Abstract
Rationale
Marijuana is believed to increase impulsivity and risk taking, but the processes whereby it affects such behaviors are not understood. Indeed, either the pharmacologic effect of delta-9-tetrahydrocannabinol (THC) or the expectancy of receiving it may lead to deficits in cognitive processing and increases in risk taking.
Objectives and methods
We examined the relative effects of expecting to receive active marijuana and the pharmacological drug effects using a balanced placebo design. Young adult regular marijuana users (N=136) were randomly assigned into one of four groups in a two × two instructional set (Told THC vs. Told no THC) by drug administration (smoked marijuana with 2.8 % THC vs. placebo) design. Dependent measures included subjective intoxication, behavioral impulsivity, and decision-making related to risky behaviors.
Results
Active THC, regardless of expectancy, impaired inhibition on the Stop Signal and Stroop Color-Word tasks. Expectancy of having smoked THC, regardless of active drug, decreased impulsive decision-making on a delay discounting task among participants reporting no deception and increased perception of sexual risk among women, consistent with a compensatory effect. Expectancy of smoking THC in combination with active THC increased negative perceptions from risky alcohol use. Active drug and expectancy independently increased subjective intoxication.
Conclusions
Results highlight the importance of marijuana expectancy effects as users believing they are smoking marijuana may compensate for expected intoxication effects when engaged in deliberate decision-making by making less impulsive and risky decisions. Effects of marijuana on impulsive disinhibition, by contrast, reflect direct pharmacologic effects for which participants did not compensate.
Keywords: THC, Cannabis, Expectancy, Impulsivity, Inhibition, Risk taking, Sexual risk
Introduction
Marijuana use has been associated with behavioral problems such as motor vehicle accidents (Drummer et al. 2004; Ramaekers et al. 2004; Wadsworth et al. 2006) and risky sexual behaviors (Costa et al. 1996; Fernandez et al. 2004; Shrier et al. 1996; Simons et al. 2010). However, the mechanisms accounting for these associations are not fully understood. In particular, the limited number of studies on marijuana and impulsivity or risk taking has not separated pharmacologic from expectancy effects. It is known that expectancies can influence responses to other drugs, such as alcohol. Alcohol expectancies can either augment or temper the expression of disinhibited behavior in a drinking situation (Testa et al. 2006). Thus, expectancies may also influence cognitive processing or risk taking after marijuana. The purpose of the present study was to examine the expectancy that marijuana was smoked independently from the pharmacologic effect of delta-9-tetrahydrocannabinol (THC) on two behavioral domains of impulsivity: disinhibition and risky decision-making (de Wit 2009; Meda et al. 2009; Reynolds et al. 2006a).
There is some evidence that marijuana impairs the capacity to inhibit already initiated responses, as measured by the Stroop Color-Word (Stroop 1935) and Stop Signal tasks (Logan et al. 1997). In one study (Hooker and Jones 1987), marijuana (1.2 % THC) impaired the ability to inhibit inappropriate responding in light users, and in another study, marijuana (Hart et al. 2001; 3.9 % THC) impaired reaction time in heavy users. Oral THC (15, 17, and 35 mg) increased impulsive responding on the Stop task in recreational marijuana users (McDonald et al. 2003; Ramaekers et al. 2006) but did not affect performance on a Go/No-Go task, which calls for cognitive in addition to motor inhibition (McDonald et al. 2003).
There is also evidence that marijuana increases risky decision-making or maladaptive choices involving the evaluation of outcomes. Chronic marijuana users display impairments in decision-making by choosing larger immediate gains despite more costly losses on the Iowa Gambling task (Bolla et al. 2005; Whitlow et al. 2004), although these differences may have pre-dated use of the drug. Findings with acute intoxication are mixed. Marijuana (17 or 35 mg THC) did not affect performance on the Iowa Gambling task in light marijuana users (Ramaekers et al. 2006) or in heavy users at similar dose levels (Vadhan et al. 2007), but performance was affected by a 17-mg THC dose in daily marijuana users (Weinstein et al. 2008). In another study (Lane et al. 2005), smoked marijuana (3.58 % THC) increased risky responding on a computerized risk task in recreational marijuana smokers, relative to a 1.77 % THC and placebo.
Acute doses of THC have also been tested using a measure of impulsive decision-making, i.e., discounting of negative consequences or of delayed rewards. Delay discounting, the tendency to choose more immediate smaller rewards over larger delayed rewards (Rachlin et al. 1991), is quantified with either hypothetical (Richards et al. 1999) or experiential (Reynolds and Schiffbauer 2004; Reynolds 2006) tasks. In one study (McDonald et al. 2003), oral THC (7.5 and 15 mg) did not alter discounting on a hypothetical discounting task in recreational smokers. It remains to be determined whether THC or whole plant marijuana affects discounting using other measures, such as the real-time measure that has been sensitive to acute alcohol effects (Reynolds et al. 2006b) and sleep deprivation (Reynolds and Schiffbauer 2004).
Several studies have examined the effects of marijuana on risk taking. Marijuana is known to impair driving (Bates and Blakely 1999; Ramaekers et al. 2000, 2004; Sexton et al. 2000), possibly due to an increase in risk taking. Its effects on other behaviors related to risk taking, such as unsafe sexual behavior, remain to be determined, especially using reliable measures of intention and perception of sexual risk taking that are known to be sensitive to the effects of drugs (e.g., alcohol; Fromme et al. 1997).
There is evidence that THC contributes to the effects of marijuana, from studies where THC content was varied under blinded conditions. However, these studies failed to take into account the participants’ expectancies of receiving an active drug. Usually, marijuana or THC studies use a double-blind placebo-controlled design with no clear instructional set manipulation (i.e., subjects told they may or may not ingest THC), so that the contribution of expectancies is unknown. The expectation of receiving a drug effect can produce subjective and behavioral effects even in the absence of the drug itself (Marlatt and Rohsenow 1980; Vogel-Sprott and Fillmore 1999). This expectancy, which may develop from either prior experiences with the drug or through learned communications about the drug’s effects, can either produce behaviors like the drug or behaviors opposite to the drug. Metrik et al. (2009, 2011a) has shown that expectancy of receiving THC produces greater THC-like subjective effects. However, expecting to receive marijuana may increase risky behavior or it may decrease it through compensatory behaviors to counteract the expected effect (e.g., exerting greater care) (e.g., Bates and Blakely 1999). The present study used the balanced placebo design (BPD) to examine marijuana’s pharmacologic and stimulus expectancy effects on impulsive disinhibition and impulsive decision-making in experienced weekly marijuana users. We hypothesized that both THC and instructions that THC was smoked (i.e., stimulus expectancy) would increase impulsive disinhibition on Stop Signal and Stroop Color-Word tasks and increase impulsive/risky decision-making on the experiential measure of delay discounting, a measure of risk taking (the Balloon Analogue Risk task) and a measure of cognitive appraisal of sexual and substance use-related risks. We predicted that both the drug and the expectation of receiving the drug would increase impulsive and risky behaviors.
Methods
Design and randomization
The study involved a two × two randomized factorial design crossing drug administration (2.8 % THC or 0 % THC) with instructional set (Told THC or Told Placebo). Using urn randomization (Wei 1978), the participants were randomized to one of four conditions (n=34 per condition) balancing on sex, college status, and tobacco smoking. The four groups were Told THC/Received THC, Told THC/Received Placebo, Told Placebo/Received THC, and Told Placebo/Received Placebo. The participants were informed that the study evaluated the effects of marijuana on mood and behavior,1 and that they would be randomly assigned to smoke one marijuana cigarette that contained THC or one marijuana placebo cigarette with THC removed.
Participants
The study was approved by the Institutional Review Board of Brown University. Marijuana smokers (N=136) were recruited through newspaper advertisements, flyers, and social media websites. The participants met the following inclusion criteria: native English speakers, 18 to 30 years of age, marijuana use at least once a week in the past month and at least ten times in the past 6 months (to generalize to regular users), and self-reported ability to abstain from marijuana for 24 h without withdrawal (to avoid confounding effects). Exclusion criteria were: history of substance abuse treatment and intent to quit or receive treatment for cannabis abuse; use of other illicit drugs; pregnancy; nursing; past month affective disorder or history of panic attacks, psychotic, or suicidal state assessed by psychiatric interview; alcohol dependence; contraindicated medical issues by physical exam; 20+ tobacco cigarettes a day; and prior knowledge about the study procedures or contact with participants.
Procedure
The participants completed a baseline non-smoking and an experimental smoking session on average of 14.7 (SD=8.4) days apart. The participants were told to abstain from marijuana and tobacco smoking for 12 h, alcohol for 24 h, and caffeine for 1 h before both sessions. An alveolar carbon monoxide (CO) of ≤6 ppm was used to confirm no recent smoking (Cooper and Haney 2009; Metrik et al. 2011a) with a Bedfont Scientific Smokelyzer®. Tobacco smokers were given an opportunity to smoke a tobacco cigarette following the CO test to prevent nicotine withdrawal at the second session. Zero breath alcohol concentration was verified with an Alco-Sensor IV (Intoximeters, Inc., St Louis, MO., USA). Positive THC urine screens were obtained from 84 % of participants at baseline and from 89 % at the beginning of the smoking session.
Sessions occurred in a 75-ft2 ventilated smoking room with a one-way mirror and intercom. At baseline, participants completed questionnaires and impulsivity and risk taking tasks to provide within-subjects control with repeated measures. At the second session, subjective effects and heart rate were assessed, and the participants were then instructed about which cigarette they were assigned to smoke. Explicit instructions about THC’s psychoactive properties were accompanied by several procedures (saliva sample post-smoking and placebo cigarettes rolled in purple grape cigarette paper) enhancing the credibility of the expectancy manipulation (for details, see Metrik et al. 2009). After smoking, we assessed the heart rate, subjective effects, impulsivity tasks in a counterbalanced order, and manipulation checks (Metrik et al. 2009). Assessments were timed to capture the peak drug effect within 90 min after smoking. The participants in Told THC or Received THC conditions remained in the laboratory for 4 h after smoking, passed a field sobriety test, and were transported home in a taxi. Participants were fully debriefed regarding the deception following the completion of the study. All were paid $145 and an additional $10–28 per session from certain impulsivity tasks.
Marijuana administration
Marijuana cigarettes (placebo or 2.8 % THC) were provided by the National Institute on Drug Abuse, rolled at both ends, humidified, and smoked according to the standardized paced puffing procedure (Foltin et al. 1987) until the ash reached a mark 10 mm from the end. This THC dose significantly affected subjective measures and impulsivity tests (e.g., Lukas et al. 1995; McDonald et al. 2003) yet permitted deception when in Told Placebo conditions (Metrik et al. 2009).
Baseline individual difference measures
The Time-Line Follow-Back (Dennis et al. 2004) assessed past 60-day number of marijuana and tobacco cigarette use days.
Dependent measures
Drug effects Subjective effects of marijuana were assessed twice with the ARCI-Marijuana scale (ARCI; Chait et al. 1985; Martin et al. 1971) at 12 min from the start of smoking and after the completion of all computer tasks, at 108 min on average. Heart rate (in beats per minute) was recorded prior to smoking and at 16 min from the start of smoking via a blood pressure cuff attached to the non-dominant arm (Datascope Accutorr Plus NIBP).
Impulsive disinhibition
Stroop Color-Word task (Stroop 1935) presented a practice trial followed by two trial blocks (48 stimuli per trial) in which the participants were asked to press as quickly as possible the designated key on the keyboard first in response to the color of a symbol string (e.g., XXXX) in four colors and then the color of the color-incongruent word. Different task versions were counterbalanced across the two study sessions. The primary dependent variable was response latency in milliseconds on color-incongruent trials. The number of correct responses was also recorded as a measure of general performance. Data for one color-blind participant were excluded. The Stop Signal task (Logan et al. 1997) measures inhibition of a prepotent response with two concurrent tasks: (1) the go task is a choice reaction–time task that requires participants to rapidly discriminate two symbols (maximum presentation 1,250 ms) and (2) the stop task involves presentation of a tone (75 ms, 1,000 Hz) that signals one to inhibit the response to the go task. The tone was presented following the visual stimuli at different delays (initial delay at 200 ms) on a quarter of the trials (32 practice trials followed by three blocks of 64 trials). The delay time to the stop signal was adjusted until the participant inhibited responses on approximately 50 % of trials. The time in milliseconds required for the participant to stop the go response (stop signal reaction time, SSRT) was calculated by subtracting the mean stop signal delay from the mean go reaction time. Data from 14 participants (7 smoked active marijuana and 7 smoked placebo) were excluded because they almost always (90–100 % of trials) failed to inhibit the stop signal, possibly due to poor comprehension of instructions (Band et al. 2003; Logan 1994; Verbruggen and Logan 2008).
Impulsive decision-making
The Experiential Discounting task (EDT; Reynolds and Schiffbauer 2004) measures tendency to choose more immediate smaller rewards over larger delayed rewards. During a block of trials, the participants were presented with a series of choices between a standard amount ($0.30) that was probabilistic (35 % chance of receiving) delivered either immediately during one block or after a delay (28 s) during another block and an adjusting amount of money (initially set to $0.15) that was always delivered immediately and was certain. The participants were told that if they chose the standard 30 cents, the next adjusting choice would increase, while choosing the immediate amount caused the next adjusting choice to decrease. The coins earned were delivered from a coin dispenser (totaling $3–25 per session). The participants made choices during both blocks (0 and 28 s delay) until the amount for an immediate choice was adjusted to a point of indifference (i.e., an equal number of choices to both the standard and immediate options). The primary dependent variable was the indifference point for the 28-s delay session adjusted by the score from the 0-s delay session (ranging from 0.0 (steepest possible discounting) to 1.0 (no discounting)). Data for one participant were lost due to technical problems. Delay Discounting Questionnaire (DDQ; Richards et al. 1999) uses a computerized adjusting amount procedure to measure discounting of delayed monetary reinforcers. In a series of choice trials, the participants were offered the hypothetical choice between $10 available after a delay (0, 2, 30, 180, and 365 days) or a smaller amount available immediately. The primary dependent variable was area under the curve connecting indifference points and the x-axis, from 0.0 (steepest discounting) to 1.0 (no discounting) (Myerson et al. 2001). The Balloon Analogue Risk Task, automatic response version (BART; Lejuez et al. 2002; Pleskac et al. 2008), is a computerized behavioral measure of risk taking. Thirty balloon images were presented one at a time with participants designating the number of pumps to have each balloon inflated. Each pump earned 1 cent, but money was lost if the balloon exploded (at 64 pumps on average). The primary dependent variable was the average number of pumps across trials. The participants received cash earned (ranging from $2 to 11) after the session. The Cognitive Appraisal of Risky Events Questionnaire-Revised (CARE-R; Fromme et al. 1997; Katz et al. 2000) assesses perceived likelihood of negative (risks) and positive consequences (benefits) of unsafe sexual behavior with a non-exclusive dating partner(s) (11 items) and includes five gender-specific questions on coercive sexual behavior, four questions on illicit drug use, and seven items on alcohol-related risky activities. All are rated on Likert scales from 1 (not at all likely) to 7 (extremely likely). The mean composite scores for risks and benefits of (1) alcohol-related behaviors such as heavy drinking and drunk driving, (2) illicit drug use, (3) unsafe sexual behaviors with non-exclusive partner, and (4) coercive sex within each gender (Cronbach’s alphas, .66–.97) were used.
Data analysis plan
Baseline differences between experimental conditions were tested with ANOVA and chi-square tests. We examined effectiveness of instruction set manipulation in terms of percent reporting deception per condition (Metrik et al. 2009). We then tested drug and stimulus expectancy effects on heart rate with linear regression covarying baseline heart rate. Subjective intoxication effects were tested with a two (times after smoking, within-groups) × two (drug, between-groups) × two (stimulus expectancy, between-groups) repeated measures ANOVA covarying baseline scores. Linear regression was used to test the two experimental effects, pharmacologic effect, and stimulus expectancy effect, on the Stroop Color-Word, Stop Signal, the EDT, the DDQ, and the BART. In these models, the centered baseline value of the respective dependent variable and dummy-coded task order (first task set to a reference group) were entered on the first step, the main effects of stimulus expectancy and drug manipulations were entered second, and the stimulus expectancy by drug manipulation interactions were added on the third step.
Four two (drug) × two (stimulus expectancy) multivariate analyses of covariance (MANCOVAs) were performed, one for each of the four risky activities on the CARE-R (alcohol risks, illicit drug use, risky sex with a non-exclusive partner, and coercive sex), entering negative and positive outcomes into each multivariate test. Baseline CARE-R risk and benefit values and task order were included as covariates in these models. Analyses of the CARE-R sexual behaviors included sex as a covariate in the models (George and Stoner 2000). Sex differences on cognitive tasks following marijuana smoking were not expected (Anderson et al. 2010) and not found when tested. Significant multivariate effects were followed with univariate tests on individual risk and benefit scales. Drug by expectancy interactions were not hypothesized and were nonsignificant across all measures, with the exception of CARE-R alcohol-related risk questions. All analyses were conducted in SPSS 19.0 for Windows.
Results
The four experimental groups did not differ on any of the descriptive variables (see Table 1), and the two experimental conditions did not differ on baseline impulsivity tasks (see Table 2).
Table 1.
Demographics and substance use sample characteristics (N=136) by four experimental groups
| Variable | TTRT
|
TPRT
|
TTRP
|
TPRP
|
||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Age | 21.3 | 3.5 | 21.3 | 3.7 | 21.8 | 2.8 | 21.2 | 2.4 |
| Marijuana initiation age | 15.4 | 1.6 | 14.9 | 1.5 | 15.3 | 1.6 | 15.5 | 1.6 |
| Marijuana regular use age | 16.6 | 0.7 | 16.4 | 0.9 | 16.4 | 1.0 | 16.3 | 1.2 |
| % marijuana use days | 42.4 | 25.4 | 39.4 | 24.1 | 39.3 | 24.9 | 43.7 | 23.9 |
| Times used marijuana on an average day | 1.9 | 1.1 | 1.7 | 0.9 | 1.8 | 1.0 | 1.8 | 0.9 |
| Number alcohol drinks/week | 7.22 | 7.76 | 7.46 | 11.26 | 7.89 | 8.07 | 8.33 | 8.94 |
| % heavy drinking days | 10.02 | 11.41 | 10.88 | 15.41 | 11.91 | 14.24 | 11.82 | 13.23 |
| % smoking tobacco days | 68.0 | 36.8 | 60.6 | 43.9 | 41.8 | 37.2 | 42.7 | 42.2 |
| Number of cigarettes per day (for n=66 tobacco smokers) | 6.87 | 5.96 | 4.67 | 4.74 | 4.79 | 4.99 | 4.16 | 3.96 |
| n | % | n | % | n | % | n | % | |
| Men | 23 | 68 | 21 | 62 | 22 | 65 | 22 | 65 |
| Caucasiana | 21 | 62 | 23 | 68 | 20 | 59 | 25 | 74 |
| African-American | 3 | 9 | 1 | 3 | 3 | 9 | 2 | 6 |
| American Indian/Alaskan Native | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asian-American | 2 | 6 | 2 | 6 | 2 | 6 | 1 | 3 |
| Mixed ethnic origin/other | 5 | 15 | 6 | 18 | 8 | 24 | 5 | 15 |
| Hispanic ethnicity | 6 | 18 | 4 | 12 | 3 | 9 | 2 | 6 |
| Married or living together | 2 | 6 | 2 | 6 | 6 | 18 | 5 | 15 |
| In college | 24 | 71 | 21 | 62 | 22 | 65 | 23 | 68 |
| Marijuana ounces used per week | ||||||||
| Less than 1/16th | 7 | 21 | 6 | 18 | 10 | 29 | 10 | 29 |
| 1/16th | 7 | 21 | 9 | 27 | 8 | 24 | 7 | 21 |
| 1/8th | 5 | 15 | 6 | 18 | 6 | 18 | 5 | 15 |
| 1/4th | 8 | 24 | 3 | 9 | 3 | 9 | 6 | 18 |
| More than 1/4th | 7 | 21 | 10 | 29 | 7 | 21 | 6 | 18 |
| DSM-IV cannabis dependenceb | 1 | 3 | 3 | 9 | 1 | 3 | 3 | 9 |
TTRT Told THC/Received THC, TPRT Told Placebo/Received THC, TTRP Told THC/Received Placebo, TPRP Told Placebo/Received Placebo
Refers to non-Hispanic White
Refers to ≥3 dependence symptoms in the past 12 months. Percentages are based on available data per group
Table 2.
Baseline and post-smoking means (SD) for stimulus expectancy (Told) and drug (Received) experimental conditions on heart rate, subjective intoxication, and impulsivity tasks
| Baseline Told
|
Post-smoking Told
|
Baseline Received
|
Post-smoking Received
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THC
|
Placebo
|
THC
|
Placebo
|
THC
|
Placebo
|
THC
|
Placebo
|
|||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Primary dependent measures | ||||||||||||||||
| SSRT | 268.46 | 70.20 | 252.63 | 57.24 | 256.39 | 61.14 | 252.73 | 48.44 | 257.72 | 76.42 | 263.11 | 49.49 | 263.75* | 61.30 | 245.31 | 46.25 |
| Stroop latency | 832.13 | 163.08 | 833.23 | 122.87 | 802.41 | 208.23 | 826.03 | 364.27 | 838.30 | 156.29 | 827.15 | 131.03 | 851.32 | 386.79 | 777.85 | 159.97 |
| EDT | 0.71 | 0.27 | 0.73 | 0.23 | 0.70* | 0.27 | 0.64 | 0.27 | 0.72 | 0.25 | 0.73 | 0.24 | 0.68 | 0.28 | 0.66 | 0.27 |
| BART | 61.80 | 9.25 | 58.65 | 13.20 | 61.78 | 9.64 | 60.10 | 12.74 | 59.61 | 13.06 | 60.84 | 9.67 | 60.87 | 12.20 | 61.00 | 10.40 |
| Other dependent measures | ||||||||||||||||
| Heart rate | 73.32 | 12.24 | 75.78 | 12.15 | 92.43 | 25.30 | 94.44 | 28.52 | 75.16 | 13.06 | 73.94 | 11.36 | 113.50*** | 22.79 | 73.37 | 10.91 |
| ARCI-M | 1.23 | 1.59 | 1.32 | 1.44 | 3.77* | 3.00 | 2.90 | 2.82 | 1.56* | 1.63 | 1.00 | 1.34 | 5.20*** | 2.72 | 1.47 | 1.71 |
| DDQ | 0.47 | 0.28 | 0.45 | 0.27 | 0.45 | 0.29 | 0.44 | 0.28 | 0.49 | 0.28 | 0.42 | 0.26 | 0.47 | 0.29 | 0.42 | 0.28 |
| Stroop accuracy | 45.09 | 5.66 | 45.94 | 2.49 | 44.78 | 4.87 | 44.96 | 4.77 | 45.33 | 5.66 | 45.71 | 2.54 | 44.04* | 6.41 | 45.68 | 2.09 |
Raw (unadjusted by covariates) means are shown. Heart rate is measured as beats per minute. ARCI-M=ARCI-Marijuana scale summary score (possible range 0–12). Stroop latency of response and SSRT (Stop Signal Reaction Time) are measured in milliseconds. Stroop accuracy represents number of correct responses on color-incongruent trials. EDT (Experiential Discounting Task) and DDQ (Delay Discounting Questionnaire) scores ranged from 0.0, steepest possible discounting, to 1.0, no discounting. BART (Balloon Analogues Risk Task) scores represent average number of pumps across balloon trials. All dependent measures are based on N=136 with the exception of Stroop variables (n=135), SSRT (n=122), and EDT (n=135)
p<.05;
p<.001 (significant pharmacologic or expectancy effect of THC relative to placebo. Post-smoking comparisons covary baseline values).
Manipulation checks
Suspicions about THC content were reported by two participants (6 %) in the Told THC/Received Placebo condition and by seven (21 %) in the Told Placebo/Received THC condition. No one in other conditions endorsed any deception. Following other BPD studies (Juliano and Brandon 2002; Kelemen and Kaighobadi 2007; Perkins et al. 2004), analyses were conducted on all participants; analyses excluding participants for whom the deception failed produced the same findings on all measures and are therefore not presented. The one exception was the EDT where the effect of expectancy manipulation became significant in the partial sample.
Heart rate and subjective intoxication effects
Table 2 lists baseline and post-smoking means (SD) for the two conditions: stimulus expectancy (Told THC vs. Placebo) and drug (Received THC vs. Placebo) on heart rate, subjective intoxication, and impulsivity tasks. THC significantly increased heart rate relative to placebo at 16 min after the start of the smoking (B=39.27, SE=2.70, sr2=.54, p<.001), with no significant main or interaction effects with stimulus expectancy. The significant main effects of drug, F (1, 125)=77.23, p<.001, and expectancy, F (1, 125)=6.32, p=.01, were seen for ARCI-M indicating that receiving THC vs. placebo and being told THC vs. placebo were independently associated with greater subjective drug effects. A significant drug by time interaction, F (1, 125)=6.67, p=.01, showed that the effects of receiving THC were greater immediately post-smoking than at the end of the post-smoking period. The stimulus expectancy manipulation by time interaction was nonsignificant.
Impulsive disinhibition tasks
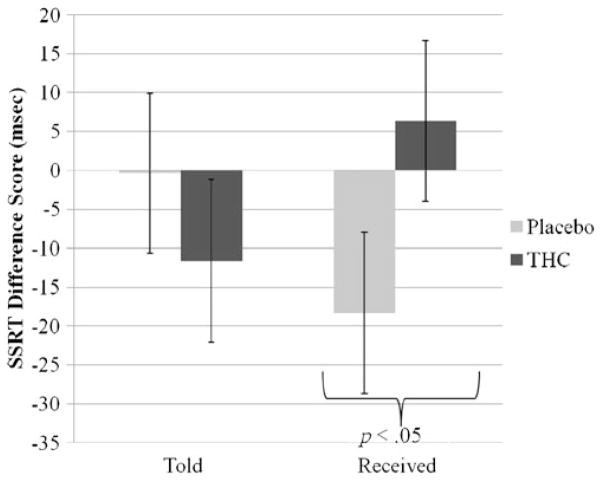
Relative to placebo, THC significantly reduced total number of correct answers on color-incongruent trials (B=−1.28, SE=.57, sr2=.02, p=.03) but not on color-congruent trials on the Stroop Color-Word task, with no effect on average response latency. Relative to placebo, THC also increased time to inhibit a prepotent response (SSRT: B= 19.76, SE=9.72, sr2=.03, p=.04, see Fig. 1), without significantly affecting the Go task reaction time. Although the THC effect was of small magnitude, a larger change from baseline to post-smoking occurred in the placebo condition (see Fig. 1). Exploratory post hoc t tests to understand this large placebo effect revealed that, compared to baseline, performance was significantly improved in the Told THC/Received Placebo condition (paired t (df=29)=3.36, p=.002) but not in the Told Placebo/Received Placebo condition. There was also a significant effect of task order (B=28.88, SE=14.12, sr2=.03, p=.04) such that, relative to Stop Signal tasks completed first after the smoking, Stop tasks completed towards the end of the session led to increased impulsivity. There was no main effect or interaction with stimulus expectancy manipulation on the Stroop or the Stop Signal tasks.
Fig. 1.
Mean Stop Signal Reaction Time (SSRT) difference scores (baseline subtracted from post-smoking values) by two experimental factors: stimulus expectancy (Told) and drug (Received). Relative to placebo, THC significantly increased SSRT (time to inhibit a prepotent response). Effect of stimulus expectancy manipulation (Told THC vs. Told Placebo) was nonsignificant
Impulsive decision-making tasks
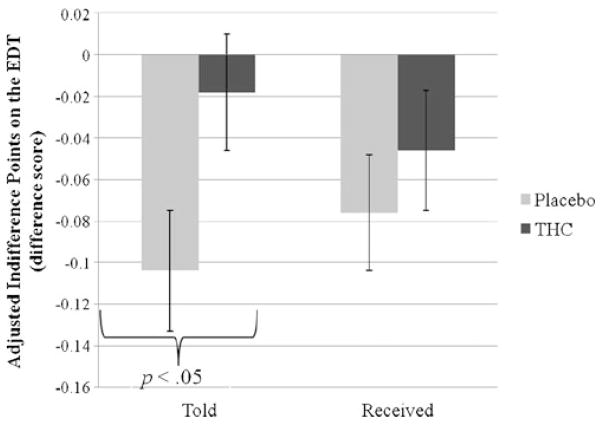
On the EDT, the main effect of the drug was nonsignificant and the main effect of stimulus expectancy was at a trend level (B=.07, SE=.04, sr2=.02, p=.06). In the EDT analysis without the participants for whom the deception failed (n =126), this effect of expectancy was significant (B=.09, SE=.04, sr2=.03, p=.02) (see Fig. 2). Those in Told THC condition discounted delayed rewards less than those in Told Placebo. On the DDQ and BART, there were no main effects of stimulus expectancy or drug manipulation.
Fig. 2.
Mean adjusted indifference point difference scores (baseline subtracted from post-smoking values) on the EDT task (ranging from 0.0 (steepest possible discounting) to 1.0 (no discounting)) by two experimental factors: stimulus expectancy (Told) and drug (Received). Relative to placebo, THC did not significantly change delayed discounting. Those in Told THC condition discounted delayed rewards less than those in Told Placebo condition
Substance use risk decision-making (CARE-R)
MANCOVA tests revealed a significant drug by expectancy interaction effect for alcohol-related risk behaviors: Wilks’ Lambda=.95, F (2, 126)=3.33, p=.04. Univariate tests (B=.82, SE=.40, sr2=.02, p=.04) indicated that among those who are in Received THC condition, those in Told THC condition rated negative consequences of alcohol risk behaviors as more likely than those in Told Placebo condition (sr2=.07, p=.01). Among those who are in Received Placebo condition, there was no significant association between the expectancy manipulation and alcohol-related risky decisions. There were no significant univariate effects for expected benefits from alcohol and no significant multivariate effects for illicit drug use.
Sexual risk decision-making (CARE-R)
MANCOVA tests revealed a significant drug effect for risky sex with non-exclusive partner: Wilks’ Lambda=.93, F (2, 125)=4.60, p=.01. Univariate tests indicated that relative to the Received Placebo condition, those in the Received THC conditions rated benefits of risky sex with a non-exclusive partner as less likely (B=−.44, SE=.14, sr2=.04, p=.003); there were no univariate effects on negative consequences from risky sex. There were no experimental main or interaction effects of stimulus expectancy. Main or interaction effects of drug were not significant in the sex-specific multivariate coercive sex models; multivariate effects of stimulus expectancy were only at a trend level (p=.07) for both men and women. Univariate tests indicated that women in the Told THC conditions significantly increased perceived likelihood of risks from coercive sex relative to women in the Told Placebo conditions (B=.79, SE=.35, sr2=.07, p=.03); there were no significant effects in the benefits model of coercive sex among women or men, or for risks for men.
Discussion
This is the first evaluation of the independent and combined effects of smoking and expecting to smoke active marijuana on multiple measures of risk taking and impulsivity, using the balanced placebo design. The pharmacological effect of THC somewhat contributed to impaired inhibition, whereas expectancy of having smoked THC affected impulsive decision-making, with a compensatory direction of effect on some measures of risky decision-making. Expectancy of smoking THC in combination with active THC increased perceptions of risk from risky alcohol use. Independently from the THC administration, expectancy also increased perceived risk from coercive sex among women. Conversely, receiving THC independent from the instructions about the THC dose decreased expected positive consequences from risky sex. Both THC dose and marijuana expectancy independently increased subjective intoxication.
Although THC significantly increased impulsive responding on two behavioral tasks of impulsive disinhibition, marijuana’s effect on these tasks was of small magnitude, and on the Stroop task, only accuracy but not response latency was somewhat affected. These changes may not be clinically meaningful and are consistent with another marijuana study (Hooker and Jones 1987). Similar findings in both light and heavy users (McDonald et al. 2003; Ramaekers et al., 2006; Ramaekers et al. 2009) indicate that THC may not reliably decrease either motor inhibition, which requires rapid response control, as measured by the Stop Signal task or affect cognitive inhibition, which controls processes such as sustained attention and interference, as indexed by the Stroop task.
The absence of THC’s pharmacologic effect on delay discounting adds to the extant empirical findings that suggest that marijuana intoxication (McDonald et al. 2003) is only weakly associated with delay discounting in comparison to other drugs such as tobacco, cocaine, opioids, and alcohol (Johnson et al. 2010). Because an Experiential Discounting Task was employed in the present study, the non-significant results cannot be attributed to the hypothetical nature of rewards, as had been suggested for other studies (McDonald et al. 2003). Results suggest that marijuana does not impair this aspect of behavior control to the extent that alcohol or other drugs do (Fillmore 2003).
Marijuana expectancy significantly affected performance on the experiential but not on the hypothetical delay discounting task in a subsample of participants for whom BPD deception worked, but in the opposite direction than was hypothesized. Across measures that involved an evaluative component, an individuals’ expectation that marijuana was used made them more cautious in decisions and led to increased awareness of risks. This trend level effect was evident on a delay discounting task and for women on measures of sexual risk-related judgment, independent of the actual THC dose, and on judgments of risks from alcohol use, when active THC was also smoked. Similarly, our exploratory analysis of the apparent improvement in inhibitory control on the Stop Signal task in response to placebo revealed that placebo participants that were told they were smoking marijuana may have compensated for expected intoxication effect by increasing inhibitory control, while placebo participants that were told they were smoking placebo did not change their performance after the smoking. Compensatory behavior has been previously demonstrated with marijuana users in relation to reported perception of safer driving due to reduced speed when under the influence of marijuana (Bates and Blakely 1999). Similarly, in an alcohol study, the participants discounted delayed rewards less steeply, and the indifference points were higher (i.e., indicating less impulsivity) in the high-alcohol than the no-alcohol condition (Ortner et al. 2003). Finally, the participants who were led to expect impairment from alcohol or caffeine performed better than those led to expect enhancement of performance (Fillmore et al. 1994; Harrell and Juliano 2009). Our findings that women expecting marijuana, relative to those expecting placebo, rated negative consequences from coercive sex as more likely are entirely consistent with an alcohol study in which placebo condition women who believed they were drinking responded to a hypothetical scenario of aggressive sexual advancement with increased vigilance and caution (Testa et al. 2006). Nevertheless, stimulus expectancy effects were mostly at a trend level or, contrary to our expectation, dependent on the drug effect, which warrants further replication.
A noteworthy pattern of findings emerges from this study, such that pharmacologic effects of small magnitude were evident on inhibition tasks, and stimulus expectancy effects were more pronounced (either in combination or independently from the drug effect) on impulsive decision-making measures that reflect deliberate choice-making involving the evaluation of outcomes. This pattern of findings is consistent with alcohol placebo meta-analyses demonstrating that alcohol’s pharmacologic effects predominate over alcohol expectancy effects in the memory or motor performance behavioral domains (Hull and Bond 1986; Testa et al. 2006). However, findings in the area of sexual risk decision-making are mixed and studies that employed placebo are too few to draw firm conclusions (Testa et al. 2006). In our study, marijuana did not acutely influence performance on the BART, suggesting that this behavioral task was not a sensitive measure of acute drug effect. Indeed, neither alcohol (Reynolds et al. 2006b) nor nicotine (Wignall and de Wit 2011) has acutely altered BART performance.
Limitations
Because deception is increasingly hard to maintain in the Told Placebo Received Drug condition with higher drug doses (Martin and Sayette 1993), we had tested a moderate concentration of THC and larger doses may have produced greater effects. Results may not generalize to less experienced users with less tolerance (Hart et al. 2001; Kirk et al. 1998; Ramaekers et al. 2009; Vadhan et al. 2007). Our sexual risk taking findings are inherently confined to hypothetical measures (Crowe and George 1989).
Conclusions
Our results are consistent with other studies that demonstrate mostly minimal or subtle deficits in executive functions due to acute effects of marijuana in regular smokers (Hart et al. 2001; Pope et al. 2001; Vadhan et al. 2007) and that show that THC can affect some mechanisms underlying impulsive behaviors while not affecting others (McDonald et al. 2003). This study extended prior research by using the BPD to derive more precise estimates of the pure pharmacological effect of marijuana separate from the potential confound of marijuana expectancy. Receiving THC, and to a lesser extent expecting that one is using marijuana, altered decision-making processes by increasing perceptions of risk, consistent with prior research on marijuana and driving (Bates and Blakely 1999; Sexton et al. 2000). Such alterations, which were generally of small magnitude, may counter the relatively mild effects of THC on inhibitory control, ultimately tempering the expression of disinhibited behaviors following marijuana use. Heightened cognitive processing of risk associated with marijuana smoking may help explain the largely negative or equivocal findings on marijuana’s associations with risky sex (Brodbeck et al. 2006; Leigh et al. 2008), smoking relapse (Metrik et al. 2011b), discounting of delayed consequences (Johnson et al. 2010), and other risk behaviors typically implicated in alcohol involvement. These conclusions about marijuana’s effects on impulsivity and risk taking are to some extent congruent with the public view of marijuana being less associated with harmful consequences than alcohol and some other drugs (Nutt et al. 2007). Future studies should consider examining individual differences (e.g., use history, executive memory, outcome expectancies, and variability in cannabinoid-related genetics) that may moderate behavioral responses to THC or placebo.
Acknowledgments
This study was supported by a grant R01 DA021403 from the National Institute on Drug Abuse to Jane Metrik, a Research Career Development Award from the Medical Research Service of the Department of Veteran Affairs to John McGeary, and Senior Research Career Scientist awards from the Department of Veteran Affairs to Drs. Monti and Rohsenow. The authors gratefully acknowledge Dr. James Harper, III, Amy Mochel, Suzanne Sales, Timothy Souza, and Adrienne Umali for their contribution to the project.
Footnotes
Participants in the “Told THC” conditions were told, “You have been assigned to the condition to smoke an active marijuana cigarette that contains THC. THC is the primary psychoactive cannabinoid that ‘gets people high.’ The dosage of THC contained in each cigarette is considered to be a moderate dose for the average person. Marijuana cigarettes came from the National Institute of Drug Abuse and marijuana was grown by the government for research purposes. In this condition, we need to test how smoking marijuana will affect your mood, responses, and behavior. Participants in this condition will be compared to others who receive placebo without any THC.” Participants in the “Told Placebo” conditions were told, “You have been assigned to the condition to smoke a placebo cigarette. A marijuana placebo cigarette does not contain THC. THC, the active ingredient that gets people high, along with other cannabinoids, was extracted from the cigarettes by the National Institute on Drug Abuse, which provides them for research. Smoking this placebo cigarette will have no effect on your mood and behavior. In this condition, we need to test how people operate in a normal state of consciousness while engaging in all other actions associated with smoking. Participants in this condition will be compared to others who receive marijuana with THC.”
Contributor Information
Jane Metrik, Email: Jane_Metrik@brown.edu, Center for Alcohol and Addiction Studies, Brown University, Box G-S121, Providence, RI 02912, USA.
Christopher W. Kahler, Center for Alcohol and Addiction Studies, Brown University, Box G-S121, Providence, RI 02912, USA
Brady Reynolds, Research Institute at Nationwide Children’s Hospital, The Ohio State University, Columbus, OH, USA.
John E. McGeary, Providence Veterans Affairs Medical Center, Providence, RI, USA. Division of Behavior Genetics, Rhode Island Hospital, Providence, RI, USA
Peter M. Monti, Center for Alcohol and Addiction Studies, Brown University, Box G-S121, Providence, RI 02912, USA. Providence Veterans Affairs Medical Center, Providence, RI, USA
Margaret Haney, New York State Psychiatric Institute, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Harriet de Wit, The University of Chicago, Chicago, IL, USA.
Damaris J. Rohsenow, Center for Alcohol and Addiction Studies, Brown University, Box G-S121, Providence, RI 02912, USA. Providence Veterans Affairs Medical Center, Providence, RI, USA
References
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O’Leary DS. Sex, drugs, and cognition: effects of marijuana. J Psychoactive Drugs. 2010;42:413–424. doi: 10.1080/02791072.2010.10400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GP, Ridderinkhof KR, Van Der Molen MW. Speed–accuracy modulation in case of conflict: the roles of activation and inhibition. Psychol Res. 2003;67:266–279. doi: 10.1007/s00426-002-0127-0. [DOI] [PubMed] [Google Scholar]
- Bates MN, Blakely TA. Role of cannabis in motor vehicle crashes. Epidemiologic Reviews. 1999;21:222–232. doi: 10.1093/oxfordjournals.epirev.a017998. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brodbeck J, Matter M, Moggi F. Association between cannabis use and sexual risk behavior among young heterosexual adults. AIDS Behav. 2006;10:599–605. doi: 10.1007/s10461-006-9103-9. [DOI] [PubMed] [Google Scholar]
- Chait LD, Fischman MW, Schuster CR. “Hangover” effects the morning after marijuana smoking. Drug Alcohol Depend. 1985;15:229–238. doi: 10.1016/0376-8716(85)90002-X. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103:107–113. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa FM, Jessor R, Fortenberry JD, Donovan JE. Psychosocial conventionality, health orientation, and contraceptive use in adolescence. J Adolesc Heal. 1996;18:404–416. doi: 10.1016/1054-139X(95)00192-U. [DOI] [PubMed] [Google Scholar]
- Crowe LC, George WH. Alcohol and human sexuality: review and integration. Psychol Bull. 1989;105:374–386. doi: 10.1037//0033-2909.105.3.374. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Funk R, Harrington Godley S, Godley MD, Waldron H. Cross-validation of the alcohol and cannabis use measures in the Global Appraisal of Individual Needs (Gain) and Timeline Followback (TLFB; Form 90) among adolescents in substance abuse treatment. Addiction. 2004;99(Suppl 2):120–128. doi: 10.1111/j.1360-0443.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- Drummer OH, Gerostamoulos J, Batziris H, Chu M, Caplehorn J, Robertson MD, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36:239–248. doi: 10.1016/S0001-4575(02)00153-7. [DOI] [PubMed] [Google Scholar]
- Fernandez MI, Collazo JB, Hernandez N, Bowen GS, Varga LM. Predictors of HIV risk among Hispanic farm workers in South Florida: women are at higher risk than men. AIDS Behav. 2004;8:165–174. doi: 10.1023/B:AIBE.0000030247.00140.62. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Mulvihill LE, Vogel-Sprott M. The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacology (Berl) 1994;115:383–388. doi: 10.1007/BF02245081. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Fromme K, Katz E, D’Amico E. Effects of alcohol intoxication on the perceived consequences of risk taking. Exp Clin Psychopharmacol. 1997;5:14–23. doi: 10.1037//1064-1297.5.1.14. [DOI] [PubMed] [Google Scholar]
- George WH, Stoner SA. Understanding acute alcohol effects on sexual behavior. Annu Rev Sex Res. 2000;11:92–124. [PubMed] [Google Scholar]
- Harrell PT, Juliano LM. Caffeine expectancies influence the subjective and behavioral effects of caffeine. Psychopharmacology (Berl) 2009;207:335–342. doi: 10.1007/s00213-009-1658-5. [DOI] [PubMed] [Google Scholar]
- Hart CL, GorpW V, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology. 1987;91:20–24. doi: 10.1007/BF00690920. [DOI] [PubMed] [Google Scholar]
- Hull JG, Bond CF. Social and behavioral consequences of alcohol consumption and expectancy: a meta-analysis. Psychol Bull. 1986;99:347–360. doi: 10.1037//0033-2909.99.3.347. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Exp Clin Psychopharmacol. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. J Abnorm Psychol. 2002;111:88–97. doi: 10.1037//0021-843X.111.1.88. [DOI] [PubMed] [Google Scholar]
- Katz EC, Fromme K, D’Amico EJ. Effects of outcome expectancies and personality on young adults’ illicit drug use, heavy drinking, and risky sexual behavior. Cogn Ther Res. 2000;24:1–22. doi: 10.1023/A:1005460107337. [DOI] [Google Scholar]
- Kelemen WL, Kaighobadi F. Expectancy and pharmacology influence the subjective effects of nicotine in a balanced-placebo design. Exp Clin Psychopharmacol. 2007;15:93–101. doi: 10.1037/1064-1297.15.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JM, Doty P, de Wit H. Effects of expectancies on subjective responses to oral delta-9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;59:287–293. doi: 10.1016/S0091-3057(97)00414-0. [DOI] [PubMed] [Google Scholar]
- Lane SD, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30:800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- Leigh BC, Ames SL, Stacy AW. Alcohol, drugs, and condom use among drug offenders: an event-based analysis. Drug Alcohol Depend. 2008;93:38–42. doi: 10.1016/j.drugalcdep.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk-taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898X.8.2.75. [DOI] [PubMed] [Google Scholar]
- Logan GD. Spatial attention and the apprehension of spatial relations. J Exp Psychol Hum Percept Perform. 1994;20:1015–1036. doi: 10.1037//0096-1523.20.5.1015. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci. 1997;8:60–64. doi: 10.1111/j.1467-9280.1997.tb00545.x. [DOI] [Google Scholar]
- Lukas SE, Mendelson JH, Benedikt R. Electroencephalographic correlates of marijuana-induced euphoria. Drug Alcohol Depend. 1995;37:131–140. doi: 10.1016/0376-8716(94)01067-u. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Rohsenow DJ. Advances in Substance Abuse: Behavioral and Biological Research. Greenwich, CT: JAI Press; 1980. Cognitive processes in alcohol use: expectancy and the balanced placebo design; pp. 159–199. [Google Scholar]
- Martin CS, Sayette MA. Experimental design in alcohol administration research: limitations and alternatives in the manipulation of dosage-set. J Stud Alcohol. 1993;54:750–761. doi: 10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- Martin W, Sloan J, Sapira J, Jasinski D. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenindate in man. Clinical Pharmacological Therapy. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Rohsenow DJ, Monti PM, McGeary J, Cook TA, de Wit H, et al. Effectiveness of a marijuana expectancy manipulation: piloting the balanced-placebo design for marijuana. Exp Clin Psychopharmacol. 2009;17:217–225. doi: 10.1037/a0016502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Kahler CW, McGeary JE, Monti PM, Rohsenow DJ. Acute effects of marijuana smoking on negative and positive affect. J Cogn Psychother. 2011a;25:1–16. doi: 10.1891/0889-8391.25.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Spillane NS, Leventhal A, Kahler CW. Marijuana use and cessation of tobacco smoking among heavy alcohol drinkers. Drug and Alcohol Dependence. 2011b;119:194–200. doi: 10.1016/j.drugalcdep.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;24:1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol. 2003;38:151–156. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Perkins K, Jacobs L, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. The influence of instructions and nicotine dose on the subjective and reinforcing effects of smoking. Exp Clin Psychopharmacol. 2004;12:91–101. doi: 10.1037/1064-1297.12.2.91. [DOI] [PubMed] [Google Scholar]
- Pleskac TJ, Wallsten TS, Wang P, Lejuez CW. Development of an automatic response mode to improve the clinical utility of sequential risk-taking tasks. Exp Clin Psychopharmacol. 2008;16:555–564. doi: 10.1037/a0014245. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Berghaus G, Van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109–119. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Robbe HWJ, O’Hanlon JF. Marijuana, alcohol, and actual driving performance. Human Psychopharmacology-Clinical And Experimental. 2000;15:551–558. doi: 10.1002/1099-1077(200010)15:7<551::AID-HUP236>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an Experiential Discounting Task. Behavioral Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Personality and Individual Difference. 2006a;40:305–315. doi: 10.1016/j.paid.2005.03.024. [DOI] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav. 2006b;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell S, de Wit H. Delay and probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton BF, Tunbridge RJ, Brook-Carter N, Jackson PG. The influence of cannabis on driving. UK DETR Road Safety Research Report. 2000 Retrieved 11-24-2004 from: www.csdporg/research/TRL477pdf.
- Shrier LA, Emans SJ, Woods ER, Durant RH. The association of sexual risk behaviors and problem drug behaviors in high school students. J Adolesc Heal. 1996;20:377–383. doi: 10.1016/S1054-139X(96)00180-2. [DOI] [PubMed] [Google Scholar]
- Simons JS, Maisto SA, Wray TB. Sexual risk taking among young adult dual alcohol and marijuana users. Addict Behav. 2010;35:533–536. doi: 10.1016/j.addbeh.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology: Applied. 1935;12:242–248. [Google Scholar]
- Testa M, Fillmore MT, Norris J, Abbey A, Curtin JJ, Leonard KE, et al. Understanding alcohol expectancy effects: revisiting the placebo condition. Alcohol Clin Exp Res. 2006;30:339–348. doi: 10.1111/j.1530-0277.2006.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. J Clin Exp Neuropsychol. 2007;29:357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Automatic and controlled response inhibition: associative learning in the Go/No-Go and Stop-Signal paradigms. J Exp Psychol Gen. 2008;137:649–672. doi: 10.1037/a0013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Sprott M, Fillmore MT. Expectancy and behavioral effects of socially used drugs. In: Kirsch EI, editor. How expectancies shape experience. Washington, DC: American Psychological Association; 1999. pp. 215–232. [DOI] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, Smith AP. A community based investigation of the association between cannabis use, injuries and accidents. J Psychopharmacol. 2006;20:5–13. doi: 10.1177/0269881105056642. [DOI] [PubMed] [Google Scholar]
- Wei Application of an urn model to the design of sequential controlled clinical trials. J Am Stat Assoc. 1978;73:559–563. doi: 10.2307/2286600. [DOI] [Google Scholar]
- Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, et al. A study investigating the acute dose-response effects of 13 mg and 17 mg delta 9-tetrahydrocannabinol on cognitive-motor skills, subjective and autonomic measures in regular users of marijuana. J Psychopharmacol. 2008;22:441–451. doi: 10.1177/0269881108088194. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, et al. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wignall ND, De Wit H. Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Exp Clin Psychopharmacol. 2011;19:183–191. doi: 10.1037/a0023292. [DOI] [PMC free article] [PubMed] [Google Scholar]