Abstract
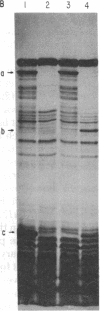
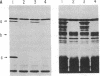
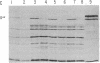
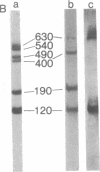
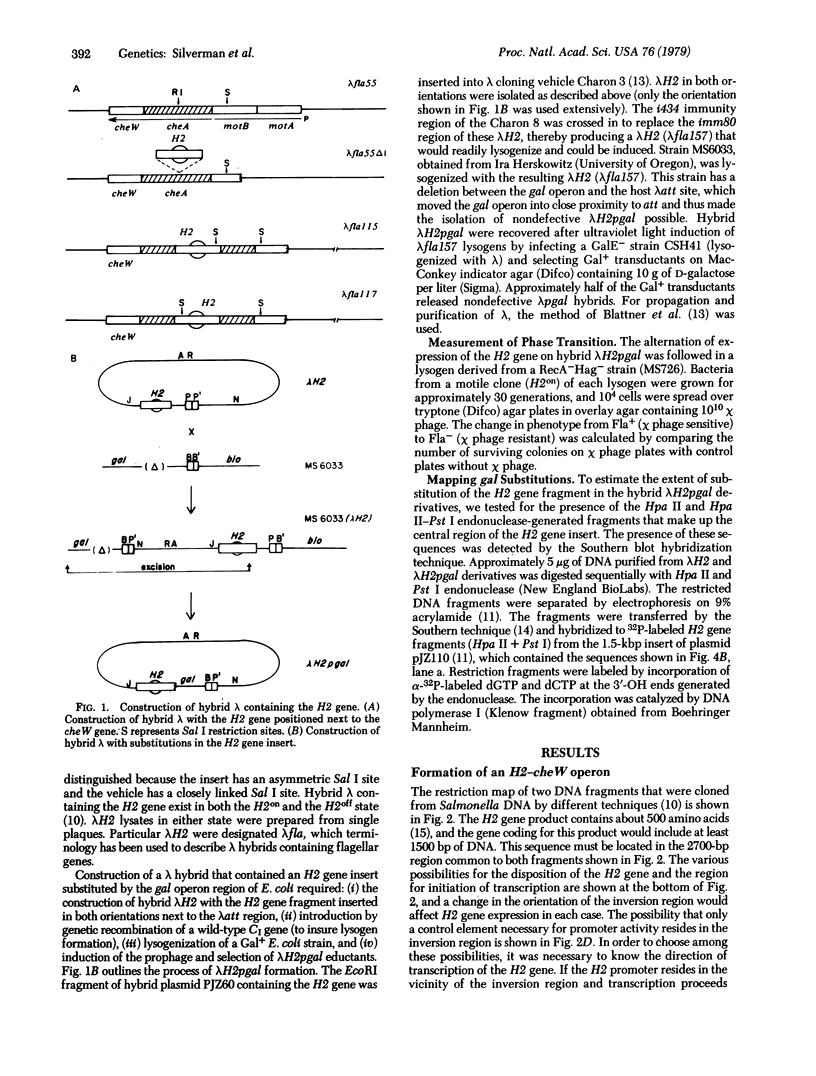
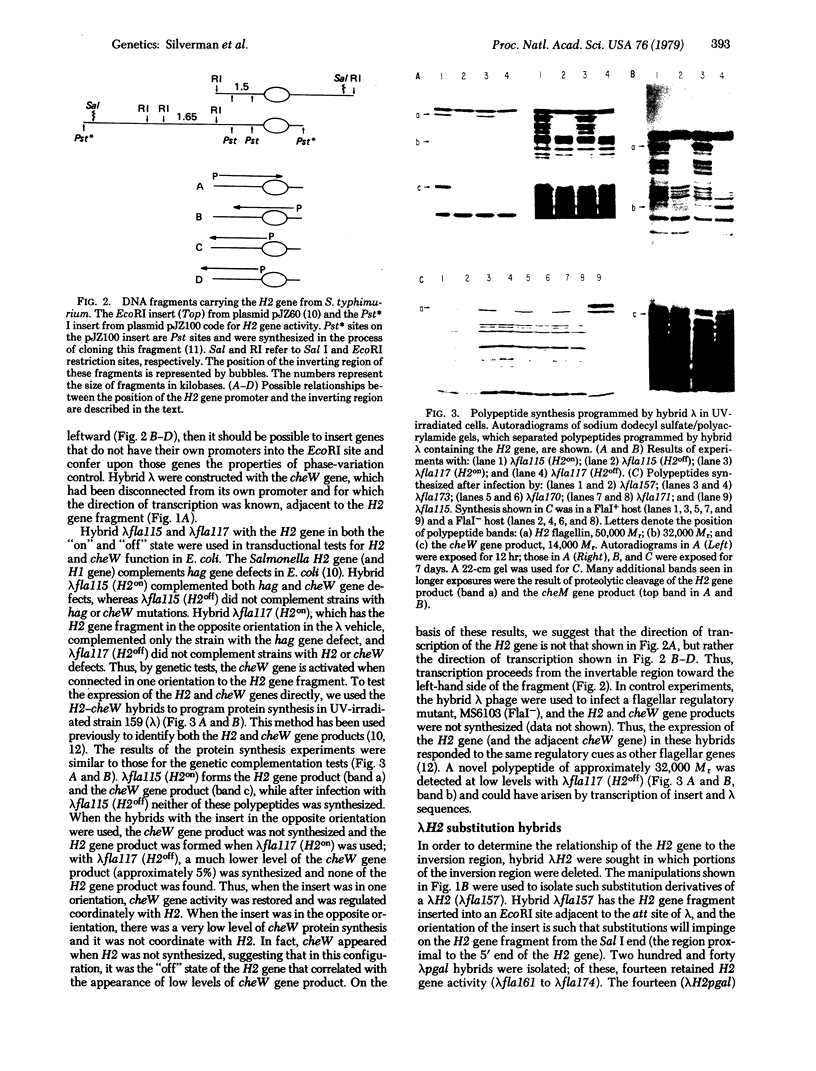
The alternative expression of Salmonella genes H1 and H2, which specify different flagellar antigens, results in the oscillation of phenotype known as phase variation. This alternation is controlled by the inversion of an 800-base-pair sequence of DNA adjacent to, or including part of, the H2 gene. The invertable region was presumed to regulate the function of a promoter and to include specific sites at which a recombinational event, resulting in the inversion, could occur. Here we report genetic manipulations of hybrid lambda phage carrying the H2 gene that were used to define the H2 promoter region and the recombinational sites. The H2 gene fragment was inserted on a hybrid lambda phage next to the cheW gene, which lacked a promoter element. In the resulting fusion, cheW gene activity was restored, the expression of the H2 and cheW genes was controlled coordinately by the inversion, and the polarity of transcription and location of the H2 gene could be determined. Evidence from this type of gene fusion suggested that the H2 gene promoter is included in the inversion region. Hybrid H2 phage were constructed that contained substitutions for regions of the H2 gene. In contrast to hybrid lambda containing the H2 gene, which alternate between "on" and "off" states, several substituted lambdaH2 were fixed in the "on" state. A site necessary for the recombinational event must have been removed in these fixed lambdaH2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Lederberg J, Iino T. Phase Variation in Salmonella. Genetics. 1956 Sep;41(5):743–757. doi: 10.1093/genetics/41.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLINTOCK B. Controlling elements and the gene. Cold Spring Harb Symp Quant Biol. 1956;21:197–216. doi: 10.1101/sqb.1956.021.01.017. [DOI] [PubMed] [Google Scholar]
- Pearce U. B., Stocker B. A. Phase variation of flagellar antigens in Salmonella: abortive transduction studies. J Gen Microbiol. 1967 Nov;49(2):335–349. doi: 10.1099/00221287-49-2-335. [DOI] [PubMed] [Google Scholar]
- Saedler H., Reif H. J., Hu S., Davidson N. IS2, a genetic element for turn-off and turn-on of gene activity in E. coli. Mol Gen Genet. 1974;132(4):265–289. doi: 10.1007/BF00268569. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Identification of polypeptides necessary for chemotaxis in Escherichia coli. J Bacteriol. 1977 Jun;130(3):1317–1325. doi: 10.1128/jb.130.3.1317-1325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thipayathasana P., Valentine R. The requirement for energy transducing ATPase for anaerobic motility in Escherichia coli. Biochim Biophys Acta. 1974 Jun 28;347(3):464–468. doi: 10.1016/0005-2728(74)90083-8. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Hozumi N., Matthyssens G., Schuller R. Somatic changes in the content and context of immunoglobulin genes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):877–889. doi: 10.1101/sqb.1977.041.01.097. [DOI] [PubMed] [Google Scholar]
- Toussaint A. The DNA modification function of temperate phage Mu-1. Virology. 1976 Mar;70(1):17–27. doi: 10.1016/0042-6822(76)90232-4. [DOI] [PubMed] [Google Scholar]
- Zieg J., Hilmen M., Simon M. Regulation of gene expression by site-specific inversion. Cell. 1978 Sep;15(1):237–244. doi: 10.1016/0092-8674(78)90098-3. [DOI] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]