single-step selection procedure for isolating clones resistant to BaP at 4 microgram/ml was designed. Those BaP-resistant variants tested had much reduced aryl hydrocarbon hydroxylase activities under both inducing and noninducing conditions and they retained their resistance to BaP and low aryl hydrocarbon hydroxylase activities over considerable periods of time of culture in the absence of BaP. The spontaneous rate of origin of the BaP-resistant clones was estimated, by Luria-Delbrück fluctuation analysis, to be 2 X 10(-7) events per cell per generation. It is concluded that the properties of the variants are consistent with the notion that they are mutational in origin and that this system is a promising one for carrying out a somatic cell genetic analysis of aryl hydrocarbon hydroxylase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., DeMars R. Somatic cell mutation. Detection and quantification of x-ray-induced mutation in cultured, diploid human fibroblasts. Mutat Res. 1973 May;18(2):199–224. doi: 10.1016/0027-5107(73)90037-7. [DOI] [PubMed] [Google Scholar]
- Aviv D., Thompson E. B. Variation in tyrosine aminotrasferase induction in HTC cell clones. Science. 1972 Sep 29;177(4055):1201–1203. doi: 10.1126/science.177.4055.1201. [DOI] [PubMed] [Google Scholar]
- Bartholomew J. C., Salmon A. G., Gamper H. B., Calvin M. Benzo(alpha)pyrene effects on mouse epithelial cells in culture. Cancer Res. 1975 Mar;35(3):851–856. [PubMed] [Google Scholar]
- Benedict W. F., Gielen J. E., Owens I. S., Niwa A., Bebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. IV. Stimulation of the enzyme activity in established cell lines derived from rat or mouse hepatoma and from normal rat liver. Biochem Pharmacol. 1973 Nov 1;22(21):2766–2769. doi: 10.1016/0006-2952(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Bernhard H. P., Darlington G. J., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells: synthesis and secretion of serum albumin. Dev Biol. 1973 Nov;35(1):83–96. doi: 10.1016/0012-1606(73)90008-0. [DOI] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. The metabolism of polycyclic hydrocarbons and its relationship to cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):149–186. doi: 10.1016/0304-419x(78)90013-6. [DOI] [PubMed] [Google Scholar]
- Diamond L. Metabolism of polycyclic hydrocarbons in mammalian cell cultures. Int J Cancer. 1971 Nov 15;8(3):451–462. doi: 10.1002/ijc.2910080313. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V., Huberman E., Sachs L. Enzymatic hydroxylation of benzopyrene and its relationship to cytotoxicity. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1188–1194. doi: 10.1073/pnas.64.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen D. A., Coon M. J. Induction of multiple forms of mouse liver cytochrome P-450. Evidence for genetically controlled de novo protein synthesis in response to treatment with beta-naphthoflavone or phenobarbital. J Biol Chem. 1976 Mar 25;251(6):1817–1827. [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Huberman E., Yamasaki H., Sachs L. Independent regulation of two types of aryl hydrocarbon (benzo(a)pyrene) hydroxylase in mammalian cells. Int J Cancer. 1976 Jul 15;18(1):76–82. doi: 10.1002/ijc.2910180111. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Demars R. Quantification of chemical mutagenesis in diploid human fibroblasts: induction of azaguanine-resistant mutants by N-methyl-N'-nitro-N-nitrosoguanidine. Mutat Res. 1978 Feb;53(1):29–53. doi: 10.1016/0165-1161(78)90377-1. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Kuroki T., Drevon C. Direct or proximate contact between cells and metabolic activation systems is required for mutagenesis. Nature. 1978 Jan 26;271(5643):368–370. doi: 10.1038/271368a0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J., Stocco D., Barron E. Spontaneous mutation rate to thioguanine resistance is decreased in polyploid hamster cells. J Cell Physiol. 1978 Jul;96(1):81–86. doi: 10.1002/jcp.1040960110. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. II. Cellular responses during enzyme induction. J Biol Chem. 1968 Dec 10;243(23):6250–6261. [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A. Discontinuous variability, in the form of a geometric progression, of albumin production in hepatoma and hybrid cells. Proc Natl Acad Sci U S A. 1974 May;71(5):2062–2066. doi: 10.1073/pnas.71.5.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., O'Farrell P. H., Friedrich U., Coffino P. Mutations causing charge alterations in regulatory subunits of the cAMP-dependent protein kinase of cultured S49 lymphoma cells. Cell. 1977 Mar;10(3):381–391. doi: 10.1016/0092-8674(77)90025-3. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Benedict W. F., Owens I. S., Nebert D. W. Aryl hydrocarcon hydroxylase and tyrosine aminotransferase activities in somatic-cell hybrids derived from hepatoma tissue culture HTC (rat) cells and 3T3 (mouse) benzo(a)pyrene-resistant cells. Biochem Biophys Res Commun. 1974 Feb 4;56(3):605–610. doi: 10.1016/0006-291x(74)90647-0. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Gelboin H. V., Coon H. G. Variation in aryl hydrocarbon (benzo(a)pyrene) hydroxylase activity in heteroploid and predominantly diploid rat liver cells in culture. J Cell Biol. 1976 Jul;70(1):217–225. doi: 10.1083/jcb.70.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. K., McCourt D. W., Roller P. P., Gelboin H. V. Enzymatic conversion of benzo(a)pyrene leading predominantly to the diol-epoxide r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene through a single enantiomer of r-7, t-8-dihydroxy-7,8-dihydrobenzo(a)pyrene. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2594–2598. doi: 10.1073/pnas.73.8.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]



