Abstract
B cell receptor (BCR)-mediated antigen processing and presentation is critical to the initiation and control of a humoral immune response. Trafficking of internalized antigen-BCR (Ag-BCR) complexes to intracellular antigen processing compartments is driven by ubiquitination of the cytoplasmic domain of the BCR. Using a biochemical approach, it is here established that ubiquitinated Ag-BCR complexes are formed via a signaling-dependent mechanism and restricted to plasma membrane lipid rafts. Since the structure of lipid rafts is temperature sensitive, the impact of physiological range temperature changes (33–39°C) on lipid raft-dependent and independent BCR functions was investigated. While the kinetics of lipid raft independent BCR internalization are unaffected by temperature changes within this range, raft-dependent BCR signaling and ubiquitination as well as BCR-mediated antigen processing are significantly impacted. When compared to 33°C (peripheral body temperature), the extent and duration of Ag-BCR ubiquitination is increased and prolonged at 37–39°C (normal to febrile temperature). As might be expected, increased temperature also accelerates the overall kinetics of Ag-BCR degradation. Interestingly, at 33°C the expression of peptide-class II complexes derived from the BCR-mediated processing of cognate antigen is profoundly slowed, whereas the kinetics of expression of peptide-class II complexes derived from fluid-phase antigen processing remain unchanged. These results establish the effect of physiological range temperature changes on multiple lipid raft-dependent BCR functions including the processing and presentation of cognate antigen, suggesting one mechanism by which physiological range temperature changes such as fever may impact the initiation and /or maturation of a humoral immune response.
Keywords: B Cells, Presentation/Processing, MHC, BCR
Introduction
The B cell receptor (BCR) is the main receptor by which B cells bind and recognize foreign antigen. Binding of antigen to the BCR initiates a series of events including endocytosis, antigen processing and formation of peptide-class II complexes, culminating in B cell-T cell interactions and development of a full blown humoral immune response. Antigen engagement of the BCR results in at least two distinct early events, BCR signaling and BCR-mediated antigen internalization, which are mediated by two distinct subsets of antigen-BCR complexes (1) that partition into distinct plasma membrane domains. Specifically, BCR signaling is highly dependent upon a subset of antigen-BCR (Ag-BCR) complexes that partition into plasma membrane lipid rafts (2–5), which are membrane domains known to act as signaling platforms. In contrast, the bulk of BCR-mediated antigen internalization occurs via plasma membrane clathrin-coated pits (6–9), by way of a mechanism that is independent of lipid rafts (5) and BCR signaling (6). Hence, while lipid rafts play a prominent role in some BCR functions such as BCR signaling, they have little if any role in other BCR functions such as the bulk of BCR-mediated antigen internalization.
As B cells re-circulate throughout the body, they encounter local temperatures ranging from a peripheral body temperature of 33°C (91.4°F) or lower to a core body temperature of 37°C (98.6°F). Moreover, fever or exercise can raise core body temperature to 39°C (102.2°F) or higher. While this physiological range of temperature fluctuations does not reach a high enough temperature to induce a full blown heat-shock stress response (i.e. 42°C), changes in temperature across the range of 33 – 39°C are known to have profound impacts on the structure and function of the lipid bilayer component of the plasma membrane. At the low end of the temperature range, the line tension generated by the height mismatch between the raft and non-raft regions of the membrane is thought to represent a barrier to raft fusion, allowing smaller rafts to exist as discrete entities (10, 11). At the higher end of the temperature scale, line tension is no longer of sufficient strength to prevent raft fusion, resulting in a dramatic remodeling of the lipid bilayer. Since some BCR functions such as signaling are highly dependent on lipid rafts, while other functions such as endocytosis appear to be raft independent, it was thus of interest to determine the impact of physiological range temperature changes (PRTC) on both lipid raft dependent and independent BCR functions.
Materials and Methods
Animals
B10.BR/SgSnJ (B10.Br) mice were purchased from Jackson Laboratory and housed in the Albany Medical College Animal Resource Facility under specific-pathogen-free conditions. MD4.B10.Br mice were bred either in the Albany Medical College Animal Resource Facility or at Taconic Farms Inc. The appropriate institutional review committee approved all reported protocols.
Cells
The murine B cell line A20μWT expressing a wild-type human IgM BCR specific for phosphorylcholine (PC) (5) was maintained in Alpha Modified Eagle’s Medium with 5% FBS and 50μM 2-mercaptoethanol in the presence of 500μg/ml of G418 (to maintain human IgM expression). For all the experiments, cells were grown to a density of <1×106/ml at 37°C, 5% CO2. Splenic B cells from B10.Br (expressing a non–HEL-specific IgMb BCR and I-Ak MHC class II molecules) and MD4 B10.Br (MD4 transgenic mice expressing an HEL-specific BCR) mice were isolated and maintained in tissue culture as previously reported (12).
Reagents
The following reagents were used for this study: hen egg lysozyme (HEL, catalog no. L-6876; Sigma, St Louis, MO), Aw3.18 mAb recognizing peptide residues 48–62 of HEL bound to MHC II I-Ak (murine IgG1, Catalog no. CRL-2826 ATCC, Manassas, VA), Goat anti-mouse IgG F(ab′)2 (Catalog no. .115-006-006; Jackson ImmunoResearch, West Grove, PA), FITC Rat anti-mouse IgG1 (A85-1, Catalog no. 553443; BD Pharmingen, San Jose, CA), FITC anti-mouse I-Ak (10-3.6, IgG2a; Catalog no. 553540; BD Pharmingen), FITC anti-mouse IgMa (DS-1, IgG1; Catalog no. 553516; BD Pharmingen), PE anti-CD45R/B220 (RA3–6B2, IgG2a; Catalog no. 553090; BD Pharmingen), Goat anti-humanIgM F(ab′)2 (Catalog no. 109-006-129; Jackson ImmunoResearch), Rabbit anti-human IgM-btn (Catalog no. 309-065-095; Jackson ImmunoResearch), Biotin anti-mouse IgMa (Catalog no. 553515; BD Pharmingen), Anti-Mouse IgMb-btn(AF6–78, catlog no.553519; BD Pharmingen), Cholera Toxin B (CTB, catalog no. 227039; Calbiochem), Horseradish peroxidase conjugated (HRP-CTB, catalog no. C3741; Sigma), PP1 and PP2 (Src Family Tyrosine Kinase Inhibitor, catalog no. BML-EI275 and BML-EI297 respectively; Enzo Life Sciences), anti-GAPDH, mouse monoclonal 6C5 (catalog no. AM4300; Ambion).
Temperature Control
Cells were cultured in bicarbonate-free RPMI 1640 media (Sigma R73388) supplemented with 10% FBS and 50 μM 2-mercaptoethanol. Culture containers were sealed and fully immersed in a heated water bath set to the indicated temperatures. A calibrated thermometer was used to set the temperature of all three baths (33, 37 and 39°C). Unless otherwise noted, cells were pre-incubated at the indicated temperature for 2–6 hours before analysis.
Isolation of Lipid Rafts / Analysis of BCR Distribution
Isolation of lipid rafts was done as previously described (5, 6) with the changes noted here. Lipid rafts were isolated by sucrose density gradient centrifugation from detergent lysates of A20μWT cells at the concentration of 108 viable cells/ml pulsed with biotinylated anti-huIgM (anti-BCR) for indicated times. In every experiment, to determine the lipid raft fraction, one tube had cells pulsed with Horseradish Peroxidase (HRP) labeled Cholera toxin B (CTB, Catalog no. C3741, Sigma) as a marker of GM1 bearing lipid rafts. After determining the raft and non-raft fractions, fractions were pooled to pull down all ubiquitinated proteins in the sample using either p62 UBA or UQ1 beads. Total BCR was determined using the pooled fractions before pull-downs, while pooled fractions after pull-down were used to determine ubiquitinated BCR. The lysates were then run on SDS-PAGE / Western blot to determine the presence or absence of ubiquitinated BCR. For temperature experiments, cells were pre-incubated at 33° or 37° C for 2 hours.
BCR Signaling
Intracellular calcium measurements were done as described previously (5, 6) with minor modifications for the temperature range changes in these experiments. Prior to preloading A20μWT cells with calcium sensitive dyes Fluo-3 AM and Fura Red (Catalog no. F1242, F3021, respectively, Invitrogen, Eugene, OR), the cells were pre-incubated in their respective temperatures 33° or 37° or 39° C for 2 hours. The cells were allowed to rest at their respective temperatures for 2 hours before analysis began. In case of Cholera toxin B (CTB)-pretreatment experiment, after resting cells at room temperature for 2 hours unlabeled cells were pre-treated with CTB at either 50μg/ml or 100μ/ml for 5 min before starting acquisition. After acquiring baseline measurements for 30 seconds, the cells were stimulated with indicated BCR ligand and data was acquired for 180 seconds. The relative intracellular calcium levels were analyzed using the FlowJo (Treestar) program.
BCR Internalization
Kinetics of antigen-BCR endocytosis was determined as described previously with modifications detailed below (5, 6, 13). A20μWT cells or Splenic B cells from B10.Br mice were pre-incubated at either 33° or 37° or 39° C for 2 hours with re-suspension every half hour. After pre-incubation, the cells were pulsed with either biotinylated anti-huIgM (anti-BCR) at a final concentration of 10μg/ml (A20μWT cells) or biotinylated anti-mouse IgMb at a final concentration of 10μg/ml (Splenic B cells) and incubated at their respective temperatures for indicated times to allow for internalization of the ligand. The reaction was stopped by adding ice-cold buffer and cells stained with Streptavidin Alexa 488 (SA, Catalog no. S32354; Invitrogen) to label ligand remaining on the surface, and all cells were stained with 0.1 μg/ml of propidium iodide (Catalog no. P3566;Invitrogen). To determine the level of fluorescence a FacScan I (BD Biosciences) was used and data analyzed using the program Flow Jo (Treestar). All analysis was performed on live cells (propidium iodide-negative). Fraction of antibody remaining at the cell surface was calculated by dividing the mean fluorescence intensity (MFI) at each time point by the MFI value at time 0.
BCR-mediated Ligand Degradation
Analysis of BCR degradation by SDS-PAGE western blot was done as previously described (14) with these noted modifications. Either A20μWT cells or splenic B cells used in the experiments were pre-incubated at their respective temperatures 33 or 37° or 39° C for 2 hr. After pre-incubation, the cells were pulsed with their respective ligands - either biotinylated anti-huIgM at a final concentration of 10μg/ml (A20μWT cells) or biotinylated anti-mouse IgMb at a final concentration of 10μg/ml (splenic B cells) for indicated times. Cells were lysed at 1x107 viable cells/ml concentration in RIPA buffer for 10 minutes on ice. After clearing the lysates by centrifugation for 15 minutes at 16,000 × g at 4°C, the total BCR was analyzed by reducing SDS-PAGE, Western blotting with SA-HRP, and Super Signal West Dura ECL substrate, using Blue Sensitive X-ray film.
BCR Ubiquitination
Analysis of BCR ubiquitination by SDS PAGE western blot was done as previously described (14) with these noted modifications. Either A20μWT cells or splenic B cells used in the experiments were pre-incubated at their respective temperatures 33° or 37° or 39° C for 2 hr. After pre-incubation, the cells were pulsed with their respective ligands - either biotinylated anti-huIgM at a final concentration of 10 μg/ml (A20uWT cells) or biotinylated anti-mouse IgMb at a final concentration of 10 μg/ml (splenic B cells) for indicated times. Cells were lysed at 1x107 viable cells/ml concentration in RIPA buffer for 10 min on ice. After clearing the lysates by centrifugation for 15 min at 16,000 × g at 4°C, the ubiquitinated proteins in the cleared lysate was precipitated by using either p62 UBA-agarose beads or UQ1 (Ubiquillin1) UBA –agarose beads (Catalog # UW9010 and UW9830; BioMol-Enzo, respectively) overnight at 4°C. After collecting the samples from ubiquitin pull-downs, they were analyzed by reducing SDS-PAGE, Western blotting with SA-HRP (Catalog no. 21124; Pierce Biotechnology), and Super Signal West Dura ECL substrate, using Blue Sensitive X-ray film. For experiments involving CTB pre-treatment, samples were pre-treated with the indicated concentration of CTB and incubated at 37°C for 5 min before addition of anti-huIgM-btn and analysis of BCR ubiquitination as detailed above. For experiments involving the inhibition of Src family kinase activity, samples were pretreated for 1 hr at 37°C with a cocktail of 10 μM PP1 and 10 μM PP2 before addition of anti-huIgM-btn and analysis of BCR ubiquitination as detailed above.
Expression of HEL46–61–I-Ak Peptide-Class II Complexes
Splenocytes from either MD4.B10.Br or B10.Br mice were pulsed with antigen (HEL, 100 nM for HEL-specific MD4.B10.Br B cells and 100 μM for non-HEL-specific B10.Br B cells) and the level of HEL46–61–I-Ak expressed at each time point was determined by staining with the HEL46–61–I-Ak-specific mAb Aw3.18 (15) as previously reported (12, 13), with the exception of the substitution of Aw3.18 (detected with A85–1 rat anti-murine IgG1-FTIC, Pharmingen 553443) for C4H3. The MFI of Aw3.18 staining is reported for live (propidium iodide negative) B220+ B cells falling within a “lymphocyte” forward and side scatter gate is reported. Total I-Ak expression was monitored by staining parallel samples with 10–3.6-FITC (Cat. #109901, BioLegend).
Results
Ubiquitinated Antigen-BCR Complexes are Restricted to Lipid Raft Membrane Domains
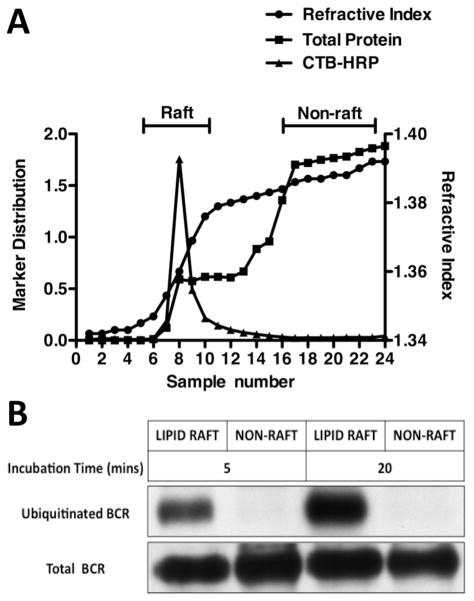
Previous studies have established that the cytoplasmic tail of a relatively small fraction of Ag-BCR complexes is ubiquitinated, directing those Ag-BCR complexes to MIIC-like late endosomes for processing to peptide-class II complexes (14, 16). Other studies have also established that a relatively small fraction of Ag-BCR complexes are found in plasma membrane domains termed “lipid raft”, which are known platforms for receptor signaling (2–5). To establish the relationship between these two sub-populations of Ag-BCR complexes, the ubiquitination state of the Ag-BCR complexes in and outside of lipid rafts was determined. As previously reported by many groups, antigen binding results in the formation of Ag-BCR complexes that can be found both in and outside of lipid rafts (figure 1). However, when an ubiquitin pull-down strategy is used to focus the analysis on the subset of ubiquitinated Ag-BCR complexes, only complexes within lipid rafts were found to be ubiquitinated (figure 1). While these results do not establish whether all raft resident Ag-BCR complexes are ubiquitinated or, in contrast, whether there also exists a fraction of raft resident complexes that are not ubiquitinated, the results do establish that the subset of ubiquitinated Ag-BCR complexes is restricted to membrane domains with the biochemical properties of lipid rafts.
Figure 1. Ubiquitinated Ag-BCR Complexes are Restricted to Lipid Raft Membrane Domains.
A20μWT B cells were surface “tagged” with either CTB-HRP (to label GM1+ lipid rafts) or anti-huIgM-btn (to label surface human IgM BCR molecules). The cells were then lysed in cold 0.1% TX-100 and lipid rafts separated from non-rafts by sucrose density gradient centrifugation (5, 6). Panel A – The refractive index, total protein and CTB-HRP level in each gradient fraction was determined. The raft region of the gradient is at the first inflection point in the refractive index curve, where CTB-HRP is exclusively found. The non-raft region of the gradient is the bottom 1/3 of the gradient (fractions 16 −24), which has a uniformly high refractive index and protein content. Panel B – Samples from pools of 100% of raft fractions and ~30% of non-raft fractions from the anti-huIgM-btn tagged cells (incubated for either 5 or 20 minutes at 37°C after tagging) were directly probed by western blot with SA-HRP to detect “Total” ligand-BCR complexes. Ubiquitinated ligand-BCR complexes were isolated from the remainder of the pools by UQ1 pull-down and detected by blotting with SA-HRP (14). Shown is a representative result from 1 of 5 independent experiments.
BCR Ubiquitination Occurs via a Src Family Kinase Signaling-Dependent Mechanism
Since it is well know that lipid rafts are critical BCR signaling platforms (3, 5, 6) and ubiquitinated Ag-BCR complexes were found restricted to these membrane domains (figure 1), it was of interest to evaluate the role of BCR signaling in the formation of ubiquitinated Ag-BCR complexes. Since the initial steps of BCR signaling center on phosphorylation of BCR ITAM tyrosine residues by Src family kinases (17), the impact of blocking this early step of BCR signaling on the formation of ubiquitinated Ag-BCR complexes was determined. Pre-treatment of A20μWT B cells with a cocktail of the two well-characterized Src family kinase inhibitors PP1 and PP2 [which blocks BCR signaling but does not alter the level of BCR expression nor the kinetics of BCR internalization (6)] results in a profound block in the formation of ubiquitinated Ag-BCR complexes (figure 2). These results establish that BCR ubiquitination occurs via a mechanism involving Src family kinase-mediated BCR signaling and are consistent with the possibility that the molecules directly involved in the process of BCR ubiquitination are present in or readily recruited to plasma membrane lipid rafts.
Figure 2. BCR Ubiquitination Occurs via a Src Family Kinase Signaling-Dependent Mechanism.
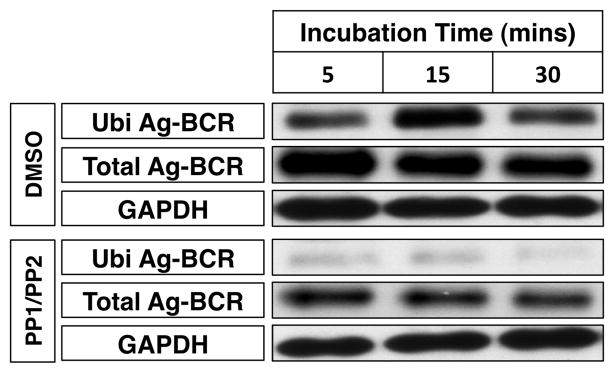
A20μWT B cells were pre-treated for 1 hour at 37°C with a cocktail of 10 μM PP1 and 10 μM PP2 or vehicle control (DMSO) and then stimulated with anti-huIgM-btn for the indicated times. The cells were then lysed and the level of total and ubiquitinated ligand-BCR complexes determined as in figure 1. Shown is a representative results from 1 of 3 independent experiments.
Physiological Range Temperature Changes Selectively Impact Lipid Raft-Dependent BCR Functions
Lipid rafts are composed of a subset of membrane lipids enriched in long-chain highly saturated fatty acid tails that under appropriate conditions form lipid “ordered” membrane micro-domains, the structure of which is highly sensitive to temperature changes over a physiological range (10, 11, 18). Therefore, the effect of physiological range temperature changes (PRTC), between 33°C (peripheral body temperature) and 39°C (mild fever), on both lipid raft-dependent as well as lipid raft-independent BCR functions was investigated.
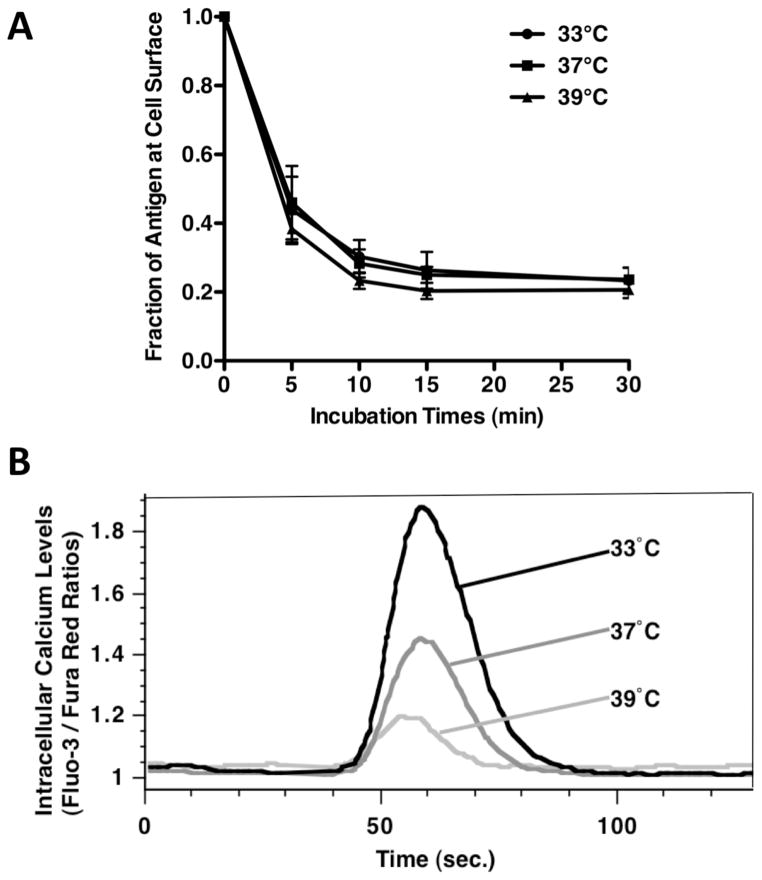
Previous work has established that while BCR signaling is highly dependent on plasma membrane lipid rafts (2–5), Ag-BCR internalization occurs predominantly via a lipid raft independent mechanism (5). Therefore the impact of PRTC on BCR signaling and internalization was investigated. As might be expected, the kinetics of lipid raft-independent Ag-BCR internalization were unaffected within the relatively narrow temperature range of 33° to 39°C (figure 3A, similar results were observed using splenic B cells, data not shown). These results show that temperature changes between 33° and 39°C do not have a profound overall effect on cell physiology (i.e., increasing temperature to 39°C does not dramatically increase the overall kinetics of cellular metabolism), suggesting that any impact that PRTC may have on lipid raft-dependent BCR functions will likely be occurring via the direct impact of temperature on membrane structure. Consistent with this scenario, analysis of lipid raft-dependent BCR-induced intracellular calcium signaling at 33 vs. 39°C revealed a significant decrease in BCR signaling at 39°C (figure 3B). These results suggest that the effect of PRTC on BCR signaling is due to the direct effect of temperature on lipid raft structure / function.
Figure 3. Lipid Raft Dependent BCR Signaling is Selectively Impacted by Physiological Range Temperature Changes.
Panel A – A20μWT B cells were pre-incubated at the indicated temperature for 2 hours, exposed to anti-huIgM-btn and the kinetics of ligand internalization determined by staining with SA-Alexa488 and analysis by flow cytometry. Shown is the average level of BCR-mediated ligand internalization from 3 independent experiments (p = 0.9991 by linear regression). The level of BCR expression subsequent to pre-incubation at the indicated temperatures but before induction of BCR internalization did not change by more than +/− 10%, when compared to the samples maintained at 37°C. Panel B – A20μWT B cells were pre-incubated at the indicated temperature for 2 hours, loaded with the calcium-sensitive dyes Fluo-3 and Fura Red, rested at the indicated temperatures for 2 hours, and then stimulated with 10μg/ml anti-BCR antibody. Shown is the intracellular calcium level as indicated by the Fluo-3: Fura Red fluorescence ratio over time for 1 representative experiment of 4.
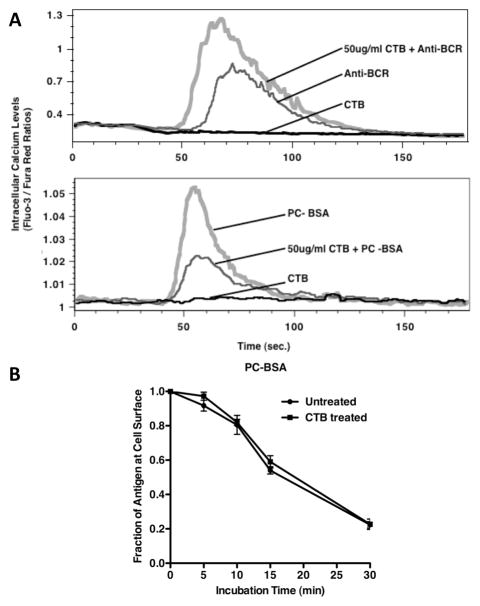
To confirm that the PRTC-induced change in BCR signaling is a direct effect of temperature on lipid rafts (as opposed to a temperature-induced change in the kinetics of BCR signaling “enzymology”) the impact of the lipid raft ligand, cholera toxin B (CTB, a lipid raft-modifying agent) on BCR signaling and internalization was investigated. Previous studies have established that binding of CTB to model membranes increases line tension at lipid raft boundaries (19) and elicits a redistribution of the liquid ordered (i.e., raft) and liquid-disordered (i.e., non-raft) phases of the bilayer (19). Consistent with these previous findings, pre-binding of 50–100 μg/ml of CTB to B cells before ligation of the BCR profoundly impacts lipid raft-dependent BCR signaling (figure 4A), while having little or no effect on lipid raft-independent BCR internalization (figure 4B). Interestingly, the impact of CTB pre-binding on raft-dependent BCR signaling can be either positive or negative, depending on the ligand used to engage the BCR. Specifically, pre-binding of CTB inhibits BCR signaling initiated by haptenated protein [i.e., PC-BSA (figure 4A, lower plot)], which will only interact with the BCR via its 2 antigen binding sites, while CTB pre-binding augments BCR signaling elicited by a polyclonal anti-BCR antibody (figure 4A, upper plot), which will bind the BCR at multiple epitopes distributed across the constant region of the molecules. While the precise reason for the differential impact of CTB on BCR signaling elicited by different BCR ligands is unclear, the results nevertheless establish that binding of the known raft “modulator” CTB can act like PRTC to selectively modulate lipid raft-dependent BCR functions. Taken together, these results establish that while PRTC between 33° and 39°C do not profoundly change overall B cell physiology and do not significantly impact lipid raft independent BCR functions such as internalization, PRTC significantly impacts lipid raft-dependent BCR functions such as BCR signaling.
Figure 4. Lipid Raft Dependent BCR Functions are Selectively Impacted by Cholera Toxin B Binding.
Panel A – Fluo-3 and Fura Red loaded A20μWT B cells were pre-treated with the indicated amount of CTB for 5 minutes, before stimulation with anti-BCR antibody or 10 μg/ml PC-BSA and the resulting intracellular calcium flux monitored as in figure 3. Shown are representative results from 1 experiment of 3. Panel B – A20μWT B cells were pre-treated with the indicated amount of CTB for 5 minutes, and the kinetics of anti-BCR-btn internalization determined as in figure 3. Shown is the average level of BCR-mediated ligand internalization from 3 independent experiments, (p = 0.8193 by linear regression).
Physiological Range Temperature Changes Regulate Ubiquitin-Directed BCR-Mediated Antigen Processing and Presentation
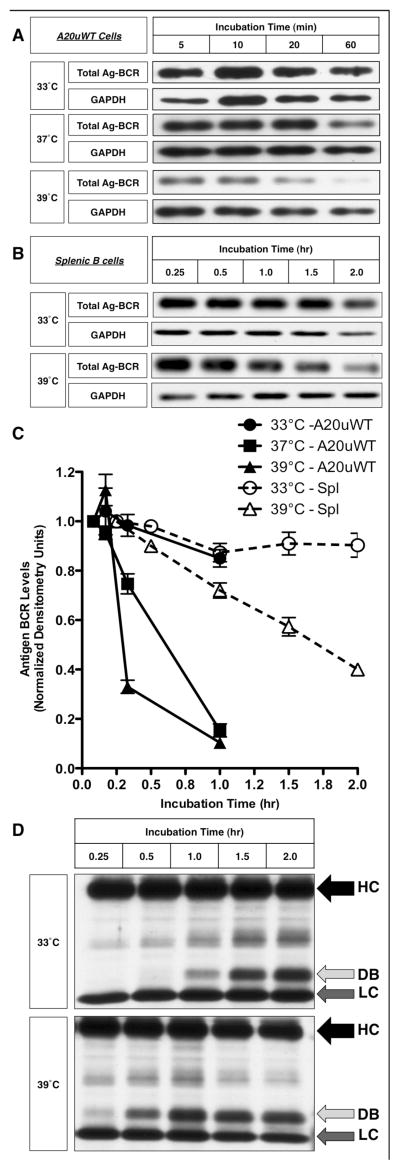
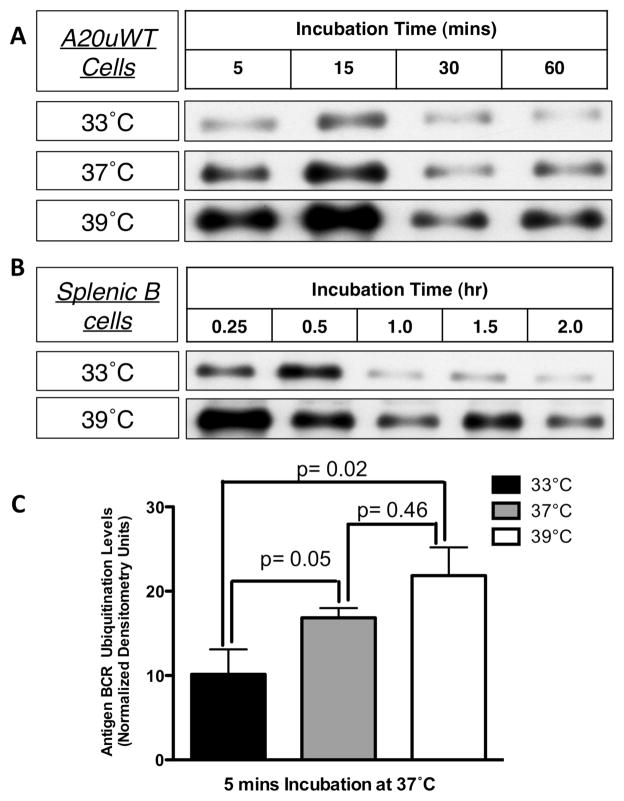
Having demonstrated that the subset of ubiquitinated Ag-BCR complexes that are the source of antigen for rapidly generated peptide-class II complexes (14, 16) are restricted to lipid raft membrane domains and that the function of these membrane domains is sensitive to PRTC, it was of interest to determine the impact of PRTC on the BCR-mediated processing and presentation of cognate antigen. One of the first steps of BCR-mediated antigen presentation is the proteolytic processing of BCR internalized ligand. As illustrated by the results presented in figure 5, changing the experimental temperature from 33°C to 39°C significantly accelerates the kinetics of BCR-mediated antigen processing, in both the well characterized A20μWT model B cell line (figure 5, panels A and C) and primary murine splenic B cells (figure 5, panels B – D). However, since previous work has established that it is not the bulk Ag-BCR complexes that are rapidly converted to peptide-class II complexes, but rather the subset of ubiquitinated Ag-BCR complexes (14, 16), the impact of PRTC on BCR ubiquitination was investigated.
Figure 5. Fever Range Temperature Accelerates the Kinetics of BCR-mediated Antigen Processing.
Panel A – A20μWT B cells were pre-incubated at the indicated temperature for 2 hours, pulsed with anti-BCR-btn at the same temperature, whole cell lysates prepared and the kinetics of anti-BCR-btn degradation determined by SDS-PAGE and blotting with SA-HRP. GAPDH was used as a loading control. Shown are representative results from 1 of 3 independent experiments. Panel B – B.10.Br splenic B cells were pre-incubated at the indicated temperature for 2 hours, and the kinetics of BCR-mediated antigen processing of anti-BCR-btn was determined by staining with SA-HRP and reducing SDS PAGE western blot. Shown are representative results from 1 of 3 independent experiments. Panel C – Densitometric analysis of the average degradation (over 3 independent experiments) of “Total Ag-BCR” by A20μWT cells and splenic B cells (spl). Bars = +/− 1 S.D. Statistical analysis: A20μWT, 33 vs. 39°C at 60 minutes, p = 0.005, splenocytes, 33 vs. 39°C at 2 hours, p = 0.006. Panel D – Longer exposure of a blot from an experiment similar to that shown in Panel B. The arrows labeled “HC” and “LC” indicate the intact heavy and light chains of the bound anti-BCR-btn antibody. The arrow labeled “DB” indicates a “degradation band” (derived from the proteolytic degradation of the intact anti-BCR-btn heavy chain, HC) that is generated more rapidly at 39°C. Shown are representative results from 1 of 3 independent experiments.
While BCR ubiquitination was detectable both at 33°C and 39°C, BCR ubiquitination was prolonged and more extensive in both transformed and normal B cells at the higher temperatures of 37–39°C (figure 6). Nevertheless, the ubiquitinated Ag-BCR complexes were found to remain restricted to membrane lipid rafts at all temperatures (supplementary figure 1). Moreover, pre-treatment of A20μWT B cells with different concentrations of the lipid raft-modifying agent CTB (which selectively enhances anti-IgM-induced lipid raft-dependent BCR functions, figure 4) results in a dose-dependent increase in the level of anti-IgM-induced BCR ubiquitination (figure 7). Taken together, these results suggest that elevated temperature, such as would be encountered during a fever, impacts the structure and function of lipid raft membrane domains in such a way as to augment the ubiquitination of internalized Ag-BCR complexes, which would be predicted to enhance the efficiency of BCR-mediated antigen processing and presentation.
Figure 6. Fever Range Temperature Increases the Level of Ag-BCR Ubiquitination.
Panel A – A20μWT B cells were pre-incubated for 2 hours at the indicated temperature. Cells were pulsed with anti-BCR-btn for the indicated times at that same temperature and ubiquitinated ligand–BCR complexes isolated by UQ1 pull-down. The level of ubiquitinated ligand–BCR complexes was determined by SDS-PAGE and blotting with SA-HRP. Panel B – Splenocytes from B10.Br mice were pre-incubated for 2 hours at the indicated temperature. The cells were then pulsed with anti-IgMb-btn at those same temperatures for the indicated times. The cells were then lysed and ubiquitinated ligand-BCR complexes were detected as in Panel A. Shown are representative results from 1 of 3 independent experiments (For panels A and B of this figure, the ubiquitin pull-down was done from the same samples as shown in figure 5A and 5B, which serves as a “loading” control for this figure). Panel C – Densitometric analysis of the average level (across 3 independent experiments) of ubiquitinated Ag-BCR complexes detected at 5 minutes with A20μWT cells (panel A). Error bars = +/− 1 S.D.
Figure 7. Cholera Toxin B Binding Increases the Level of Ag-BCR Ubiquitination.
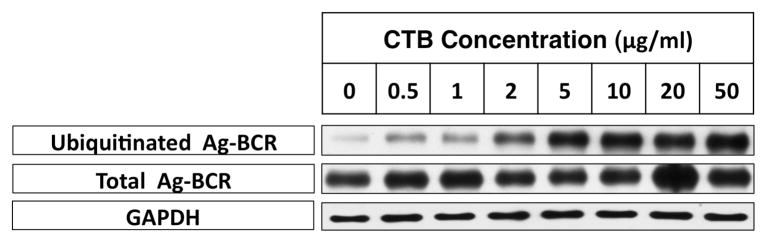
A20μWT B cells were pre-incubated with the indicated dose of CTB for 5 minutes at 37°C. Cells were then pulsed with anti-BCR-btn for 20 min at 37°C. The level of total and ubiquitinated Ag-BCR complexes was determined as in figure 1. Shown are representative results from 1 of 3 independent experiments. Similar results were observed with splenic B cells (data not shown)
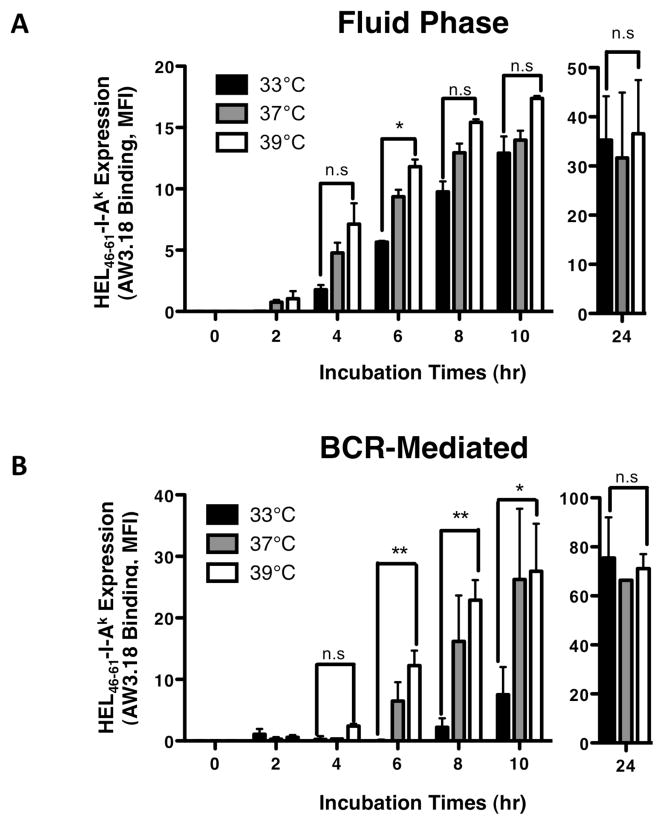
To further characterize the immunological impact of PRTC on the BCR-mediated processing and presentation of cognate antigen, the impact of temperature on the kinetics of cell surface expression of peptide-MHC class II complexes derived from ubiquitinated Ag-BCR complexes was investigated. At all three temperatures tested (i.e., 33, 37 and 39°C) the kinetics of the BCR-independent processing and presentation of non-cognate antigen internalized by fluid-phase endocytosis was essentially unaffected (figure 8A), establishing that (as observed for lipid raft-independent BCR internalization, figure 3A) these PRTC have a minor (if any) impact on lipid raft-independent B cell functions. In stark contrast, the kinetics of BCR-mediated antigen processing is much more sensitive to PRTC. Specifically, at 39°C the kinetics of BCR-mediated antigen presentation is slightly accelerated when compared to the kinetics observed at 37°C, while at 33°C BCR-mediated antigen processing is essentially blocked for the first 8–10 hours of antigen exposure (figure 8B). Nevertheless, after 24 hours at 33°C, BCR-mediated antigen presentation ultimately recovers to the same level as observed at the higher temperatures (figure 8B). Taken together, the results presented in figures 5 through 8 establishes the selective impact of PRTC on the BCR-mediated processing and presentation of cognate antigen, which is mediated by a ubiquitinated subset of Ag-BCR complexes that are restricted to temperature sensitive lipid raft membrane domains.
Figure 8. Fever Range Temperature Accelerates the Kinetics of BCR-mediated Antigen Presentation.
Splenocytes from B10.Br (Panel A) or MD4.B10.Br (Panel B) mice were pre-incubated for 2 hours at the indicated temperature. Cells were then pulsed with antigen [MD4.B10.Br – 100 nM HEL for BCR-mediated processing, B10.Br – 100 μM HEL + 10 μg/ml anti-BCR for fluid phase processing and BCR signaling, respectively (12)] for the indicated time at the indicated temperature. Cells were harvested and the level of HEL46–61–I-Ak complex expression was determined by staining with the HEL46–61–I-Ak complex-specific mAb Aw3.18 (15) and analysis of Aw3.18 binding by flow cytometry (12). Shown is the average MFI of Aw3.18 staining of B220+ B cells +/− S.E.M. for 3 independent experiments. The level of Aw3.18 binding detected with the 33°C and 39°C samples at each time point/antigen dose was compared by a Student’s t-test (** = p value of <0.01, *= p value of <0.05, n.s = not significant with a p value of >0.05). The total level of I-Ak class II expression was also monitored and did not vary by more than +/− 10% between samples.
Discussion
BCR-mediated antigen processing entails Ag-BCR endocytosis, delivery of ubiquitinated Ag-BCR complexes to MIIC where antigen is converted to antigenic peptide-MHC class II complexes, and finally delivery of these peptide-class II complexes to the surface of the cell. Previous work by this lab and others has established that while some aspects of BCR-mediated antigen processing are highly dependent on lipid rafts [e.g., BCR signaling (2–5)], other BCR functions such as endocytosis are predominantly lipid raft independent events (5). Since the structure and function of lipid raft membrane micro-domains are known to be temperature sensitive (10, 18), the present study was undertaken to determine the effect of physiological range temperature changes (PRTC) on both lipid raft dependent and independent BCR functions.
The results presented in this report establish two important features of B cell immunobiology. First, biochemical analysis established that ubiquitination of Ag-BCR complexes occurs via a signaling-dependent mechanism and that the subset of ubiquitinated Ag-BCR complexes that mediates the relatively rapid processing of antigen and conversion to peptide-class II complexes (14) are completely contained within the subset of Ag-BCR complexes found in plasma membrane lipid rafts that are known to be intimately involved in BCR signaling. Second, physiologically relevant changes in temperature, which have previously shown to impact the structure and function of membrane lipid rafts (10, 18), have profound yet selective effects on lipid raft-dependent BCR functions such as signaling (2–5) and receptor ubiquitination, while having limited or no effect on lipid raft independent BCR functions such as clathrin-coated pit-mediated internalization (5, 6). These findings significantly extend our understanding of B lymphocyte immunobiology and provide insight into how B cell responses may be tailored by environmental cues.
Two previous reports have established that while ubiquitination of the cytoplasmic tail of the BCR (i.e., IgH/L–CD79A/B) is necessary for the trafficking of Ag-BCR complexes to DM+ MIIC antigen processing compartments and subsequent conversion to peptide-class II complexes, BCR ubiquitination is not necessary for Ag-BCR internalization (14, 16). Since BCR signaling and internalization are mutually exclusive events (1) [with BCR signaling being highly dependent on membrane lipid raft domains (2–5), while the bulk of BCR internalization occurs via a lipid raft /BCR signaling independent mechanism (5–7)], it was of great interest to establish the role of lipid rafts and signaling in BCR ubiquitination. The results presented in this report establish the restriction of ubiquitinated Ag-BCR complexes to lipid raft membrane domains and a role for Src family kinase-based BCR signaling in the mechanism of BCR ubiquitination. This finding is consistent with the published observation that blocking BCR ubiquitination has no effect on BCR internalization [which occurs predominantly via a lipid raft- (14) and signaling (6) independent mechanism], and supports the published suggestion that BCR ubiquitination may occur via a BCR signaling / lipid raft dependent pathway (14). Studies to further establish the molecular mechanism of BCR ubiquitination are presently underway.
As lymphocytes circulate throughout the body, they can be exposed to temperatures ranging from a peripheral body temperature of 33°C (91.4°F) or less to a core body temperature of 37°C (98.6°F). Moreover, during infection or exercise core body temperature can rise to 39°C (102.2°F) or higher. While temperature changes across the range of 33 – 39°C are below the level that induces a “heat shock” response (e.g., 42°C), such changes will have an impact on cellular structures and functions. One cellular structure that is exquisitely sensitive to temperature is the lipid bilayer of cellular membranes.
Lipid rafts are sub-domains of the lipid bilayer enriched in lipids possessing long-chain fully saturated fatty acid tails, making this region of the bilayer thicker than the non-raft region of the bilayer. The height mismatch between the lipid raft and non-raft regions of the plasma membrane generates a “line tension” that prevents smaller raft regions from fusing into one large lipid raft (11). However, changes in temperature across the range of 33 – 39°C (i.e., PRTC) can overcome this energy barrier, allowing re-organizations of the raft and non-raft regions of the membrane (18). It was therefore of interest to determine the effect of PRTC on both lipid raft dependent as well as lipid raft independent BCR functions. The prototypical lipid raft dependent BCR function is signaling (2–5), which was found to be highly sensitive to PRTC. Interestingly, calcium-based BCR signaling is greatest at lower temperature (i.e., 33°) where lipid rafts would be expected to exist as small independent structures (as opposed to a single large raft), suggesting that the lipid raft organization that prevails at 33°C is the most conducive to this aspect of BCR signaling. Moreover, the observation that BCR signaling is greatest at lower temperature argues that the effect of temperature on signaling is not simply due to a temperature-induced acceleration of the rate of enzyme-based signaling (since this scenario would predict greater BCR signaling at 37 – 39°C), but rather supports the notion that the change in signaling is due to a temperature-induced change in raft structure / function. Consistent with the scenario is the observation that pre-binding of CTB [which like temperature is known to be a raft-modifying agent (19)] also selectively impacts lipid raft dependent BCR signaling. In contrast to BCR signaling, the bulk of internalization of Ag-BCR complexes has been shown to occur independent of both BCR signaling (6) as well as plasma membrane lipid rafts (5). Accordingly, modification of lipid rafts by either PRTC or CTB pre-binding has no significant effect on the kinetics of Ag-BCR internalization. Taken together, these results establish that PRTC, which are known to have significant effect on the structure and function of lipid rafts (18), have a selective effect on lipid raft-dependent BCR functions such as BCR signaling, while leaving lipid raft independent BCR functions such as Ag-BCR internalization relatively unchanged.
Trafficking of internalized Ag-BCR complexes to DM+ MIIC where they are converted from Ag-BCR complexes to antigenic peptide-class II complexes is a critical aspect of BCR-mediated antigen processing that has been shown to be dependent on BCR ubiquitination (14, 16), while fluid-phase processing and presentation of non-cognate antigen occurs independent of BCR signaling and/or ubiquitination (14, 20). In light of the observation reported herein that ubiquitinated Ag-BCR complexes are restricted to lipid raft membrane domains, it was of interest to determine the relative impact of PRTC on BCR-mediated vs. fluid-phase antigen processing. Consistent with the high level partitioning of ubiquitinated Ag-BCR complexes into temperature sensitive lipid rafts, changes across the physiological temperature range profoundly alter the kinetics of BCR-mediated antigen processing. Contrastingly, PRTC has essentially no effect on the kinetics of the lipid raft-independent fluid-phase processing of non-cognate antigen. These results extend the observation that ubiquitinated Ag-BCR complexes are restricted to temperature-sensitive lipid raft domains, and establishes that the BCR-mediated processing of cognate antigen, which results in the formation of peptide-class II complexes with unique biological properties (12), occurs via lipid raft-dependent pathway that is highly sensitive to PRTC.
Two questions raised by the results presented in this report are the relative roles of ubiquitinated vs. non-ubiquitinated Ag-BCR complexes in the formation of antigenic peptide-class II complexes as well as the mechanism of internalization of these distinct subsets of Ag-BCR complexes. Previous work from this laboratory has established that blocking BCR ubiquitination profoundly inhibits the rapid formation of antigen-class II complexes (14), while having little impact on the intracellular persistence of internalized Ag-BCR complexes that are slowly processed to peptide-class II complexes (13). This suggests that ubiquitinated Ag-BCR complexes are efficiently recognized by the ubiquitin-dependent ESCRT sorting machinery upon internalization and thus efficiently sorted to or within multi-vesicular body (MVB)-like MIIC for the rapid conversion into antigenic peptide-MHC class II complexes, whereas non-ubiquitinated Ag-BCR complexes are initially sequestered within a distinct endocytic compartment and then slowly delivered to or trafficked within MIIC for processing to peptide-class II complexes. This scenario is consistent with the finding reported herein that incubation of B cells at 33°C, which results in the lowest level of Ag-BCR ubiquitination, results in a delay in the cell surface expression of derivative antigenic peptide-class II complexes, but has no effect on the level of peptide-class II complexes ultimately expressed on the surface of the cell (figure 8).
Internalization of the non-ubiquitinated Ag-BCR complexes [which have been estimated to comprise greater than 90% of all cell surface Ag-BCR complexes (14)] occurs via plasma membrane clathrin coated pits (6–9), likely using an adaptor protein such as the well-characterized endocytic adaptor AP-2. Ubiquitinated Ag-BCR complexes [which are estimated to comprises between 1 and 10% of all cell surface Ag-BCR complexes (14)] are likely also internalized via plasma membrane clathrin coated pits [possibly via clathrin recruited to lipid rafts (21)]. Internalization of these complexes likely occurs either via direct interaction of the ubiquitinated Ag-BCR complexes with the adaptor AP-2 or possibly via the endocytic adaptor EPS15, which binds AP-2 and possesses an ubiquitin interacting motif. The existence of these two pathways of Ag-BCR internalization and trafficking would allow B cells to both rapidly express cell surface of peptide-class II complexes (which would be beneficial under conditions such as those in a sensitized animal where T cell help is pre-existing) and to express these peptide-class II complexes for a prolonged period of time (which would be beneficial under conditions such as those in a naïve animal where CD4 T cell help may not be available for 3–4 days). The precise molecular mechanisms controlling the internalization and intracellular trafficking of these distinct Ag-BCR complexes are currently under study.
Taken in total, the results presented in this report establish the selective impact of PRTC across the range of 33 – 39°C on lipid raft dependent BCR functions such as BCR signaling and BCR-mediated antigen processing and presentation. Somewhat surprisingly, lipid raft dependent BCR signaling was actually augmented at the low range physiological temperature of 33°C, suggesting that the earliest aspects of B cell activation may have evolved to occur at locations in the body that are normally maintained at this temperature (i.e., peripheral locations such as the skin, hands or feet). In contrast, the kinetics of BCR-mediated antigen processing and presentation are more rapid at the upper range of physiological temperatures (i.e., 37 – 39°C), suggesting that the latter aspects of BCR activation such as antigen presentation to CD4 T cells may have evolved to be optimal at locations in the body normally maintained at this higher temperature, such as lymph nodes within the core of the body or during fever. Together, these results highlight the impact of physiological range temperature changes on the cell biology of BCR-mediated antigen processing and suggest at least one mechanism by which physiologically relevant changes in body temperature such as fever can fine-tune the immune response.
Supplementary Material
Acknowledgments
The authors would like to thank the Center for Immunology and Microbial Disease Flow Cytometry Core Facility as well as the Albany Medical College Animal Resources Facility. They would also like to thank Lisa Drake for excellent technical assistance and Kathleen McCabe for critical reading of the manuscript.
Abbreviations
- Ag
antigen
- CTB
cholera toxin B
- HEL
hen egg lysozyme
- HRP
horseradish peroxidase
- MFI
mean fluorescence intensity
- MIIC
MHC class II-enriched compartment
- MVB
multi-vesicular body
- PC
phosphorylcholine
- PRTC
physiological range temperature change
- SA
streptavidin
- TX-100
Triton X-100
Footnotes
This work was supported by a N.I.H. grant (AI-065773) to J.R.D.
References
- 1.Hou P, Araujo E, Zhao T, Zhang M, Massenburg D, Veselits M, Doyle C, Dinner AR, Clark MR. B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 2006;4:e200. doi: 10.1371/journal.pbio.0040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awasthi-Kalia M, Schnetkamp PP, Deans JP. Differential effects of filipin and methyl-beta-cyclodextrin on B cell receptor signaling. Biochem Biophys Res Commun. 2001;287:77–82. doi: 10.1006/bbrc.2001.5536. [DOI] [PubMed] [Google Scholar]
- 3.Cheng PC, Cherukuri A, Dykstra M, Malapati S, Sproul T, Chen MR, Pierce SK. Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin Immunol. 2001;13:107–114. doi: 10.1006/smim.2000.0302. [DOI] [PubMed] [Google Scholar]
- 4.Petrie RJ, Schnetkamp PP, Patel KD, Awasthi-Kalia M, Deans JP. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J Immunol. 2000;165:1220–1227. doi: 10.4049/jimmunol.165.3.1220. [DOI] [PubMed] [Google Scholar]
- 5.Putnam MA, Moquin AE, Merrihew M, Outcalt C, Sorge E, Caballero A, Gondre-Lewis TA, Drake JR. Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J Immunol. 2003;170:905–912. doi: 10.4049/jimmunol.170.2.905. [DOI] [PubMed] [Google Scholar]
- 6.Caballero A, Katkere B, Wen XY, Drake L, Nashar TO, Drake JR. Functional and structural requirements for the internalization of distinct BCR-ligand complexes. Eur J Immunol. 2006;36:3131–3145. doi: 10.1002/eji.200636447. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Cramer L, Mueller H, Wilson B, Vilen BJ. Independent trafficking of Ig-alpha/Ig-beta and mu-heavy chain is facilitated by dissociation of the B cell antigen receptor complex. J Immunol. 2005;175:147–154. doi: 10.4049/jimmunol.175.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salisbury JL, Condeelis JS, Maihle NJ, Satir P. Receptor-mediated endocytosis by clathrin-coated vesicles: evidence for a dynamic pathway. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):733–741. doi: 10.1101/sqb.1982.046.01.070. [DOI] [PubMed] [Google Scholar]
- 9.Salisbury JL, Condeelis JS, Satir P. Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J Cell Biol. 1980;87:132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Saez AJ, Chiantia S, Schwille P. Effect of line tension on the lateral organization of lipid membranes. J Biol Chem. 2007;282:33537–33544. doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]
- 11.Kuzmin PI, Akimov SA, Chizmadzhev YA, Zimmerberg J, Cohen FS. Line tension and interaction energies of membrane rafts calculated from lipid splay and tilt. Biophys J. 2005;88:1120–1133. doi: 10.1529/biophysj.104.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nashar TO, Drake JR. The Pathway of Antigen Uptake and Processing Dictates MHC Class II-Mediated B Cell Survival and Activation. J Immunol. 2005;174:1306–1316. doi: 10.4049/jimmunol.174.3.1306. [DOI] [PubMed] [Google Scholar]
- 13.Gondré-Lewis TA, Moquin AE, Drake JR. Prolonged antigen persistence within nonterminal late endocytic compartments of antigen-specific B lymphocytes. J Immunol. 2001;166:6657–6664. doi: 10.4049/jimmunol.166.11.6657. [DOI] [PubMed] [Google Scholar]
- 14.Drake L, McGovern-Brindisi EM, Drake JR. BCR ubiquitination controls BCR-mediated antigen processing and presentation. Blood. 2006;108:4086–4093. doi: 10.1182/blood-2006-05-025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadaglio G, Nelson CA, Deck MB, Petzold SJ, Unanue ER. Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Veselits M, O’Neill S, Hou P, Reddi AL, Berlin I, Ikeda M, Nash PD, Longnecker R, Band H, Clark MR. Ubiquitinylation of Ig beta dictates the endocytic fate of the B cell antigen receptor. J Immunol. 2007;179:4435–4443. doi: 10.4049/jimmunol.179.7.4435. [DOI] [PubMed] [Google Scholar]
- 17.DeFranco AL, Richards JD, Blum JH, Stevens TL, Law DA, Chan VW, Datta SK, Foy SP, Hourihane SL, Gold MR, et al. Signal transduction by the B-cell antigen receptor. Ann N Y Acad Sci. 1995;766:195–201. doi: 10.1111/j.1749-6632.1995.tb26662.x. [DOI] [PubMed] [Google Scholar]
- 18.Weise K, Triola G, Janosch S, Waldmann H, Winter R. Visualizing association of lipidated signaling proteins in heterogeneous membranes-Partitioning into subdomains, lipid sorting, interfacial adsorption, and protein association. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbamem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Akimov SA, Hlaponin EA, Bashkirov PV, Boldyrev IA, Mikhalyov II, Telford WG, Molotkovskaya IM. Ganglioside GM1 Increases Line Tension at Raft Boundary in Model Membranes. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology. 2009;3:216–222. [Google Scholar]
- 20.McGovern EM, Moquin AE, Caballero A, Drake JR. The effect of B cell receptor signaling on antigen endocytosis and processing. Immunol Invest. 2004;33:143–156. doi: 10.1081/imm-120030733. [DOI] [PubMed] [Google Scholar]
- 21.Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–462. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.