Background: The plasma membrane calcium ATPase (PMCA) reaction cycle is associated with conformational changes.
Results: We identified different conformations after the association of Ca2+, ATP, and vanadate to PMCA.
Conclusion: PMCA forms a stable complex with Ca2+ and vanadate; ATP can bind to all pump conformations.
Significance: This study found a new intermediate in the PMCA reaction cycle; all of the intermediates interact with ATP.
Keywords: ATP, Calcium, Calcium ATPase, Calcium Transport, Protein Conformation, Reaction Cycle, Vanadate
Abstract
The aim of this work was to study the plasma membrane calcium pump (PMCA) reaction cycle by characterizing conformational changes associated with calcium, ATP, and vanadate binding to purified PMCA. This was accomplished by studying the exposure of PMCA to surrounding phospholipids by measuring the incorporation of the photoactivatable phosphatidylcholine analog 1-O-hexadecanoyl-2-O-[9-[[[2-[125I]iodo-4-(trifluoromethyl-3H-diazirin-3-yl)benzyl]oxy]carbonyl]nonanoyl]-sn-glycero-3-phosphocholine to the protein. ATP could bind to the different vanadate-bound states of the enzyme either in the presence or in the absence of Ca2+ with high apparent affinity. Conformational movements of the ATP binding domain were determined using the fluorescent analog 2′(3′)-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate. To assess the conformational behavior of the Ca2+ binding domain, we also studied the occlusion of Ca2+, both in the presence and in the absence of ATP and with or without vanadate. Results show the existence of occluded species in the presence of vanadate and/or ATP. This allowed the development of a model that describes the transport of Ca2+ and its relation with ATP hydrolysis. This is the first approach that uses a conformational study to describe the PMCA P-type ATPase reaction cycle, adding important features to the classical E1-E2 model devised using kinetics methodology only.
Introduction
P-type ATPases are a group of enzymes responsible for active transport of cations across the cell membrane. They use the hydrolysis of ATP as a source of energy and share in common the formation of an acid-stable phosphorylated intermediate as part of their reaction cycle. The plasma membrane calcium pump (PMCA)4 is a P-type ATPase that participates as an integral part of the Ca2+ signaling mechanism from eukaryotic cells (1) and is thus a crucial component of cell function. Detailed structural information about PMCA is currently lacking. Its abundance is ∼0.1% of the total protein in the red cell membrane, although it is much more abundant in some specialized cells. Unfortunately, these latter cells are not available in quantity, hampering efforts to produce suitable crystals for x-ray structure analysis. Although PMCA could not yet be crystallized, insight into the structural organization of the sarcoplasmic reticulum Ca2+-ATPase (SERCA), a member of the same family, has come from the elucidation of several crystal structures at atomic resolution, representing the pump in various intermediate states (see Refs. 2 and 3). The membrane-buried region of SERCA is made up of 10 membrane-spanning helices and is connected to a large cytoplasmic headpiece, which is further separated into three distinct domains, denoted A (“actuator”), P (“phosphorylation”), and N (“nucleotide binding”). However, unlike SERCA, PMCA is highly regulated by calmodulin, which activates this protein by binding to an autoinhibitory region (4) and changes the conformation of the pump from an inhibited state, E1I, to an activated one, E1A (4–6).
The current kinetic model for the PMCA proposes that the enzyme exists in two main conformations, E1 and E2. E1 has a high affinity for Ca2+ and is readily phosphorylated by ATP, whereas E2 has a low affinity for Ca2+ and can be phosphorylated by Pi. After binding of intracellular Ca2+ to high affinity sites, E1 can be phosphorylated by ATP with formation of the intermediate E1P. A subsequent conformational transition to E2P would allow Ca2+ to be released to the extracellular medium from low affinity sites, followed by the hydrolysis of the phosphoenzyme to E2 and a new conformational transition to E1 (7). During some stages of the reaction cycle, Ca2+ becomes occluded (i.e. trapped in the enzyme machinery) while it is transported from one side to the other side of the membrane (8). It has been described that in addition to being the substrate in the phosphorylation of the E1Ca state, ATP also functions in a non-phosphorylating mode by enhancing the rates of the steps involved in phosphoenzyme turnover (E1PCa → E2P and E2P → E2) as well as the E2 → E1Ca transition of the dephosphoenzyme (9–20). In PMCA, the mechanisms underlying these modulatory effects of ATP remain largely unresolved.
By using [125I]TID-PC/16, we were previously able to assess different transmembrane conformations in PMCA: a first one in which the protein displays maximum lipid exposure corresponding to an autoinhibited state of the enzyme (E1CaI, in the presence of Ca2+ alone, hereafter denoted as E1Ca), a second one in which protein-lipid interactions are markedly decreased corresponding to an activated state (E1CaA, presence of Ca2+ and calmodulin), and a third one in the absence of Ca2+ (E2) (21). Using the same experimental approach, we were also able to measure equilibrium constants for different ligands through the change of transmembrane conformations in PMCA (22). In order to obtain a complete understanding of the physical processes that involve ATP hydrolysis and Ca2+ transport, it is necessary to know more about the structure of the enzyme. The above results highlight the convenience of directly exploring the effects of different ligands on the PMCA transmembrane region. Applying this technique to PMCA, we were able to measure equilibrium constants for the dissociation of ligands from PMCA complexes and to draw structural conclusions about the regulation of the transport of Ca2+ in the presence of different ligands, such as ATP and vanadate, a well known inhibitor of P-ATPases, which forms a complex analogous to the phosphorylated intermediate E2P. Conformational movement of the ATP binding domain was determined using the fluorescent analog TNP-ATP. To assess the conformational behavior of the Ca2+ domain, we also studied the occlusion of Ca2+ in experimental conditions that lead the PMCA to different intermediates of the reaction cycle.
EXPERIMENTAL PROCEDURES
Reagents
All chemicals used in this work were of analytical grade and purchased mostly from Sigma. Recently drawn human blood for the isolation of PMCA was obtained from the Hematology Section of Fundacion Fundosol (Argentina). Blood donation in Argentina is voluntary, and therefore the donor provides informed consent for the donation of blood and for the subsequent legitimate use of the blood by the transfusion service. None of the experiments described in this paper included calmodulin in the incubation media, and therefore, all results refer to the autoinhibited form of the PMCA.
Purification of PMCA from Human Erythrocytes
PMCA was isolated from calmodulin-depleted erythrocyte membranes by the calmodulin affinity chromatography procedure (23). Protein concentration after purification was about 10 μg/ml. No phospholipids were added at any step along the purification procedure. The purification procedure described preserves transport activity and the kinetic properties and regulatory characteristics of the enzyme in its native milieu (23, 24).
Measurement of Ca2+-ATPase Activity
Ca2+-ATPase activity was measured at 37 °C as the initial velocity of release of Pi from ATP, as described previously (7). The incubation medium contained 40 μm DMPC, 120 μm C12E10, 120 mm KCl, 30 mm MOPS-K (pH 7.4 at 37 °C), 2 mm ATP, 3.75 mm MgCl2, 2 mm EGTA, and enough CaCl2 to give 100 μm final free [Ca2+]. When necessary, sodium orthovanadate ((VO4)3−, also named vanadate hereafter) or lanthanum chloride (LaIII) was added at the concentrations indicated in the figures. Release of Pi was estimated according to the procedure of Fiske and SubbaRow (25). Measurements were performed in a Jasco V-630 Bio spectrophotometer.
Determination of Phosphorylated Intermediates
The phosphorylated intermediates (EP) were measured as the amount of acid-stable 32P incorporated into the enzyme (0.9 μg/ml), according to the method described by Echarte et al. (26). The phosphorylation was measured at 4 °C in a medium containing 30 mm MOPS-K (pH 7.4 at 4 °C), 120 mm KCl, 3.75 mm MgCl2, 120 μm C12E10, 40 μm DMPC, 2 mm EGTA, 30 μm [γ-32P]ATP, and enough CaCl2 to obtain 100 μm final free [Ca2+]. The reaction was started by the addition of [γ-32P]ATP under vigorous stirring, and after 1 min, it was stopped with an ice-cold solution of TCA (10% (w/v) final concentration). The tubes were centrifuged at 7000 rpm for 3.5 min at 4 °C. The samples were then washed once with 7% TCA, 150 mm H3PO4 and once with double-distilled water and processed for SDS-PAGE. For this purpose, the pellets were dissolved in a medium containing 150 mm Tris-HCl (pH 6.5 at 14 °C), 5% SDS, 5% DTT, 10% glycerol, and bromphenol blue (sample buffer). Electrophoresis was performed at pH 6.3 (14 °C) in a 7.5% polyacrylamide gel. The reservoir buffer was 0.1 m sodium phosphate, pH 6.3, with 0.1% SDS. Migration of the sample components took place at 14 °C, with a current of 60 mA until the tracking dye reached a distance of about 10 cm from the top of the gel. Gels were stained, dried, and exposed to a Storage Phospho Screen (Amersham Biosciences). Unsaturated autoradiograms and stained gels were scanned with an HP Scanjet G2410 scanner. Analysis of the images was performed with GelPro Analyzer. EP quantification was achieved as described by Echarte et al. (26).
Preparation of [125I]TID-PC/16
TTD-PC/16 (tin precursor) was a kind gift of Dr. J. Brunner (ETH Zentrum, Zurich, Switzerland). [125I]TID-PC/16 was prepared by radioiodination of its tin precursor according to Weber and Brunner (27).
Labeling
A photolabeling assay was carried out as described by Mangialavori et al. (21, 22). A dried film of the photoactivatable reagent was suspended in PC/C12E10 mixed micelles containing 10 μg/ml of the membrane protein. The samples were incubated in the presence of necessary components for 10 min at 25 °C before being irradiated for 10 min with light from a filtered UV source (λ = 360 nm).
Radioactivity and Protein Determination
Electrophoresis was performed according to the Tris-Tricine SDS-PAGE method (28). Polypeptides were stained with Coomassie Blue R; the isolated bands were excised from the gel, and the incorporation of radioactivity was directly measured on a γ-counter. The amount of protein was quantified by eluting each stained band, as described previously (29), including bovine serum albumin in each gel as a standard for protein quantification. Specific incorporation was calculated as the ratio between the measured radioactivity and the amount of protein determined for each band. In all cases, a sample in the presence of 2 mm EGTA was included as a control and was taken as 100% of the specific incorporation. This condition was included in the figures only when it was necessary for the result analysis.
Measurements of Occluded Calcium
The procedure for measuring the occlusion of Ca2+ in microsomes containing PMCA involves a system for overexpression of the PMCA and the use of a rapid mixing device combined with a filtration chamber, allowing the isolation of the enzyme and quantification of calcium occluded (8, 30). In a typical experiment, one volume of a microsomal preparation suspended in a solution with 30 mm MOPS-K (pH 7.4 at 25 °C), 120 mm KCl, and 400 nm thapsigargin was mixed with the same volume of a solution containing the same concentrations of MOPS-K and KCl plus 3.75 mm MgCl2, 2 mm ATP, and enough [45Ca2+]CaCl2 to obtain the concentrations of free Ca2+ indicated in the figures. For some experiments, either 50 μm (VO4)3− or 50 μm LaIII (in this case, to minimize the possibility of precipitation with LaIII, ATP was 25 μm instead of 2 mm) was also included in the latter solution. Measurements were carried out in equilibrium conditions at 25 °C. To ensure the achievement of equilibrium, a reaction time of 3 s was selected after measuring the time courses of occluded calcium in different incubation media. Reactions were quenched by injecting the reaction mixture into the filtration chamber (quenching and washing chamber) at a flow rate of 1–5 ml/s. During the injection process, the fluid was mixed with an ice-cold washing solution flowing at a rate of 30–40 ml/s and then filtered through a Millipore filter (AA, 0.8-μm pore size) placed in the quenching and washing chamber in order to retain the microsomal suspension that included the enzyme. To ensure that the initial temperature in the quenching and washing chamber was 1–2 °C and that the flow was constant, about 50 ml of washing solution was allowed to run through the filter prior to the injection of the reaction mixture, and 240 ml of washing solution was applied to the filter thereafter. The composition of the washing solution was 10 mm Tris (pH 7.4 at 2 °C) and 10 mm EDTA. After the washing solution was drained, the filter was removed, dried under a lamp, and counted for 45Ca2+ radioactivity in a scintillation counter. This was converted into nmol of Ca2+ using the specific activity value of the 45Ca2+ in the reaction mixture. Ca2+ occluded was considered equal to the 45Ca2+ radioactivity retained by the enzyme after subtracting the blank values. These were estimated from the amount of 45Ca2+ retained by the filters in the presence of enzyme that was heat-inactivated for 2 h at 50 °C.
Spectroscopic Measurements
The fluorescence measurements were made in a quartz cell of 3 × 3 mm using an SLM- spectrofluorometer AMINCO BOWMAN Series 2 (Spectronic Instrument Inc., Rochester, NY). The excitation and emission bandwidths were set at 4 nm.
Determination of PMCA Apparent Affinity for TNP-ATP
Purified PMCA (10 μg/ml) was reconstituted in a medium containing 40 μm DMPC, 120 μm C12E10, 120 mm KCl, 30 mm MOPS-K (pH 7.4 at 25 °C), 1 mm MgCl2 and then incubated with one of the following components for 10 min at 25 °C: (i) 2 mm EGTA, (ii) 100 μm free Ca2+, (iii) 2 mm EGTA plus 50 μm (VO4)3−, or (iv) 100 μm free Ca2+ plus 50 μm (VO4)3−. After incubation, increasing concentrations of TNP-ATP were added to each condition as described previously by Qu et al. (31). The fluorescence emission was measured at 539 nm after excitation at 495 nm and corrected for light scattering by background subtraction. An aliquot of the same solution without protein was titrated as a control in all experiments. In this case, the fluorescence emission behavior of TNP-ATP was linear and less than 15% of the fluorescence emission in the presence of protein.
As described previously by Pérez-Gordones et al. (32), in the presence of calcium, no hydrolysis was observed of TNP-ATP. As was reported for other ATPases (33–37), PMCA Ca2+-ATPase activity is completely inhibited by this analog.
Analysis of SERCA Structure and Accessible Surface Area (ASA)
The ASAs of two crystal structures of SERCA were compared as an approach to analyze the changes in incorporation of [125I]TID-PC/16 in the PMCA intermediates E1Ca and E1PCa. The crystal structures used for comparison were those of E1·2Ca (Protein Data Bank code 1su4) (38) and Ca2E1P (Protein Data Bank code 3ba6) (39). The transmembrane regions were taken as explicitly defined in Uniprot for sarcoplasmic/endoplasmic reticulum calcium ATPase 2, accession number P20647. The ASA of the transmembrane helices was calculated with MolMol (40, 41) with a solvent radius of 1.4 Å.
Data Analysis
Theoretical equations were fitted to the results by nonlinear regression based on the Gauss-Newton algorithm using commercial programs (Excel and Sigma-Plot for Windows, the latter providing not only the best fitting values of the parameters but also their S.E.). The goodness of fit of a given equation to the experimental results was evaluated by the corrected AIC criterion (42) defined as AICC = N ln(SS/N) + 2P N/(N − P − 1), where N is the number of data, P is the number of parameters plus one, and SS is the sum of weighted square residual errors. Unitary weights were considered in all cases, and the best equation was chosen as that giving the lower value of AICC. The AIC criterion is based on information theory and selects an equation among several possible equations on the basis of its capacity to explain the results using a minimal number of parameters.
RESULTS
Effect of Vanadate and Lanthanum on PMCA Ca2+-ATPase Activity
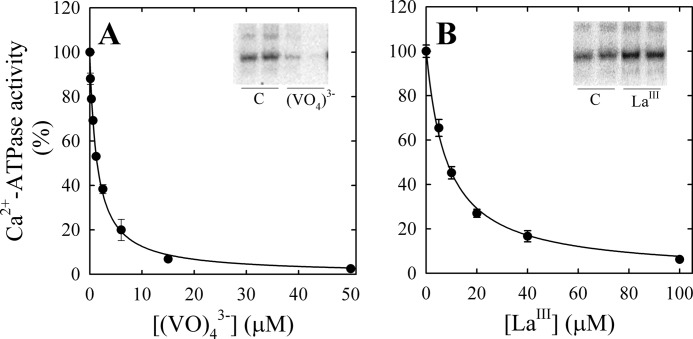
Ca2+-ATPase activity of purified PMCA was measured in the presence of 100 μm free Ca2+ and increasing concentrations of vanadate (Fig. 1A) or LaIII (Fig. 1B). Equation 1 was fitted to the experimental data (continuous lines),
 |
where [I] is the concentration of inhibitor (either (VO4)3− or LaIII), V0 is the Ca2+-ATPase activity in the absence of inhibitor, and KI is the concentration of inhibitor needed for half-maximal effect. The value of KI for (VO4)3− was 1.5 ± 0.1 μm, and it was similar to that reported previously (43), and the value of KI found for LaIII was 8.2 ± 0.5 μm.
FIGURE 1.
Effect of vanadate (A) and lanthanum (B) on PMCA Ca2+-ATPase activity. PMCA Ca2+-ATPase activity was determined as described under “Experimental Procedures” and normalized according to the activity obtained in the absence of (VO4)3− or LaIII. The continuous line represents the fit of Equation 1 to the experimental values. The apparent Ki for the complexes of PMCA with (VO4)3− and LaIII were 1.5 ± 0.1 and 8.2 ± 0.5 μm, respectively. Insets, gel autoradiographs of the phosphorylated intermediates of PMCA obtained in the absence of vanadate (control (C)) or in the presence of 50 μm (VO4)3− (A) or 100 μm LaIII (B).
Although these inhibitors have similar effects on the PMCA activity, vanadate mimics the phosphoryl group in the transition state of E2P, preventing the enzyme phosphorylation (Fig. 1A, inset) (43–45), whereas LaIII inhibits by blocking the transition E1P → E2P, acting noncompetitively with respect to Ca2+ and ATP and bringing the enzyme phosphorylation level to a maximum (Fig. 1B, inset) (46, 47).
Effects of Vanadate and Lanthanum on the PMCA Transmembrane Domain Conformation
To investigate the structure-function relationship of intermediates of the reaction cycle of PMCA, we studied the effects of (VO4)3− and LaIII on the conformation of the transmembrane domain of the pump under conditions similar to those used in the inhibition experiments of Fig. 1, except for the fact that all experiments were performed in equilibrium. To this end, we used a hydrophobic photolabeling strategy with [125I]TID-PC/16, a photoactivatable reagent that has been previously shown to behave as phosphatidylcholine with regard to protein-lipid interactions (48, 49). It is thus possible to assess lipid exposure of transmembrane protein regions by quantifying the amount of reagent that becomes covalently attached to the protein upon photolysis (6, 21, 22, 48–52). In order to ensure equilibrium conditions, ATP was omitted from the media except when LaIII was present.
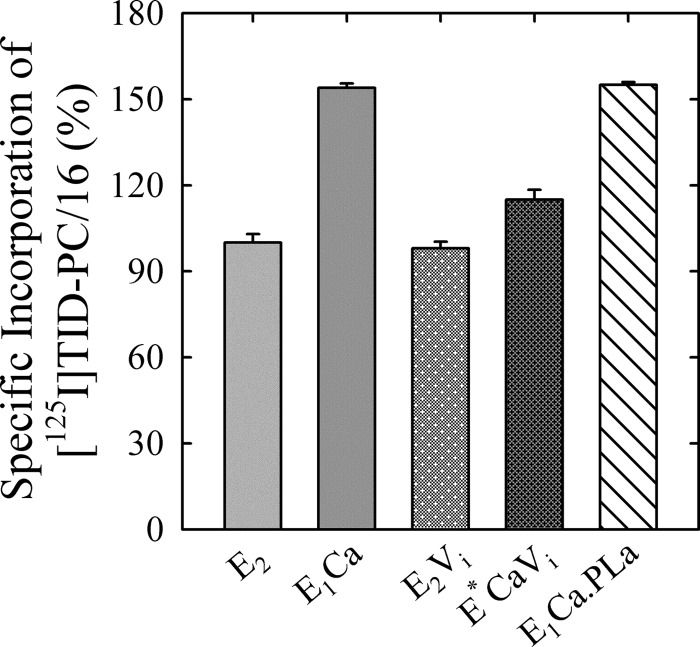
First, we determined the extent of [125I]TID-PC/16 labeling of the PMCA in its major known conformational states. The E1Ca conformer is obtained by incubating the enzyme in the presence of Ca2+ alone, whereas in the presence of 2 mm EGTA, the conformational equilibrium is shifted toward the E2 state (in the absence of Ca2+), whose level of [125I]TID-PC/16 incorporation was taken as 100% (21). Because in this work we do not analyze the effect of calmodulin, we simply refer to the state of PMCA plus Ca2+ as E1Ca. In the E1Ca conformer, the incorporation of [125I]TID-PC/16 increases by 50% as compared with that of E2. The E1PCa·La conformer was obtained by phosphorylation in the presence of LaIII, whereas E2P was mimicked by the complexes of PMCA with (VO4)3−, both with and without Ca2+. Fig. 2 shows the specific incorporation of [125I]TID-PC/16 to PMCA in the presence of EGTA (E2); Ca2+ (E1Ca); (VO4)3− plus EGTA (E2Vi); (VO4)3− plus Ca2+ (E*ViCa); or LaIII, Ca2+, and ATP (E1PCa·La). In agreement with reported data (21) specific incorporation of [125I]TID-PC/16 to PMCA was about 55% higher in the E1Ca conformation than in E2. In the presence of EGTA, vanadate has no significant effect on the specific incorporation of [125I]TID-PC/16 (98 ± 2.3%). However, whenever Ca2+ was present together with vanadate, the area accessible to lipids was different from that obtained in the presence of vanadate or Ca2+ alone (115 ± 3.4%). On the other hand, the specific incorporation of [125I]TID-PC/16 in the presence of Ca2+, LaIII, and ATP was 155.0 ± 1.0%, a value similar to that obtained in the presence of Ca2+ alone.
FIGURE 2.
Effect of different ligands on the conformation of PMCA. Specific incorporation of [125I]TID-PC/16 into PMCA was determined as described under “Experimental Procedures” in the presence of 2 mm EGTA (E2); 100 μm free Ca2+ (E1Ca); 50 μm (VO4)3− plus 2 mm EGTA (E2Vi); 50 μm (VO4)3− plus 100 μm free Ca2+ (E*CaVi); or 50 μm LaIII, 100 μm free Ca2+ plus 25 μm ATP (E1PCa·La). Specific incorporation of the probe in the presence of 2 mm EGTA (E2) was taken as 100%. Error bars, S.E.
Earlier experiments on the kinetics of vanadate inhibition (53) showed antagonism by Ca2+, consistent with Ca2+ and (VO4)3− binding to alternate conformations. Results obtained with vanadate plus calcium might be explained by two possible hypotheses: (i) the state denoted as E*ViCa is actually an equilibrium mixture of mutually exclusive states of PMCA, E2Vi, and E1Ca, or (ii) both Ca2+ and (VO4)3− can bind together to PMCA in a state, E*ViCa, where the asterisk indicates a distinct conformation with characteristics of both E1 and E2.
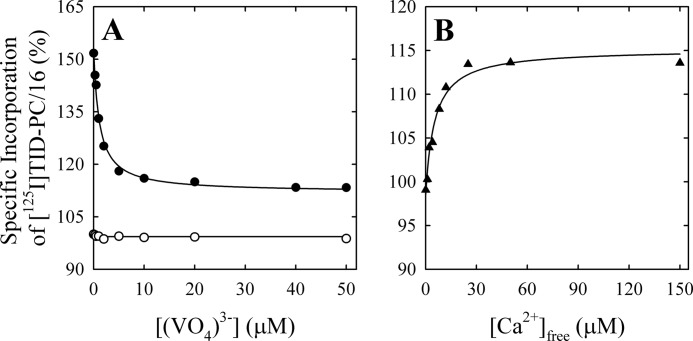
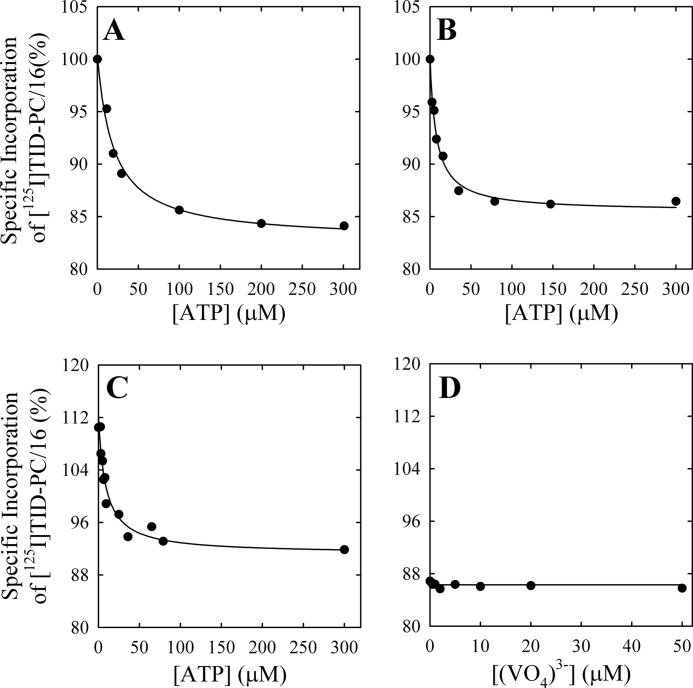
In order to test these hypotheses, we measured the specific incorporation of [125I]TID-PC/16 as a function of vanadate concentration (Fig. 3A) in media with (closed circles) and without (open circles) Ca2+. It can be seen that in the latter case, specific incorporation is independent of [(VO4)3−], indicating that the E2 and the E2P analog conformations have similar areas accessible to lipids. The average value of the specific incorporation is around 100% (99.3 ± 0.5%; Table 1). In the presence of Ca2+, however, there is a decrease of specific incorporation that tends to a level significantly higher than 100%. Results in Fig. 3A (closed circles) were analyzed by nonlinear fitting of the following decreasing function of [(VO4)3−],
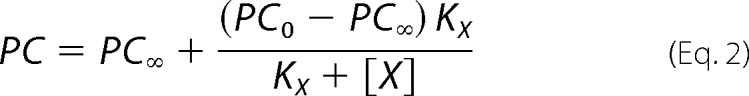 |
where PC is the specific incorporation of [125I]TID-PC/16; [X] is the concentration of vanadate; PC0 and PC∞ are specific incorporations in the absence of vanadate and at nonlimiting concentration of the inhibitor, respectively; and KX is the concentration of vanadate for half-maximal effect. This analysis shows (Table 1) that the value of PC∞ is significantly higher than 100%, making hypothesis (i) above untenable because if E2Vi and E1Ca were two mutually exclusive species in equilibrium, the addition of enough vanadate should bring the entire enzyme to the E2Vi state. We therefore conclude that there exists a ternary complex, E*ViCa, which is confirmed by results of titration by Ca2+ of E2Vi (Fig. 3B). To analyze these results, Equation 2 above was rearranged to the following increasing hyperbolic function of [Ca2+],
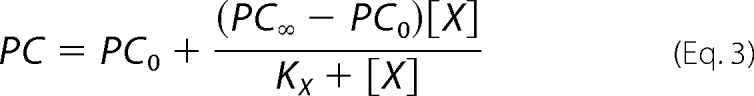 |
where [X] is now the concentration of Ca2+, PC0 and PC∞ are specific incorporations of [125I]TID-PC/16 in the absence of Ca2+ and at [Ca2+] tending to infinity, respectively, and KX is the concentration of Ca2+ for half-maximal effect. In this case, the value of PC∞ is 114.1 ± 2.2%, which is not significantly different from the value of 112 ± 0.8% obtained from the titration of E1Ca with vanadate. These values indicate that the addition of enough Ca2+ to the enzyme-vanadate complex (Fig. 3B) and the addition of enough vanadate to the E1Ca complex (Fig. 3A, closed circles) lead to the same ternary complex, E*ViCa.
FIGURE 3.
Effect of vanadate on the conformation of the transmembrane domain of PMCA. A, specific incorporation of [125I]TID-PC/16 to PMCA in the presence of 2 mm EGTA (open circles) or 100 μm free Ca2+ (closed circles) at increasing (VO4)3− concentrations. The continuous line in the presence of calcium is the graph of Equation 2 using the best fitting parameters in Table 1 (Reaction ii). Specific incorporation of [125I]TID-PC/16 to PMCA in the presence of 2 mm EGTA as a function of [(VO4)3−] was constant (continuous line; Reaction vi), and thus Kx could not be experimentally determined. B, specific incorporation of [125I]TID-PC/16 to PMCA in the presence of 50 μm (VO4)3− and increasing free Ca2+ concentrations. The continuous line is the graph of Equation 3 using the best fitting parameters shown in Table 1 (Reaction iii). Specific incorporation of the probe in the presence of 2 mm EGTA was taken as 100%.
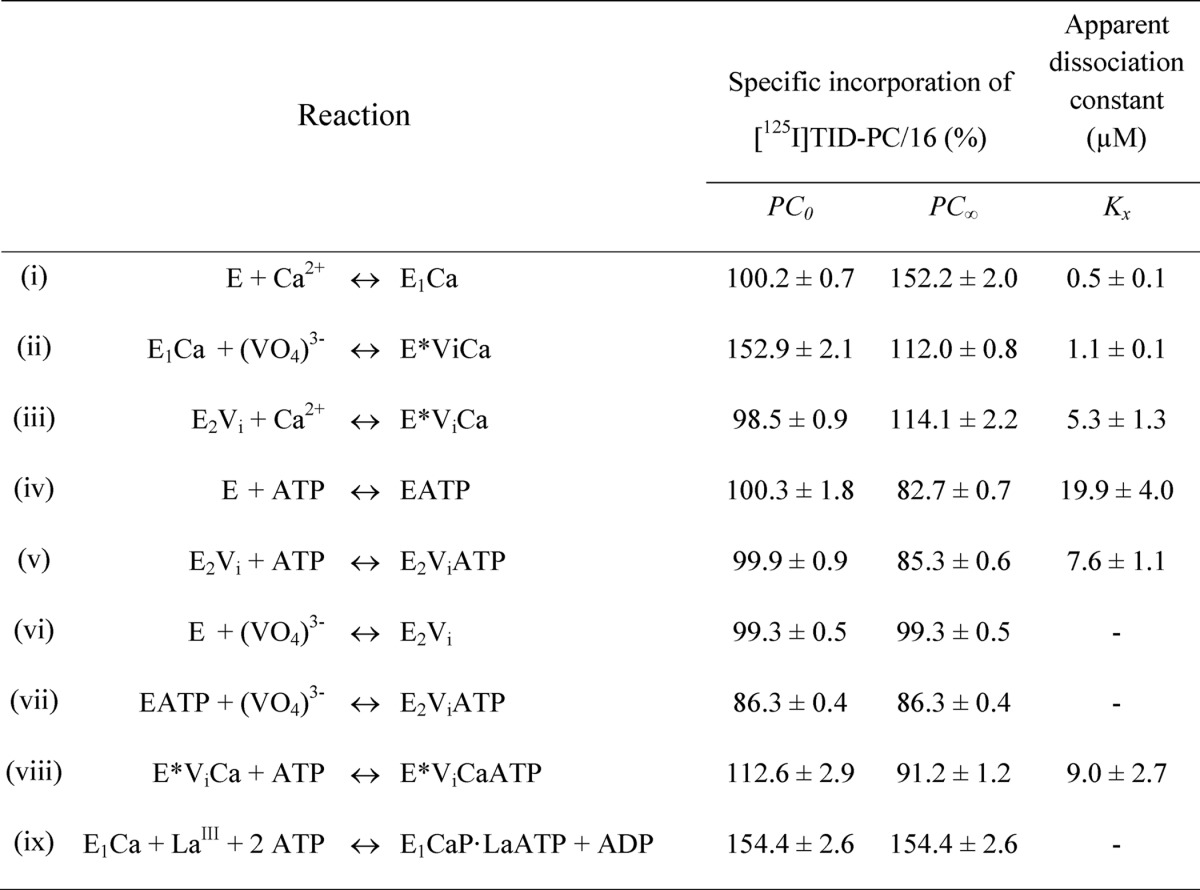
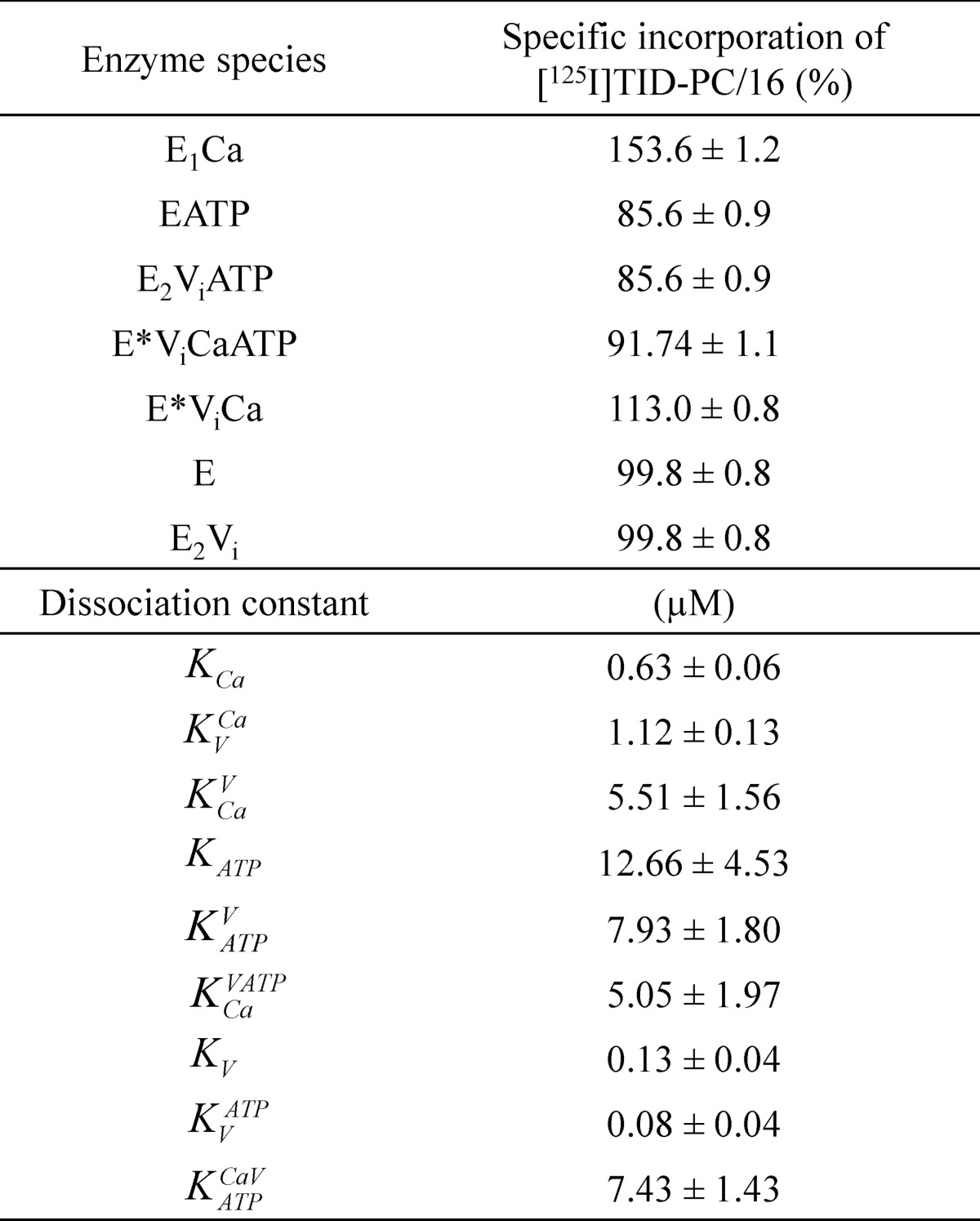
TABLE 1.
Parameters for the interaction of PMCA with calcium, vanadate, and ATP determined from the specific incorporation of [125I]TID-PC/16 under equilibrium conditions (Equations 2 and 3)
E represents the enzyme in the absence of calcium and is supposed to be an equilibrium mixture of the conformational states E1 and E2 ([E] = [E1] + [E2]), which is shifted toward E2. E* represents a state formed in the presence of vanadate and calcium whose conformation is different from that of E1 and E2. The parameters PC0 and PC∞ are the specific incorporation of [125I]TID-PC/16 in the absence of reactants or when their concentration tends to infinity, respectively. Kx is the apparent dissociation constant of the complex of PMCA formed through the corresponding equilibrium reaction on the left. Data for reaction (i), which were included for comparison, were taken from Ref. 22.
These results suggest that, despite the apparent lack of effect of vanadate in Fig. 3A (open circles), the addition of Ca2+ reveals that E2 and E2Vi are different conformers of the pump. From the results in Fig. 3, we postulate the existence of two phosphoenzyme analogs with vanadate: E2Vi in the absence of calcium and E*ViCa in its presence.
Effect of Vanadate on Occluded Intermediates of PMCA
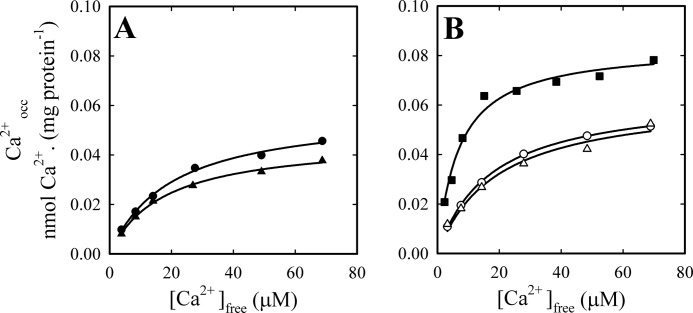
We have previously identified and characterized the calcium occluded intermediate(s) of PMCA using a rapid mixing device combined with a filtration chamber, allowing the isolation of the enzyme and the quantification of occluded calcium (8).
To confirm the existence of the E*ViCa intermediate in equilibrium and steady-state conditions, with or without ATP, we measured the amount of occluded calcium in the absence and in the presence of vanadate for different concentrations of Ca2+, in media either without (Fig. 4A) or with 2 mm ATP (Fig. 4B). We also included a control experiment performed in the absence of vanadate and in the presence of 25 μm ATP plus 50 μm LaIII. As expected, experimental data for each condition can be described by a rectangular hyperbola as a function of [Ca2+]. In the absence of ATP, vanadate slightly reduces (around 20%) the amount of intermediate with calcium occluded (Fig. 4A), whereas the inhibitor has no significant effect in the presence of ATP (Fig. 4B). In accordance with the increase of phosphorylated intermediate shown in Fig. 1B, the maximal level of occluded intermediate obtained in the presence of LaIII and ATP is about 30% higher than in the absence of the inhibitor. The addition of lanthanum also increased more than 2.5-fold the apparent affinity for Ca2+.
FIGURE 4.
Effect of vanadate on the level of calcium occluded by the PMCA. Occluded calcium was determined for increasing Ca2+ concentrations, with (triangles) or without (circles and squares) 50 μm (VO4)3− (see “Experimental Procedures” for details). Determinations where performed in the absence (A) and in the presence (B) of 2 mm ATP, except for a control experiment (closed squares), where media contained 25 μm ATP and 50 μm LaIII. The continuous lines represent the fit of a Michaelis-Menten-like function of [Ca2+] to the experimental data. Best fitting values of the parameters were as follows. A, closed circles, Caocc,max = 0.058 ± 0.002 nmol of 45Ca (mg of protein)−1, K0.5 = 19.7 ± 1.9 μm; closed triangles, Caocc,max = 0.046 ± 0.001 nmol of 45Ca (mg of protein)−1, K0.5 = 17.4 ± 1.4 μm. B, open circles, Caocc,max = 0.064 ± 0.001 nmol of 45Ca (mg of protein)−1, K0.5 = 17.2 ± 0.7 μm; open triangles, Caocc,max = 0.063 ± 0.005 nmol of 45Ca (mg of protein)−1, K0.5 = 19.1 ± 4 μm; closed squares, Caocc,max = 0.084 ± 0.003 nmol of 45Ca (mg of protein)−1, K0.5 = 6.8 ± 0.9 μm.
The apparent affinity for Ca2+ obtained from these experiments was lower than that observed for the purified enzyme, and the effect of vanadate on this was only marginal. Despite the difficulty of comparing data coming from very different preparations (see “Experimental Procedures”), these results confirm that calcium is occluded not only in E1Ca but also in the E*ViCa ternary complex. The higher level of calcium occlusion obtained in the presence of ATP (Fig. 4B) would indicate that the nucleotide is able to bind to E*ViCa to form a quaternary complex, E*ViCaATP.
Effects of ATP
In addition to its role as substrate in the phosphorylation of the E1 state, ATP also operates in a non-phosphorylating mode (modulatory ATP), enhancing the rates of the steps involved in phosphoenzyme turnover (E1P → E2P and E2P → E2) as well as the E2 → E1 transition of the dephosphoenzyme (7, 54, 55) The mechanisms underlying these modulatory effects of ATP have been extensively studied in SERCA (9–20). However, for PMCA, this question remains unsolved. Therefore, we employed a strategy similar to that described above to study the transmembrane conformational changes associated with the binding of ATP to different states of PMCA.
E1PCa State
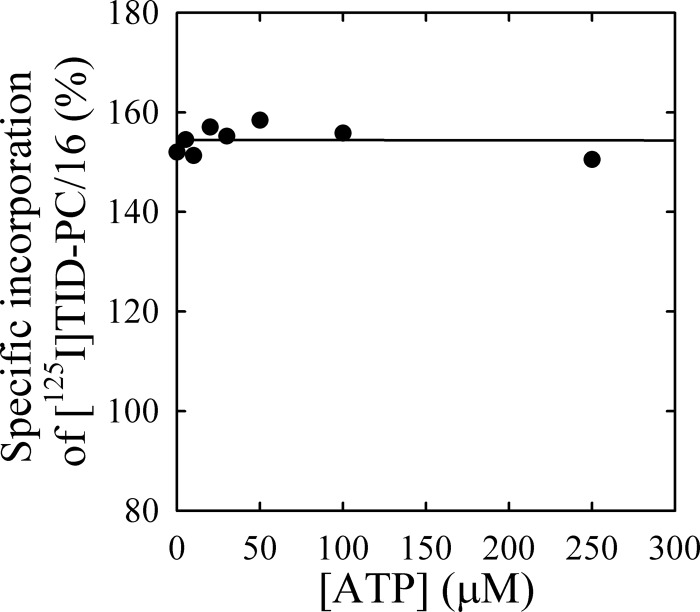
In order to study the effect of ATP on the conformation of the transmembrane domain during formation of E1PCa, we measured the specific incorporation of [125I]TID-PC/16 into PMCA as a function of [ATP] in the presence of LaIII and Ca2+ (Fig. 5). In the initial condition, PMCA is in the E1Ca conformation (46, 47), and as ATP concentration increases, the reaction is shifted toward the formation of the phosphorylated intermediate, E1PCa·La (see Fig. 1B, inset). Although ATP can also bind to this intermediate with low affinity (55), only marginal effects in specific incorporation of [125I]TID-PC/16 are detected. There appears to be a slight increase at low concentrations of ATP followed by a low affinity decrease at concentrations higher than 50 μm. However, these changes are difficult to ascertain, and it seems safer to describe the phenomenon by saying that the level of [125I]TID-PC/16 incorporation remains approximately constant around the value obtained for E1Ca (155 ± 1%).
FIGURE 5.
Effect of ATP on the conformation of the transmembrane domain of the E1PCa analog. Specific incorporation of [125I]TID-PC/16 to PMCA was determined as a function of increasing ATP concentrations in the presence of 100 μm free Ca2+ plus 50 μm LaIII. Specific incorporation of the probe into PMCA in the presence of 2 mm EGTA was taken as 100%. Results were approximately constant (continuous line, but see “Discussion”), and a precise value of Kx could not be determined (see Table 1, Reaction ix).
E2 and E2P Analog States
On the contrary, titration of the E2 state with ATP in the absence of Ca2+ (Fig. 6A) leads to a decrease in specific incorporation of [125I]TID-PC/16. Results were analyzed by nonlinear fitting of Equation 2, where the variable X stands for ATP. The best fitting values of the parameters are shown in Table 1. These results show that ATP can bind to PMCA, at micromolar concentrations, in the absence of Ca2+, causing a conformational change in the transmembrane domain.
FIGURE 6.
Effect of ATP on the conformation of the PMCA transmembrane domain. Specific incorporation of [125I]TID-PC/16 to PMCA was determined as a function of increasing ATP concentrations in the presence of 2 mm EGTA (A), 2 mm EGTA plus 50 μm (VO4)3− (B), or 100 μm free Ca2+ plus 50 μm (VO4)3− (C). The continuous lines are the graphs of Equation 2 for the best fitting parameter values shown in Table 1 (Reactions iv, v, and viii, respectively). D, the specific incorporation of [125I]TID-PC/16 to PMCA in media with 2 mm EGTA and 2 mm ATP did not vary with the concentration of (VO4)3− (continuous line), and thus Kx could not be experimentally determined (see Table 1, Reaction vii).
Fig. 6, B and C, shows the specific incorporation of [125I]TID-PC/16 to PMCA as a function of [ATP] when the enzyme was preincubated in the presence of vanadate with and without Ca2+, respectively. Nonlinear fitting of Equation 2 (Table 1) yields values of KX for ATP that are at least 50% lower than that obtained in the absence of vanadate. In addition, the simultaneous presence of vanadate and Ca2+ causes the levels of specific incorporation at both zero and non-limiting [ATP] to be higher than the corresponding levels obtained in the absence of Ca2+.
When the pump was incubated in the absence of Ca2+ and in the presence of saturating [ATP], the specific incorporation of [125I]TID-PC/16 to PMCA was around 85% (Fig. 6, A and D). The addition of increasing vanadate concentrations has no effect on the incorporation of the probe (Fig. 6D). This result shows that in the absence of calcium, vanadate does not produce changes in the area accessible to the lipid environment of PMCA transmembrane domain, regardless of the conformation reached by the pump in the presence of ATP. As will be shown below, this lack of effect does not imply the absence of binding of vanadate to PMCA.
A Model for the Interaction of PMCA with Calcium, Vanadate, and ATP
Our findings can be summarized by the scheme shown in Fig. 7. In the absence of Ca2+, PMCA is an equilibrium mixture of 10% E1 and 90% E2 forms (56). However, because our experiments do not include information on this equilibrium, we refer to the enzyme in the presence of EGTA as “E”, which is an equilibrium mixture of the E2 and E1 forms. A similar practice is used for the equilibrium between E1ATP and E2ATP, which will depend on the apparent affinities of these forms for the nucleotide, and thus in the absence of calcium, these will simply be denoted as “EATP.” On the other hand, our results show that, regarding the conformation of the transmembrane domain, the ternary complex E*ViCa is different from E1Ca and E2Vi and is therefore denoted with an asterisk, as described above.
FIGURE 7.
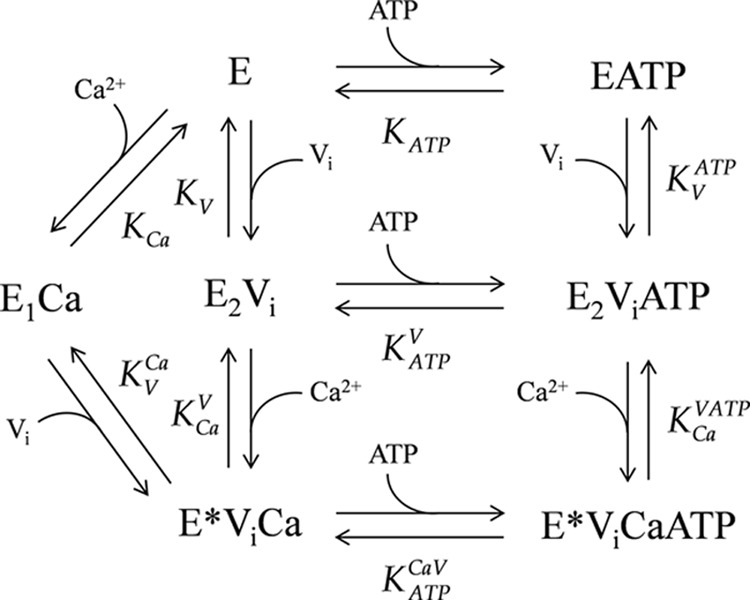
Scheme of the model proposed for the interaction of PMCA with calcium, vanadate, and ATP. The model was used to perform a global fitting analysis of the results shown in Figs. 3, 5, and 6, and the best fitting values of the parameters are shown in Table 2.
Each step in the model represents the equilibrium binding of a single ligand to PMCA. A global fitting of the parameters of the equilibrium equations arising from the model was performed on the results shown in Figs. 3, 5, and 6. The best fitting values for the specific incorporation of [125I]TID-PC/16 (percentage) in the presence of the different reactants and their apparent dissociation constants are shown in Table 2. The parameter values obtained from this global fitting correlate very well with those obtained from each experiment (Table 1), which were taken as initial values for the fitting. The correlation is indicative that this global model is appropriate to describe the interactions of PMCA with Ca2+, vanadate, and ATP. The model allows the determination of the apparent dissociation constants for steps 6, 7, and 8 (i.e. for the binding of Ca2+ to E2ViATP, for the binding of vanadate to E, and for the binding of vanadate to EATP, where the latter two could not be experimentally measured). Results summarized in Table 2 show that (i) the apparent affinity of PMCA for vanadate decreases in the presence of Ca2+, (ii) the apparent affinity of PMCA for Ca2+ decreases in the presence of vanadate, (iii) ATP does not modify the apparent affinity for Ca2+ of the E2Vi form, and (iv) the apparent affinity of E2Vi and E*ViCa forms for ATP were similar, meaning that Ca2+ does not modify the apparent affinity for ATP of E2Vi.
TABLE 2.
Best fitting values of specific incorporation of [125I]TID-PC/16 and dissociation constants for calcium, vanadate, and ATP predicted by the global model shown in Fig. 7
Constants KV, KtATP and KCaVATP were calculated as a combination of the rest of the equilibrium constants forming part of the same closed reaction cycle according to the equations, K>V = KVCaKCa/KCaV, KVATP = KATPVKV/KATP, and KCaVATP = KATPCaV KCaV/KATPV.
Interaction of TNP-ATP with Different PMCA Intermediates
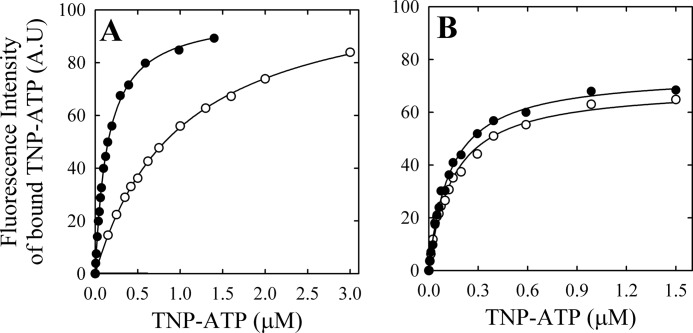
The previous strategy does not allow for obtaining information about the relationship between ATP and PMCA in the presence of Ca2+, the catalytic role of ATP, because this nucleotide is rapidly hydrolyzed. Therefore, in order to obtain more information on the interaction of E1Ca or E2 with ATP, we studied the binding of its non-hydrolyzable, fluorescent analog, TNP-ATP, to PMCA in different conditions. This approach was used for studying the interaction with ATP in several proteins (reviewed in Ref. 57). As was previously reported for PMCA (32) and other proteins (57), TNP-ATP is not a substrate for the pump and inhibits its ATPase activity. Fig. 8 shows the fluorescence at 539 nm as a function of [TNP-ATP] in media where PMCA was incubated with and without 100 μm Ca2+ and without (A) or with (B) 50 μm vanadate. Experimental data can be described by the equation,
 |
which represents the interaction with a single type of binding sites, where ΔF represents the change in fluorescence intensity following the addition of TNP-ATP at a concentration [S], ΔFmax is the maximum change in fluorescence intensity, and KD is the apparent dissociation constant for the probe. The best fitting values of KD and ΔFmax are shown in Table 3.
FIGURE 8.
Binding of TNP-ATP to PMCA. Fluorescence intensity of bound TNP-ATP to PMCA as a function of the concentration of TNP-ATP was determined as described under “Experimental Procedures” in the presence of 100 μm free Ca2+ (closed circles) or 2 mm EGTA (open circles) (A) and in the presence of 50 μm (VO4)3− and either 100 μm free Ca2+ (closed triangles) or 2 mm EGTA (open triangles) (B). The continuous line is the graph of Equation 4 using the best fitting parameters shown in Table 3.
TABLE 3.
PMCA apparent affinity for TNP-ATP under equilibrium conditions
ΔFmax corresponds to the fluorescence intensity when the concentration of TNP-ATP tends to infinity, and KD represents the apparent dissociation constant for the complex formed between PMCA and TNP-ATP. For the meaning of E and E*, see the legend to Table 1.
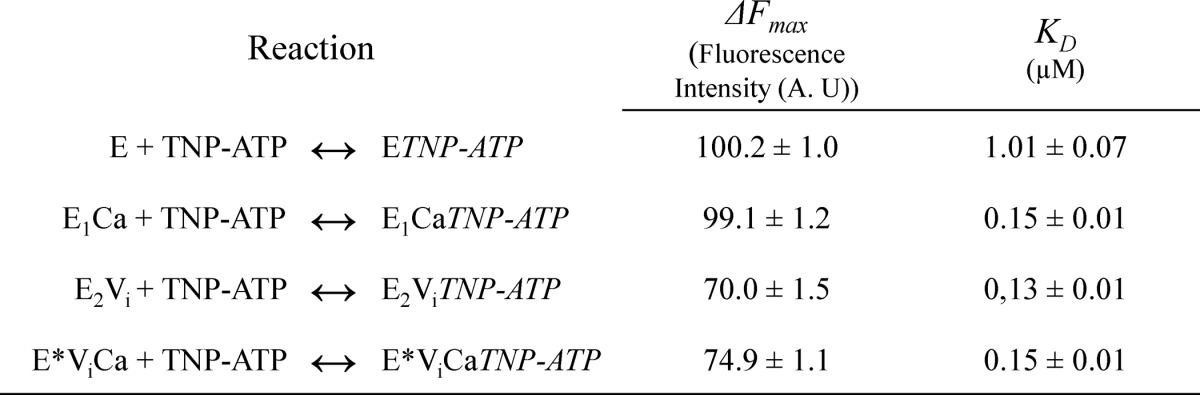
Results show that the apparent affinity of PMCA for TNP-ATP is 10-fold higher in the presence of Ca2+ than that in its absence. However, in the presence of vanadate, the apparent affinity for this ATP analog did not change with the addition of Ca2+ and was similar to that of E1Ca in Fig. 8A. These results can be compared with those in Fig. 6, A–C, where the affinity for ATP is clearly higher in the presence of vanadate (B and C) than in its absence (A), showing a parallel behavior for the affinities of TNP-ATP. It could be surprising that the affinity for ATP observed in Fig. 6 is considerably lower than that for TNP-ATP in Fig. 8. However, Moutin et al. (58) reported that the apparent affinity of sarcoplasmic reticulum Ca2+-ATPase for TNP-ATP is significantly higher than that for the unmodified nucleotide.
On the other hand, TNP-ATP fluorescence intensity at saturating concentrations of the probe was similar for E1Ca and E2, whereas this value was 40% lower in the presence of vanadate. These results suggest that there is a change in the hydrophobic environment of the TNP-ATP and therefore a different environment for the ATP binding domain as a consequence of the different conformations.
The change in ΔFmax upon the addition of vanadate proves that the lack of response to the inhibitor observed in Fig. 3A is not due to its inability to bind to PMCA. In other words, vanadate apparently does not modify the transmembrane domain, but it does produce a conformational change on the cytoplasmic domain, thus justifying the hypothesis of the existence of a different conformation, E*ViCaATP.
DISCUSSION
There are several crystal structures of the Ca2+ pump of sarcoplasmic reticulum corresponding to different conformations reached during the reaction cycle (2, 3), and crystal structures of the H+-ATPase (59) and of the Na+/K+-ATPase were reported as well (60, 61). However, there are as yet no high resolution structures for the majority of the P-type ATPases, including the family of PMCAs. This underlines the continued need to develop new tools to probe the structure-function relationship in these proteins by alternative and complementary methods. Of particular interest for a better understanding of the pump mechanism are methods that yield information on the membrane-embedded part of the pump, which is the most difficult region to study by traditional methods of protein expression and analysis.
Effects of Lanthanum and Vanadate on the Transmembrane Domain
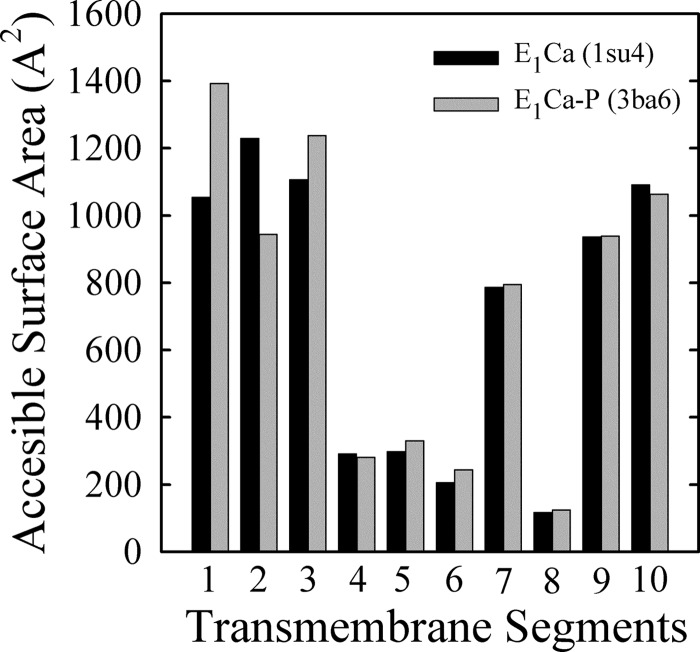
For PMCA, the amount of EP obtained in the presence of lanthanum is usually considered as a valid calculation of the total active enzyme concentration (55). It has been proposed that LaIII would act as a dead end inhibitor capable of bringing the entire amount of functionally active enzyme into the E1PCa form. This phenomenon is consistent with the fact that specific incorporation of [125I]TID-PC/16 in the presence of Ca2+, LaIII, and ATP was 155.0 ± 1.0%, a maximal value that is similar to that obtained in the absence of ATP. Like the phosphoenzyme level, occlusion of Ca2+ is also maximal in the presence of LaIII and ATP (8). Fig. 5 shows that increasing ATP concentrations produced only marginal changes in the incorporation of [125I]TID-PC/16. This can mean that either (i) significant changes occur in the transmembrane segments, but these are internally counterbalanced, or (ii) LaIII hinders the transmembrane conformational changes elicited by ATP. As an approach to answer this question, we evaluated the possible extent of this change by calculating the ASA of the transmembrane region using crystallographic data from SERCA. Note that in Protein Data Bank code, the structure of Ca2E1P (our E1PCa) is known as 3ba6, whereas E1·2Ca (our E1Ca) is represented by the structure known as 1su4. Results in Fig. 9 show that most significant changes are found in helices M1–M3, with an increase of 338 Å2 (32%) for M1, a decrease of 285 Å2 (30%) for M2, and an increase of 131 Å2 (12%) for M3. Thus, the overall change in ASA values when the enzyme goes from E1·2Ca (7113.9 Å2) to Ca2E1P (7349.6 Å2) is 236 Å2, just about 3% of the total ASA. These results for SERCA support the idea that our method could be not precise enough to resolve such a small overall difference in areas exposed to lipids in PMCA, despite the important changes that can occur in some of the transmembrane segments.
FIGURE 9.
ASAs of the transmembrane domains (helices M1–M10) calculated from the crystal structures 1su4 (E1Ca2) and 3ba6 (E1P(Ca2)) of SERCA. For details, see “Experimental Procedures.”
An important conclusion from the above analysis is that, although a difference in the area accessible to lipids can only occur as a consequence of the existence of different conformations of the pump, the opposite is not necessarily true (i.e. a similar value of specific incorporation of [125I]TID-PC/16 does not necessarily imply similar conformations), because different arrangements of the transmembrane helices could present a similar surface accessible to lipids.
Vanadate is considered to mimic a pentacoordinated transition state of the phosphoryl group, binding to E2 in a reaction that requires Mg2+ (43, 44). In the absence of Ca2+, vanadate does not produce any detectable change in the specific incorporation of [125I]TID-PC/16 (Fig. 3A, open circles). However, in the presence of Ca2+, increasing concentrations of vanadate produce a decrease in the specific incorporation of [125I]TID-PC/16 (Fig. 3A, closed circles). A similar result was obtained when PMCA was incubated with vanadate, and increasing Ca2+ concentrations were added (Fig. 3B). Inhibition of Ca2+-ATPase activity by vanadate is commonly explained as the result of its binding to the enzyme, leading to a conformation analogous to E2P. Early experiments on the kinetics of vanadate in SERCA showed that the inhibition is antagonized by Ca2+ (44, 53), a result consistent with the idea that Ca2+ and vanadate bind to different, mutually exclusive conformations. Experiments by Krebs et al. (62) performed in calmodulin-activated PMCA show that vanadate prevents the fluorescence changes that accompany phosphorylation by ATP. However, fluorescence experiments in SERCA (63) suggest the existence of a calcium-enzyme-vanadate complex whose fluorescence properties are “E1-like” rather than “E2-like.” Our results confirm that in the presence of Ca2+ and vanadate, PMCA forms a stable ternary complex, E*ViCa, whose conformational state is different from that of E2 and E1Ca and that we tentatively designated “E*”. Importantly, this ternary complex could be formed in the absence and in the presence of ATP. In agreement with results reported by Daiho et al. (64, 65), who obtained a stable transition phosphorylation state of SERCA in the presence of Ca2+, the ternary complex E*ViCa could be interpreted as an intermediate state interposed between E1PCa and the Ca2+-free state E2P.
While forming the E*ViCa complex from E2Vi, we found that the apparent dissociation constant for Ca2+ is higher than in the absence of vanadate (5.1 μm versus 1 μm; see Ref. 22). In SERCA (66), biochemical data obtained in the absence of thapsigargin, a potent inhibitor that binds to the transmembrane domain and leads the pump to the E2 conformation, support the conclusion that the dephosphorylation transition state of E2P represents a proton-occluded state. This idea arises from the fact that in contrast to E2, this state has a low affinity for Ca2+. Our results show that a similar state could exist for PMCA.
Ca2+ Occlusion
We report here results on Ca2+ occlusion obtained in equilibrium conditions, in the absence of ATP or in the presence of ATP plus vanadate. It is a generally accepted idea that in P-type ATPases, occlusion of the cation transported from the cytoplasmic side occurs concomitantly with the formation of E1P and that the release of the occluded cation toward the opposite side takes place after the E1P to E2P conformational transition. This has been proposed both for the occlusion of two calcium ions in the SERCA and of three sodium ions in the Na,K-ATPase. Using membrane-bound PMCA preparations in media with ATP and lanthanum, which blocks the conformational transition from E1P to E2P, we found that Ca2+ becomes occluded concomitantly with the formation of E1P with a stoichiometry of one Ca2+ per phosphorylated enzyme unit (8). However, this experimental evidence does not exclude the possibility of occlusion of Ca2+ in non-phosphorylated intermediates. In this sense, in the absence of ATP, occlusion of Ca2+ in SERCA (67) and of Na+ in oligomycin-inhibited Na,K-ATPase (68) has been reported. This is not surprising if one admits the idea that cation occlusion precedes phosphorylation (or dephosphorylation, depending on the transported cation), thus triggering the reaction (e.g. see Refs. 60 and 69).
The increase in affinity for Ca2+ observed in the presence of LaIII can be explained on the basis of the irreversibility of the phosphorylation reaction and the blocking effect of this inhibitor on the E1P to E2P conformational transition, which prevents the return of the enzyme to calcium-free states.
Binding of ATP
Toyoshima et al. (70) described that in SERCA, the reaction cycle is regulated essentially by Ca2+ alone. ATP can bind to the enzyme even when Ca2+ is absent, but without Ca2+, the reaction cycle cannot progress. Binding of Ca2+ will cause the movement of the transmembrane helices. Fig. 6 shows that ATP binds to PMCA in the absence of Ca2+, and this triggers a change in the transmembrane domain. The apparent dissociation constant for ATP measured with [125I]TID-PC/16 is in agreement with previous reports for SERCA (20). Our results (Fig. 6, B and C) show that ATP also binds to E2Vi (an E2P-like state) and to E*ViCa (an E*P(Ca)-like state), producing conformational changes in the PMCA transmembrane domain. Vanadate increases 3-fold the apparent affinity for ATP, both in the presence and in the absence of Ca2+. This is less than the 10-fold increase of the apparent affinity for ATP in SERCA. However, the structural and kinetic analyses of the E2 conformation of SERCA were performed in the presence of thapsigargin. It was recently suggested (71) that thapsigargin produces stiffness at the transmembrane domain of SERCA, making it unresponsive to conformational changes occurring within the cytosolic domain, and it is unclear how closely these inhibitor-bound structures resemble other, perhaps more physiological, states of the enzyme. In addition, it has been described (72) that in the absence of Ca2+, affinity of SERCA for ATP is lower when thapsigargin is present.
Therefore, those results should not be compared directly with our studies, which were performed in the absence of an inhibitor that leads PMCA to the E2 conformation. On the contrary, our approach measures the actual substrate affinity through the binding of trace amounts of [125I]TID-PC/16, which is directly proportional to the transmembrane surface of PMCA exposed to surrounding lipids.
A most important point of interest is whether binding of nucleotide produces an induced fit prior to the phosphoryl transfer reaction. However, binding of ATP to E1Ca cannot be measured directly due to the fast setting of a steady state of transport of Ca2+ and ATP hydrolysis. We therefore extended our studies of specific conformational effects produced by ATP using its analog TNP-ATP. This fluorescent probe has been used for studying the interaction of several proteins with ATP (53) and is not hydrolyzed by PMCA.
Results shown in Fig. 8 confirm that TNP-ATP binds to PMCA in the absence of Ca2+, although with a higher apparent dissociation constant than that observed in the presence of Ca2+ (E1Ca). At saturating TNP-ATP concentrations, the fluorescence intensity was similar in both conditions, indicating that the hydrophobic environment of the N-domain is also similar, in agreement with results reported in SERCA (2). In PMCA, the apparent KD values for TNP-ATP of the E2Vi and E*ViCa states were similar to that obtained for the E1Ca state but 7-fold lower than for E2.
The maximal fluorescence intensity of the high affinity component for the binding of TNP-ATP was similar for E2Vi and E*ViCa states but significantly lower than that obtained for E1Ca. This indicates that the N-domain environment is more hydrophobic in the presence of Ca2+ (E1Ca).
A Functional Cycle for PMCA
Scarborough (73) has observed the inadequacy of the E1/E2 nomenclature to describe the intermediates involved in the reaction cycle of P-type ATPases. In this work, we have demonstrated the existence of species of PMCA that can be seen as different conformers, depending on whether the measurements were sensing changes in the cytoplasmic (experiments with TNP-ATP) or in the transmembrane ([125I]TID-PC/16 photolabeling) domains of the enzyme. For instance, the apparent lack of effect of vanadate on the transmembrane domain of EATP contrasts with the effect of the inhibitor on the maximal fluorescence change upon binding of TNP-ATP. Although this could mean that significant changes in the transmembrane domain cancel each other regarding their exposure to lipids, as shown above in the case of the structures of E1·2Ca and Ca2E1P of SERCA, a possible uncoupling between conformational changes in the cytoplasmic and membrane domains cannot be ruled out and is worth considering. Evidence adding important features to the E1-E2 model is that E2Vi, E2ViATP, and E*ViCaATP, all of them intermediates that in principle would be thought of as E2 conformers, actually exhibit different surface areas accessible to phospholipids, which reveals that they should at least be considered as different subconformations.
We have also studied in this work for the first time the effect of ATP on the structure of these subconformations, showing that they are all able to bind ATP at physiological concentrations (see Figs. 6 (A–C) and 8). This notion questions the physiological relevance of the E1Ca conformation as an intermediate in the transport scheme, because under physiological conditions, with a concentration of ATP high enough and a large ATP/ADP ratio, the modulatory site will be saturated by ATP, allowing the E2 state to circumvent the E1Ca conformation and to transition directly into the E1ATPCa state.
Taking this evidence together, Fig. 10 shows a functional cycle of ATP binding and hydrolysis by PMCA. Note that (i) we have included a new intermediate, E*P(Ca)ATP, between E1P(Ca)ATP and E2PATP·Ca, whose conformation is different from that of E1 and E2, and (ii) with the exception of a transient ADP-bound state, all conformers are bound to a molecule of ATP and can be simultaneously phosphorylated. Conversely, Ca2+ can be occluded in or simply bound to the enzyme. In its resting condition, PMCA would mainly exist as two intermediates in equilibrium, E1ATP and E2ATP, until a signal of Ca2+ or calmodulin-Ca2+ initiates the transport of the cation driven by the ATP hydrolysis.
FIGURE 10.
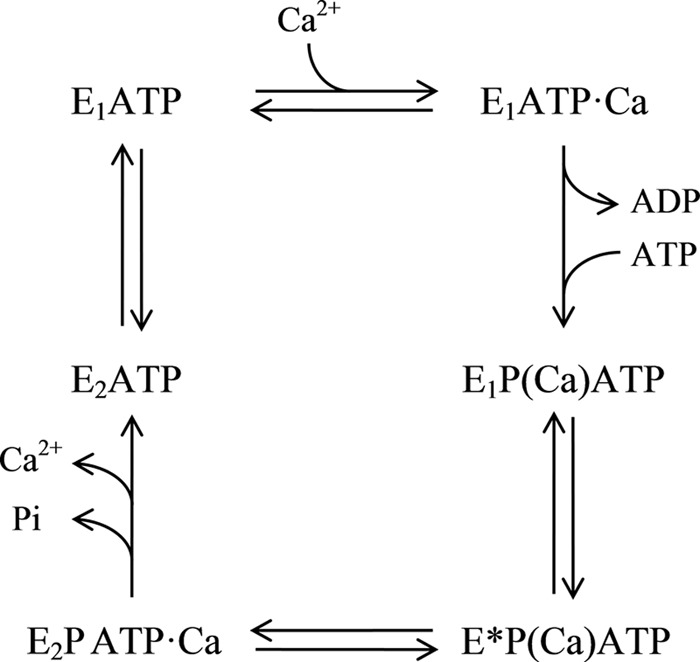
Scheme of the model proposed for the PMCA functional reaction cycle. Parentheses denote cation occlusion.
In this study, we focus on the role of ATP in the reaction cycle, leaving aside the effect of cofactors like Mg2+ and activators such as calmodulin, which are worthy of future study using the findings of this work as a basis.
Acknowledgments
We are greatly indebted to Dr. Emanuel E. Strehler (Mayo Clinic/Foundation, Rochester, MN) for helpful comments and to Dr. J. Brunner (Swiss Federal Institute of Technology, Zürich) for the kind gift of TTD-PC/16 (tin precursor) and to Fundosol (Argentina).
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica, Consejo Nacional de Investigaciones Científicas y Técnicas, and Universidad de Buenos Aires Ciencia y Técnica (UBACYT; Argentina).
- PMCA
- plasma membrane calcium pump
- DMPC
- 1,2-dimyristoyl-sn-glycero-3-phosphocholine
- C12E10
- polyoxyethylene-10-dodecyl ether
- [125I]TID-PC/16
- 1-O-hexadecanoyl-2-O-[9-[[[2-[125I]iodo-4-(trifluoromethyl-3H-diazirin-3-yl)benzyl]oxy]carbonyl]nonanoyl]-sn-glycero-3-phosphocholine
- SERCA
- sarcoplasmic reticulum Ca2+-ATPase
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- ASA
- accessible surface area
- TNP-ATP
- 2′(3′)-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate.
REFERENCES
- 1. Strehler E. E., Caride A. J., Filoteo A. G., Xiong Y., Penniston J. T., Enyedi A. (2007) Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann. N.Y. Acad. Sci. 1099, 226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toyoshima C. (2009) How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 1793, 941–946 [DOI] [PubMed] [Google Scholar]
- 3. Møller J. V., Olesen C., Winther A. M., Nissen P. (2010) The sarcoplasmic Ca2+-ATPase. Design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 [DOI] [PubMed] [Google Scholar]
- 4. Sarkadi B., Enyedi A., Földes-Papp Z., Gárdos G. (1986) Molecular characterization of the in situ red cell membrane calcium pump by limited proteolysis. J. Biol. Chem. 261, 9552–9557 [PubMed] [Google Scholar]
- 5. Corradi G. R., Adamo H. P. (2007) Intramolecular fluorescence resonance energy transfer between fused autofluorescent proteins reveals rearrangements of the N- and C-terminal segments of the plasma membrane Ca2+ pump involved in the activation. J. Biol. Chem. 282, 35440–35448 [DOI] [PubMed] [Google Scholar]
- 6. Mangialavori I., Villamil-Giraldo A. M., Pignataro M. F., Ferreira-Gomes M., Caride A. J., Rossi J. P. (2011) Plasma membrane calcium pump (PMCA) differential exposure of hydrophobic domains after calmodulin and phosphatidic acid activation. J. Biol. Chem. 286, 18397–18404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rega A. F., Garrahan P. J. (1986) The Ca2+ Pump of Plasma Membranes, pp. 115–125, CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 8. Ferreira-Gomes M. S., González-Lebrero R., de la Fuente M. C., Strehler E. E., Rossi R. C., Rossi J. P. (2011) Calcium occlusion in plasma membrane Ca2+-ATPase. J. Biol. Chem. 286, 32018–32025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scofano H. M., Vieyra A., de Meis L. (1979) Substrate regulation of the sarcoplasmic reticulum ATPase. Transient kinetic studies. J. Biol. Chem. 254, 10227–10231 [PubMed] [Google Scholar]
- 10. Wakabayashi S., Shigekawa M. (1990) Mechanism for activation of the 4-nitrobenzo-2-oxa-1,3-diazole-labeled sarcoplasmic reticulum ATPase by Ca2+ and its modulation by nucleotides. Biochemistry 29, 7309–7318 [DOI] [PubMed] [Google Scholar]
- 11. McIntosh D. B., Boyer P. D. (1983) Adenosine 5′-triphosphate modulation of catalytic intermediates of calcium ion activated adenosinetriphosphatase of sarcoplasmic reticulum subsequent to enzyme phosphorylation. Biochemistry 22, 2867–2875 [DOI] [PubMed] [Google Scholar]
- 12. Wakabayashi S., Ogurusu T., Shigekawa M. (1986) Factors influencing calcium release from the ADP-sensitive phosphoenzyme intermediate of the sarcoplasmic reticulum ATPase. J. Biol. Chem. 261, 9762–9769 [PubMed] [Google Scholar]
- 13. Champeil P., Guillain F. (1986) Rapid filtration study of the phosphorylation-dependent dissociation of calcium from transport sites of purified sarcoplasmic reticulum ATPase and ATP modulation of the catalytic cycle. Biochemistry 25, 7623–7633 [DOI] [PubMed] [Google Scholar]
- 14. Bodley A. L., Jencks W. P. (1987) Acetyl phosphate as a substrate for the calcium ATPase of sarcoplasmic reticulum. J. Biol. Chem. 262, 13997–14004 [PubMed] [Google Scholar]
- 15. Shigekawa M., Dougherty J. P. (1978) Reaction mechanism of Ca2+-dependent ATP hydrolysis by skeletal muscle sarcoplasmic reticulum in the absence of added alkali metal salts. II. Kinetic properties of the phosphoenzyme formed at the steady state in high Mg2+ and low Ca2+ concentrations. J. Biol. Chem. 253, 1451–1457 [PubMed] [Google Scholar]
- 16. Ariki M., Boyer P. D. (1980) Characterization of medium inorganic phosphate-water exchange catalyzed by sarcoplasmic reticulum vesicles. Biochemistry 19, 2001–2004 [DOI] [PubMed] [Google Scholar]
- 17. Champeil P., Riollet S., Orlowski S., Guillain F., Seebregts C. J., McIntosh D. B. (1988) ATP regulation of sarcoplasmic reticulum Ca2+-ATPase. Metal-free ATP and 8-bromo-ATP bind with high affinity to the catalytic site of phosphorylated ATPase and accelerate dephosphorylation. J. Biol. Chem. 263, 12288–12294 [PubMed] [Google Scholar]
- 18. Andersen J. P., Møller J. V. (1985) The role of Mg2+ and Ca2+ in the simultaneous binding of vanadate and ATP at the phosphorylation site of sarcoplasmic reticulum Ca2+-ATPase. Biochim. Biophys. Acta 815, 9–15 [DOI] [PubMed] [Google Scholar]
- 19. Clausen J. D., McIntosh D. B., Anthonisen A. N., Woolley D. G., Vilsen B., Andersen J. P. (2007) ATP-binding modes and functionally important interdomain bonds of sarcoplasmic reticulum Ca2+-ATPase revealed by mutation of glycine 438, glutamate 439, and arginine 678. J. Biol. Chem. 282, 20686–20697 [DOI] [PubMed] [Google Scholar]
- 20. Clausen J. D., McIntosh D. B., Woolley D. G., Andersen J. P. (2011) Modulatory ATP binding affinity in intermediate states of E2P dephosphorylation of sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 286, 11792–11802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mangialavori I., Villamil Giraldo A. M., Marino Buslje C., Ferreira Gomes M., Caride A. J., Rossi J. P. (2009) A new conformation in sarcoplasmic reticulum calcium pump and plasma membrane Ca2+ pumps revealed by a photoactivatable phospholipidic probe. J. Biol. Chem. 284, 4823–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mangialavori I. C., Ferreira-Gomes M., Pignataro M. F., Strehler E. E., Rossi J. P. (2010) Determination of the dissociation constants for Ca2+ and calmodulin from the plasma membrane Ca2+ pump by a lipid probe that senses membrane domain changes. J. Biol. Chem. 285, 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niggli V., Penniston J. T., Carafoli E. (1979) Purification of the (Ca2+-Mg2+)-ATPase from human erythrocyte membranes using a calmodulin affinity column. J. Biol. Chem. 254, 9955–9958 [PubMed] [Google Scholar]
- 24. Filomatori C. V., Rega A. F. (2003) On the mechanism of activation of the plasma membrane Ca2+-ATPase by ATP and acidic phospholipids. J. Biol. Chem. 278, 22265–22271 [DOI] [PubMed] [Google Scholar]
- 25. Fiske C. H., SubbaRow Y. (1925) The colorimetric determination of phosphorus. J. Biol. Chem. 66, 375–400 [Google Scholar]
- 26. Echarte M. M., Levi V., Villamil A. M., Rossi R. C., Rossi J. P. (2001) Quantitation of plasma membrane calcium pump phosphorylated intermediates by electrophoresis. Anal. Biochem. 289, 267–273 [DOI] [PubMed] [Google Scholar]
- 27. Durrer P., Gaudin Y., Ruigrok R. W., Graf R., Brunner J. (1995) Photolabeling identifies a putative fusion domain in the envelope glycoprotein of rabies and vesicular stomatitis viruses. J. Biol. Chem. 270, 17575–17581 [DOI] [PubMed] [Google Scholar]
- 28. Schägger H., von Jagow G. (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 [DOI] [PubMed] [Google Scholar]
- 29. Ball E. H. (1986) Quantitation of proteins by elution of Coomassie Brilliant Blue R from stained bands after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 155, 23–27 [DOI] [PubMed] [Google Scholar]
- 30. Rossi R. C., Kaufman S. B., González-Lebrero R. M., Nørby J. G., Garrahan P. J. (1999) An attachment for nondestructive, fast quenching of samples in rapid-mixing experiments. Anal. Biochem. 270, 276–285 [DOI] [PubMed] [Google Scholar]
- 31. Qu Q., Russell P. L., Sharom F. J. (2003) Stoichiometry and affinity of nucleotide binding to P-glycoprotein during the catalytic cycle. Biochemistry 42, 1170–1177 [DOI] [PubMed] [Google Scholar]
- 32. Pérez-Gordones M. C., Lugo M. R., Winkler M., Cervino V., Benaim G. (2009) Diacylglycerol regulates the plasma membrane calcium pump from human erythrocytes by direct interaction. Arch. Biochem. Biophys. 489, 55–61 [DOI] [PubMed] [Google Scholar]
- 33. Lin S. H., Faller L. D. (1996) Estimation of the distance change between cysteine-457 and the nucleotide binding site when sodium pump changes conformation from E1 to E2 by fluorescence energy transfer measurements. Biochemistry 35, 8419–8428 [DOI] [PubMed] [Google Scholar]
- 34. Cho Y. K., Ríos S. E., Kim J. J., Miziorko H. M. (2001) Estimation of the distance change between cysteine-457 and the nucleotide binding site when sodium pump changes conformation from E1 to E2 by fluorescence energy transfer measurements. J. Biol. Chem. 276, 12573–12578 [DOI] [PubMed] [Google Scholar]
- 35. Vas M., Merli A., Rossi G. L. (1994) Antagonistic binding of substrates to 3-phosphoglycerate kinase monitored by the fluorescent analogue 2′(3′)-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate. Biochem. J. 301, 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang S. G., Weisshart K., Fanning E. (1998) Characterization of the nucleotide binding properties of SV40 T antigen using fluorescent 3′(2′)-O-(2,4,6-trinitrophenyl)adenine nucleotide analogues. Biochemistry 37, 15336–15344 [DOI] [PubMed] [Google Scholar]
- 37. Stewart R. C., VanBruggen R., Ellefson D. D., Wolfe A. J. (1998) TNP-ATP and TNP-ADP as probes of the nucleotide binding site of CheA, the histidine protein kinase in the chemotaxis signal transduction pathway of Escherichia coli. Biochemistry 37, 12269–12279 [DOI] [PubMed] [Google Scholar]
- 38. Toyoshima C., Nakasako M., Nomura H., Ogawa H. (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 [DOI] [PubMed] [Google Scholar]
- 39. Olesen C., Picard M., Winther A. M., Gyrup C., Morth J. P., Oxvig C., Møller J. V., Nissen P. (2007) The structural basis of calcium transport by the calcium pump. Nature 450, 1036–1042 [DOI] [PubMed] [Google Scholar]
- 40. Lee B., Richards F. M. (1971) The interpretation of protein structures. Estimation of static accessibility. J. Mol. Biol. 55, 379–400 [DOI] [PubMed] [Google Scholar]
- 41. Koradi R., Billeter M., Wüthrich K. (1996) MOLMOL. A program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55 [DOI] [PubMed] [Google Scholar]
- 42. Burnham K., P., Anderson D., R. (2002) Model Selection and Multimodel Inference, 2nd Ed., pp. 60–85, Springer, New York [Google Scholar]
- 43. Cantley L. C., Jr., Cantley L. G., Josephson L. (1978) A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J. Biol. Chem. 253, 7361–7368 [PubMed] [Google Scholar]
- 44. Pick U. (1982) The interaction of vanadate ions with the Ca-ATPase from sarcoplasmic reticulum. J. Biol. Chem. 257, 6111–6119 [PubMed] [Google Scholar]
- 45. Rossi J. P., Garrahan P. J., Rega A. F. (1981) Vanadate inhibition of active Ca2+ transport across human red cell membranes. Biochim. Biophys. Acta 648, 145–150 [DOI] [PubMed] [Google Scholar]
- 46. Luterbacher S., Schatzmann H. J. (1983) The site of action of La3+ in the reaction cycle of the human red cell membrane Ca2+-pump ATPase. Experientia 39, 311–312 [DOI] [PubMed] [Google Scholar]
- 47. Herscher C. J., Rega A. F. (1996) Pre-steady-state kinetic study of the mechanism of inhibition of the plasma membrane Ca2+-ATPase by lanthanum. Biochemistry 35, 14917–14922 [DOI] [PubMed] [Google Scholar]
- 48. Durrer P., Galli C., Hoenke S., Corti C., Glück R., Vorherr T., Brunner J. (1996) H+-induced membrane insertion of influenza virus hemagglutinin involves the HA2 amino-terminal fusion peptide but not the coiled coil region. J. Biol. Chem. 271, 13417–13421 [DOI] [PubMed] [Google Scholar]
- 49. Villamil Giraldo A. M., Castello P. R., González-Flecha F. L., Moeller J. V., Delfino J. M., Rossi J. P. (2006) Stoichiometry of lipid-protein interaction assessed by hydrophobic photolabeling. FEBS Lett. 580, 607–612 [DOI] [PubMed] [Google Scholar]
- 50. Mangialavori I., Montes M. R., Rossi R. C., Fedosova N. U., Rossi J. P. (2011) Dynamic lipid-protein stoichiometry on E1 and E2 conformations of the Na+/K+-ATPase. FEBS Lett. 585, 1153–1157 [DOI] [PubMed] [Google Scholar]
- 51. Mangialavori I. C., Corradi G., Rinaldi D. E., de la Fuente M. C., Adamo H. P., Rossi J. P. (2012) Autoinhibition mechanism of the plasma membrane calcium pump isoforms 2 and 4 studied through lipid-protein interaction. Biochem. J. 443, 125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vanagas L., de La Fuente M. C., Dalghi M., Ferreira-Gomes M., Rossi R. C., Strehler E. E., Mangialavori I. C., Rossi J. P. (2013) Differential effects of G- and F-actin on the plasma membrane calcium pump activity. Cell Biochem. Biophys. 66, 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. (1992) Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 267, 14483–14489 [PubMed] [Google Scholar]
- 54. Reynolds J. A., Johnson E. A., Tanford C. (1985) Application of the principle of linked functions to ATP-driven ion pumps. Kinetics of activation by ATP. Proc. Natl. Acad. Sci. U.S.A. 82, 3658–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Echarte M. M., Rossi R. C., Rossi J. P. (2007) Phosphorylation of the plasma membrane calcium pump at high ATP concentration. On the mechanism of ATP hydrolysis. Biochemistry 46, 1034–1041 [DOI] [PubMed] [Google Scholar]
- 56. Adamo H. P., Rega A. F., Garrahan P. J. (1990) The E2 in equilibrium E1 transition of the Ca2+-ATPase from plasma membranes studied by phosphorylation. J. Biol. Chem. 265, 3789–3792 [PubMed] [Google Scholar]
- 57. Hiratsuka T. (2003) Fluorescent and colored trinitrophenylated analogs of ATP and GTP. Eur. J. Biochem. 270, 3479–3485 [DOI] [PubMed] [Google Scholar]
- 58. Moutin M. J., Cuillel M., Rapin C., Miras R., Anger M., Lompré A. M., Dupont Y. (1994) Measurements of ATP binding on the large cytoplasmic loop of the sarcoplasmic reticulum Ca2+-ATPase overexpressed in Escherichia coli. J. Biol. Chem. 269, 11147–11154 [PubMed] [Google Scholar]
- 59. Pedersen B. P., Buch-Pedersen M. J., Morth J. P., Palmgren M. G., Nissen P. (2007) Crystal structure of the plasma membrane proton pump. Nature 450, 1111–1114 [DOI] [PubMed] [Google Scholar]
- 60. Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sørensen T. L., Petersen J., Andersen J. P., Vilsen B., Nissen P. (2007) Crystal structure of the sodium-potassium pump. Nature 450, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 61. Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009) Crystal structure of the sodium-potassium pump at 2.4 Å resolution. Nature 459, 446–450 [DOI] [PubMed] [Google Scholar]
- 62. Krebs J., Vasak M., Scarpa A., Carafoli E. (1987) Conformational differences between the El and E2 states of the calcium adenosinetriphosphatase of the erythrocyte plasma membrane as revealed by circular dichroism and fluorescence spectroscopy. Biochemistry 26, 3921–3926 [DOI] [PubMed] [Google Scholar]
- 63. Markus S., Priel Z., Chipman D. M. (1989) Interaction of calcium and vanadate with fluorescein isothiocyanate labeled Ca2+-ATPase from sarcoplasmic reticulum. Kinetics and equilibria. Biochemistry 28, 793–799 [DOI] [PubMed] [Google Scholar]
- 64. Daiho T., Yamasaki K., Danko S., Suzuki H. (2007) Critical role of Glu40–Ser48 loop linking actuator domain and first transmembrane helix of Ca2+-ATPase in Ca2+ deocclusion and release from ADP-insensitive phosphoenzyme. J. Biol. Chem. 282, 34429–34447 [DOI] [PubMed] [Google Scholar]
- 65. Daiho T., Danko S., Yamasaki K., Suzuki H. (2010) Stable structural analog of Ca2+-ATPase ADP-insensitive phosphoenzyme with occluded Ca2+ formed by elongation of A-domain/M1′-linker and beryllium fluoride binding. J. Biol. Chem. 285, 24538–24547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Olesen C., Sørensen T. L., Nielsen R. C., Møller J. V., Nissen P. (2004) Dephosphorylation of calcium pump coupled to counterion occlusion. Science 306, 2251–2255 [DOI] [PubMed] [Google Scholar]
- 67. Nakamura J. (1987) Calcium-dependent calcium occlusion in the sarcoplasmic reticulum Ca2+-ATPase. Its enhancement by phosphorylation of the enzyme. J. Biol. Chem. 262, 14492–14497 [PubMed] [Google Scholar]
- 68. Esmann M., Skou J. C. (1985) Occlusion of Na+ by the Na,K-ATPase in the presence of oligomycin. Biochem. Biophys. Res. Commun. 127, 857–863 [DOI] [PubMed] [Google Scholar]
- 69. Forbush B., 3rd (1988) Occluded ions and Na,K-ATPase. in The Na+,K+-Pump, Part A: Molecular Aspects (Skou J. C., Nørby J. G., Maunsbach A. B., Esmann M., eds) pp. 229–248, Alan R. Liss Inc., New York [Google Scholar]
- 70. Toyoshima C., Inesi G. (2004) Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu. Rev. Biochem. 73, 269–292 [DOI] [PubMed] [Google Scholar]
- 71. Akin B. L., Chen Z., Jones L. R. (2010) Superinhibitory phospholamban mutants compete with Ca2+ for binding to SERCA2a by stabilizing a unique nucleotide-dependent conformational state. J. Biol. Chem. 285, 28540–28552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Inesi G., Lewis D., Ma H., Prasad A., Toyoshima C. (2006) Concerted conformational effects of Ca2+ and ATP are required for activation of sequential reactions in the Ca2+ ATPase (SERCA) catalytic cycle. Biochemistry 45, 13769–13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Scarborough G. A. (2003) Rethinking the P-ATPases problem. Trends Biochem. Sci. 28, 581–594 [DOI] [PubMed] [Google Scholar]