Abstract
OBJECTIVES
In coronary artery bypass grafting (CABG), graft flow distal to a mild stenosis can compete with relatively preserved native flow through the stenosis and the competition can result in graft stenosis. In chronic total occlusion (CTO), coronary collateral circulation, which is essential to maintain myocardial viability distal to CTO, varies in extent among patients and the extent can be scored by Rentrop grade in coronary angiography. We investigated whether rich collateral circulation distal to CTO competes with distally anastomosed graft flow in association with Rentrop grade.
METHODS
Of 666 patients who underwent CABG from January 2001 to December 2012, 70 patients whose left internal thoracic artery (ITA) was grafted distal to CTO in the left anterior descending artery (LAD) were divided into three groups: Poor collaterals (Rentrop grades 0 and 1, Group P, n = 22), Moderate collaterals (grade 2, Group M, n = 23) and Rich collaterals (grade 3, Group R, n = 25). The intraoperative measurements of mean graft flow (MGF) and pulsatility index (PI) of left ITA grafts, early graft patency and long-term clinical outcomes were compared.
RESULTS
The MGF and PI of left ITA grafts differed significantly among the three groups (P = 0.025 and P = 0.046, respectively). Lower Rentrop grade was associated with preferable results of higher MGF and lower PI. The graft flow pattern in Group P showed a significantly higher MGF (P = 0.020) and lower PI (P = 0.041) than those in Group R. All early postoperative coronary angiograms showed patent left ITA grafts. Serial echocardiographic evaluations, survival rates and cardiac event-free rates were comparable with the follow-up of 5.00 ± 3.11 years.
CONCLUSIONS
Rich collateral circulation distal to CTO in LADs can potentially compete with graft flow, although the competition seems not to affect clinical outcomes probably due to the regression of collaterals surmounted by the graft flow. Rentrop grade is shown to certainly reflect the degree of collateral haemodynamic circulation distal to CTO and especially important to evaluate intraoperative graft flow appropriately, considering the possible phenomenon of graft flow competition.
Keywords: Collateral circulation, Coronary artery bypass grafting, Coronary circulation, Coronary occlusion, Pulse wave analysis
INTRODUCTION
In coronary artery bypass grafting (CABG), graft flow can be compromised by the remaining native flow depending on their relative balance [1–5]. Previous reports demonstrated that graft flow distal to a mild stenosis can compete with native coronary flow, especially when the stenosis is so mild as to be insignificant, and, furthermore, such graft flow competition can result in graft stenosis and subsequent occlusion [3–6]. Therefore, it is important to preoperatively evaluate the severity of stenoses, namely the extent of the remaining perfusion in the ischaemic area, in association with bypass arrangements [7, 8]. As an intraoperative method to assess graft quality and predict graft flow competition, transit time flow measurements of grafts, such as mean graft flow (MGF) and pulsatility index (PI), are reportedly useful and have been examined in various studies [2, 3, 9–12].
In chronic total occlusion (CTO), coronary collaterals, which play an important role as the only source of blood supply to distal ischaemic territories to maintain myocardial viability, vary in extent from patient to patient. Well-developed collaterals can supply sufficient perfusion without leading to any symptoms, although lack of collaterals can cause fatal ischaemia and subsequent infarction. Previous reports demonstrated that the degree of coronary collateral perfusion itself could influence clinical outcomes in patients with coronary artery stenosis [13, 14]. Therefore, the degree of coronary collateral development should be precisely evaluated to establish optimal treatment strategies. The Rentrop grade is a well-established means of scoring the degree of development of coronary collaterals according to the extent of contrast medium in the territory distal to CTO and has recently been applied in various studies [2, 13–16].
In CABG distal to CTO, whether well-developed collateral flow competes with graft flow is unknown. Thus, in the present study, we allocated patients with CTO in the left anterior descending artery (LAD) according to Rentrop grade, and comparatively examined the MGF and PI of left internal thoracic artery (ITA) grafts anastomosed distal to the CTO, early graft patency and long-term clinical outcomes.
MATERIALS AND METHODS
Patient selection
From January 2001 to December 2012, 666 patients underwent primary CABGs in Osaka City University Hospital (Osaka, Japan). Of these patients, 70 with CTO in a high segment of the LAD (Segment 6 or 7) and whose left ITA was grafted to the LAD were selected. Their preoperative coronary angiographies were evaluated by cardiologists, and the extent of perfusion distal to CTO in the LAD through collateral feeding arteries was scored according to the criteria of Rentrop et al. as follows: grade 0 = no filling; grade 1 = filling of side branches only, without visualization of the epicardial segment; grade 2 = partial epicardial vessel filling by collaterals and grade 3 = complete epicardial vessel filling by collaterals [2, 13–16]. The patients were then divided into one of the following three groups according to Rentrop grade: Poor collaterals (grades 0 and 1, Group P, n = 22); Moderate collaterals (grade 2, Group M, n = 23) and Rich collaterals (grade 3, Group R, n = 25). This study was conducted in accordance with the guidelines of the ethics committee of our institution. Informed consent was obtained from each patient.
Operative procedures and transit time flow measurements of mean graft flow and pulsatility index
Anesthesia was induced with intravenous midazolam and fentanyl and maintained with fentanyl, propofol, vecuronium bromide and inhalation of sevoflurane. A median sternotomy approach was used in all patients. Coronary distal anastomotic sites and graft materials designed to accomplish complete revascularization were selected. An off-pump technique was used in 22 patients (31.4%): 8 (36.4%), 5 (21.7%) and 9 patients (36%) in Groups P, M and R, respectively (P = 0.48). All other patients underwent CABG with standard cardiopulmonary bypass and cardiac arrest.
The intraoperative MGF and PI were measured with a transit time flow measurement device (MedStim, Oslo, Norway) after haemodynamic stabilization to a cardiac index of >2.0 l/min/m2 and a systolic blood pressure of 100–120 mmHg. MGF was calculated across five cardiac cycles, and PI was calculated as the ratio, the difference between the maximum velocity and minimum velocity divided by the mean velocity in one cardiac cycle.
Early postoperative coronary angiography and long-term follow-up
Postoperative coronary angiography was performed ∼2 weeks after surgery in all patients without renal dysfunction who consented to this procedure. All patients were followed at least annually as outpatients and examined by echocardiography ∼2 weeks, 1 year and 5 years postoperatively. The survival and cardiac event-free rates were compared among the three groups. In this study, cardiac events were defined as cardiac death, acute coronary syndrome, rehospitalization due to congestive heart failure and reintervention. The remote follow-up data were compiled by December 2012.
Statistical analysis
All statistical analyses were performed using the JMP (version 8) statistical software from SAS Institute (Cary, NC, USA). All data were expressed as means ± standard deviation (SD). The clinical profiles of the three groups were compared using one-way factorial analysis of variance or the Kruskal–Wallis test for analysis of continuous variables and the χ2 test or Fisher's exact test for analysis of discrete variables. Within the groups, post hoc multiple comparisons were made by using the Tukey–Kramer test. The Kaplan–Meier method was used to determine the cumulative survival rate, and the log-rank test was used to compare the groups. A P-value of <0.05 was considered to be statistically significant.
RESULTS
Preoperative patient data and coronary angiography
Preoperative patient characteristics are presented in Table 1. In Group P, 3 (13.6%) and 19 (86.4%) patients had collaterals of Rentrop grades 0 and 1, respectively. The only significant difference in clinical variables concerned the distribution of the collateral source, which could be confirmed only in patients of Groups M and R. The percentages of the collateral source distal to CTO in the LAD from the left circumflex artery (LCX) or right coronary artery (RCA) were significantly different between these groups (P = 0.0082). Of these 48 patients, 42 (87.5%) had collaterals from the RCA and only 6 (12.5%) from the LCX; all of the latter were in Group M.
Table 1:
Preoperative patient characteristics
| Variables | Group P (n = 22) | Group M (n = 23) | Group R (n = 25) | P-value |
|---|---|---|---|---|
| Age (years) | 64.2 ± 10.1 | 62.7 ± 10.9 | 65.1 ± 8.42 | 0.70 |
| Male | 21 (95.4) | 21 (91.3) | 23 (92.0) | 0.85 |
| CCS classification >3 | 6 (27.2) | 5 (22.9) | 7 (28.0) | 0.84 |
| Unstable angina pectoris | 4 (18.2) | 3 (13.0) | 4 (16.0) | 0.92 |
| Left main trunk | 2 (9.1) | 2 (8.7) | 1 (4.0) | 0.73 |
| LVEF | 0.50 ± 0.12 | 0.48 ± 0.10 | 0.48 ± 0.11 | 0.52 |
| LVEF < 0.30 | 1 (4.5) | 1 (4.3) | 3 (12.0) | 0.61 |
| Number of diseased vessels | 2.50 ± 0.74 | 2.70 ± 0.63 | 2.72 ± 0.54 | 0.47 |
| Collateral source to LAD | ||||
| LCX | NA | 6 (26.1) | 0 (0.0) | 0.0082 |
| RCA | NA | 17 (73.9) | 25 (100) | 0.0082 |
| Lesions in LCX and RCA | ||||
| LCX and RCA | 11 (50.0) | 16 (69.6) | 12 (48.0) | 0.28 |
| LCX | 4 (18.2) | 3 (13.0) | 5 (20.0) | 0.85 |
| RCA | 4 (18.2) | 2 (8.7) | 6 (24.0) | 0.38 |
| Comorbidities | ||||
| Hypertension | 20 (90.1) | 17 (73.9) | 19 (76.0) | 0.34 |
| Hyperlipidaemia | 10 (45.5) | 13 (36.5) | 11 (44.0) | 0.69 |
| Diabetes mellitus | 10 (45.5) | 11 (47.8) | 8 (32.0) | 0.51 |
| Haemodialysis | 0 (0.0) | 2 (8.7) | 1 (4.0) | 0.52 |
Categorical data are presented as number (%); continuous data are presented as mean ± SD. CCS: Canadian Cardiovascular Society; LAD: left anterior descending artery; LCX: left circumflex artery; LVEF: left ventricular ejection fraction; NA: not applicable; RCA: right coronary artery.
Intraoperative data including mean graft flow and pulsatility index
All patients survived their surgery and were discharged from the hospital. Intraoperative variables did not differ significantly among the three groups with the exception of MGF (P = 0.025) and PI (P = 0.046) of the left ITA grafts (Table 2). We found that the higher the Rentrop grade, the lower and higher the MGF and PI, respectively. MGF and PI in Group P were significantly higher (50.4 ± 26.3 vs 32.6 ± 14.4 ml/min, P = 0.020) and lower (1.69 ± 0.62 vs 2.54 ± 1.60, P = 0.041) than those in Group R, respectively, as shown in Fig. 1 (C and D). The MGF and PI of grafts in other territories of the LCX and RCA were comparable among the three groups (Table 2).
Table 2:
Intraoperative and postoperative outcomes
| Variables | Group P (n = 22) | Group M (n = 23) | Group R (n = 25) | P-value |
|---|---|---|---|---|
| Number of distal anastomoses | 2.86 ± 1.21 | 3.30 ± 1.02 | 3.08 ± 1.15 | 0.38 |
| Graft materials | ||||
| Left ITA | 22 (100) | 23 (100) | 25 (100) | NA |
| Right ITA | 2 (9.1) | 1 (4.3) | 1 (4.0) | 0.68 |
| Radial artery | 8 (36.4) | 16 (69.6) | 12 (48.0) | 0.079 |
| Gastroepiploic artery | 1 (4.5) | 0 (0.0) | 1 (4.0) | 0.76 |
| Saphenous vein | 16 (72.7) | 20 (87.0) | 21 (84.0) | 0.47 |
| MGF, (ml/min) | ||||
| LAD with left ITA | 50.4 ± 26.3 | 43.1 ± 24.1 | 32.6 ± 14.4 | 0.025 |
| LCX | 37.5 ± 14.6 | 43.7 ± 15.2 | 36.2 ± 13.5 | 0.25 |
| RCA | 44.9 ± 29.8 | 39.7 ± 26.7 | 35.8 ± 15.4 | 0.57 |
| PI | ||||
| LAD with left ITA | 1.69 ± 0.62 | 2.29 ± 1.01 | 2.54 ± 1.60 | 0.046 |
| LCX | 2.09 ± 1.12 | 1.94 ± 0.63 | 2.44 ± 1.12 | 0.29 |
| RCA | 2.04 ± 0.63 | 1.64 ± 0.88 | 2.07 ± 0.91 | 0.26 |
| Graft patency | ||||
| Left ITA to LAD | 19 (100) | 20 (100) | 21 (100) | NA |
| Graft to LCX | 13 (86.7) | 18 (94.7) | 16 (100) | 0.38 |
| Graft to RCA | 14 (93.3) | 16 (94.1) | 17 (100) | 0.75 |
| Postoperative ejection fraction | ||||
| 2 weeks | 0.48 ± 0.12 | 0.49 ± 0.12 | 0.47 ± 0.08 | 0.48 |
| 1 year | 0.50 ± 0.11 | 0.49 ± 0.11 | 0.48 ± 0.09 | 0.43 |
| 5 years | 0.47 ± 0.11 | 0.46 ± 0.08 | 0.51 ± 0.06 | 0.33 |
Categorical data are presented as number (%); continuous data are presented as mean ± SD. ITA: internal thoracic artery; LAD: left anterior descending artery; LCX: left circumflex artery; MGF: mean graft flow; NA: not applicable; PI: pulsatility index; RCA: right coronary artery.
Figure 1:
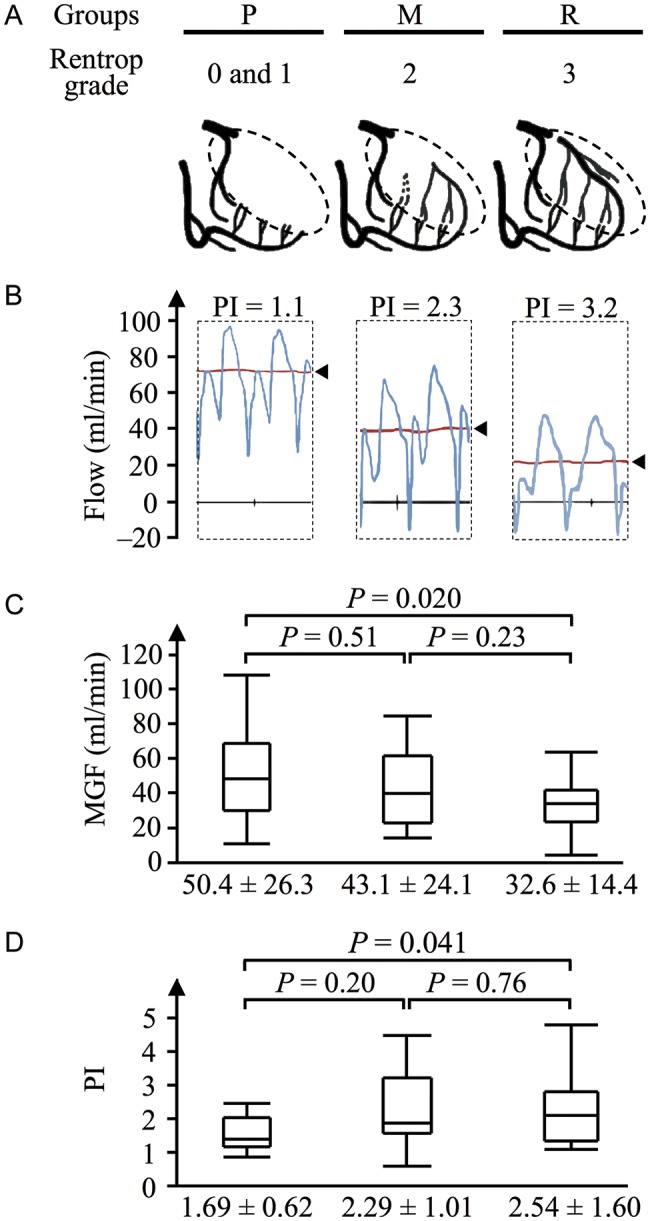
Mean graft flow (MGF) and pulsatility index (PI) of left internal thoracic artery (ITA) grafts are shown for the three groups: Poor collaterals (Rentrop grades 0 and 1, Group P, n = 22), Moderate collaterals (Grade 2, Group M, n = 23); and Rich collaterals (Grade 3, Group R, n = 25). (A) Schemas depicting the collaterals in each group. Dotted circles indicate myocardial territories distal to chronic total occlusion, where collaterals develop. (B) Representative tracings of left ITA flow in each group (arrowheads, MGF). (C) MGF was significantly different among the three groups (P = 0.025), tending to be lower with increasing Rentrop grade. MGF in Group P was significantly higher than that in Group R (P = 0.020). (D) PI was also significantly different among the three groups (P = 0.046), tending to be higher with increasing Rentrop grade. PI in Group P was significantly lower than that in Group R (P = 0.041). M: moderate; P: poor; R: rich.
Early postoperative coronary angiography and long-term clinical outcomes
Postoperative coronary angiography was performed in 60 of 70 patients (85.7%): 19 (86.4%), 20 (87.0%) and 21 (84.0%) patients in Groups P, M and R, respectively. All coronary angiographies showed patent left ITA grafts (Table 2); however, 1 patient in Group R (4.0%) showed a string sign. The collateral feeding arteries distal to CTO in the LAD that had been detected preoperatively in Groups M and R were not observed postoperatively in all patients.
The mean duration of follow-up was 5.00 ± 3.11 (maximum 10.3) years: 4.80 ± 3.02 (maximum 10.3), 4.14 ± 2.78 (maximum 9.9) and 4.56 ± 2.99 (maximum 8.7) years in Groups P, M and R, respectively. The ejection fraction was comparably preserved for up to 5 years postoperatively in all groups (Table 2). The long-term survival and cardiac event-free rates were comparable among the three groups (Fig. 2A and B).
Figure 2:
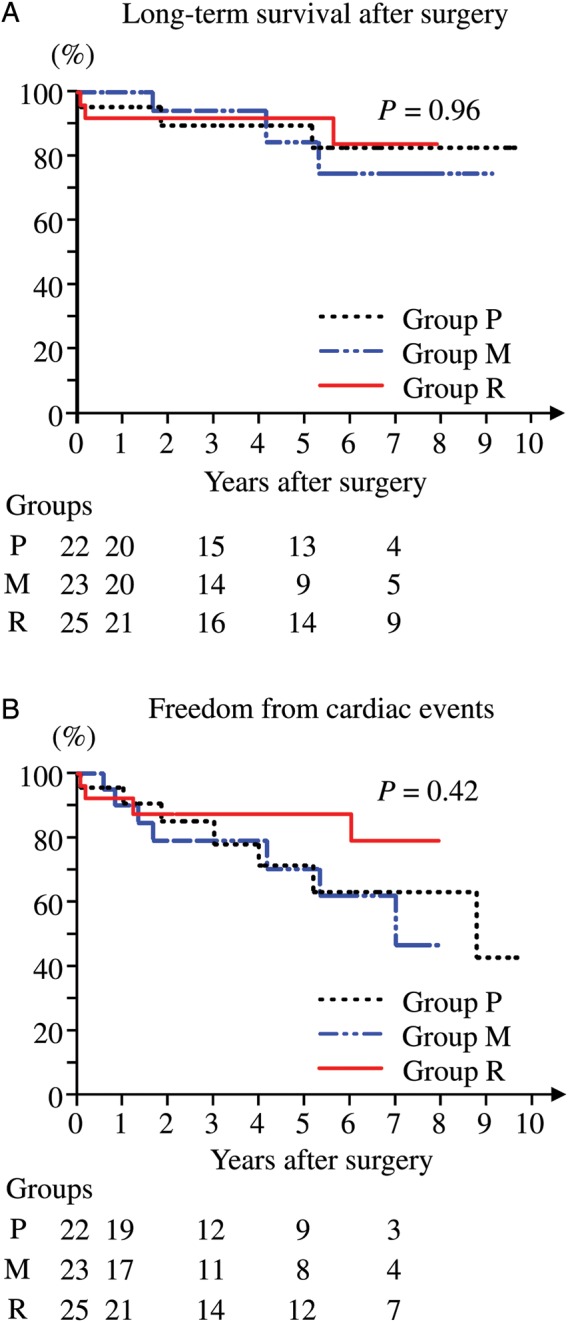
The cumulative survival rate (A) and cardiac event-free rate (B) are shown. (A) Long-term survival after surgery did not differ significantly among Groups P, M and R. The cumulative survival rates at 5 years were 83.2, 75.6 and 92.0% for Groups P, M and R, respectively. (B) Freedom from cardiac events did not differ significantly among Groups P, M and R. The cumulative cardiac event-free rates at 5 years were 63.1, 61.5 and 87.4% in Groups P, M and R, respectively. Numbers at risk are listed below the x-axis.
DISCUSSION
The present study demonstrates that the MGF of the left ITA graft distal to CTO in the LAD decreases and its PI increases with increasing Rentrop grade. We found that when collateral perfusion distal to CTO is well developed (high Rentrop grade), there is potential competition between the rich collateral flow and left ITA graft flow. The preoperative evaluation of collateral development distal to CTO using Rentrop grade is, therefore, important to appropriately assess intraoperative graft flow in consideration of the resultant possibility of graft flow competition. Our findings also indirectly clarify that a Rentrop grade showing angiographic collateral circulation certainly reflects the degree of haemodynamic collateral perfusion, inversely reflects the extent of myocardial ischaemia and could thus be helpful in deciding optimal timing of surgical treatment.
Previous reports have demonstrated that the transit time flow measurements of MGF and PI are appropriate for assessing graft quality and predicting graft failure and, furthermore, that a combination of high MGF and low PI is associated with long-term graft patency [2, 3, 9–12]. With regard to graft performance, the MGF and PI of left ITA grafts distal to chronically occluded LADs in Group R were less desirable than those in Group P. We propose that the less desirable pattern of MGF and PI in Group R resulted from competition between the rich collateral perfusion and ITA graft flow in this group. Tokuda et al. reported that an MGF of 15 ml/min or less and a PI of 5.1 or greater in ITA grafts to the LAD are the optimal cut-off criteria for predicting early graft failure [11]. In the current study, both of these markers were within the above-specified cut-off criteria for all but 4 patients. Only the MGF value was below the cut-off criterion in 1 patient each in Groups P and M, whereas both of the markers were outside the cut-off criteria in 2 patients in Group R, despite the fact that all of these patients’ early postoperative coronary angiographies showed patent left ITA grafts. In addition, another patient in Group R showed the string sign in the left ITA graft. Researchers have previously demonstrated that string sign in grafts can result from graft flow competition distal to a mild stenosis [5, 6]. In the patient, both MGF and PI (52 ml/min and 1.15, respectively) were within the cut-off criteria and the collateral feeding arteries disappeared in the postoperative angiography. Thus, his string sign in the ITA graft seemed not to result from the currently demonstrated graft flow competition with rich collateral circulation distal to CTO. Considering all of our findings together, we could say that there is potential competition between rich collateral flow (high Rentrop grade) distal to CTO in the LAD and left ITA graft flow, although the competition might not influence the graft patency.
This study does not show any demonstrable influence of the potential graft flow competition distal to CTO in the LAD on early graft patency, although graft failure related to the competition could not be apparent until the long-term postoperative period. Graft flow competition distal to a mild stenosis is reportedly associated with graft failure [3–6]. Graft flow distal to a mild stenosis competes with antegrade native flow through the mild stenosis, whereas graft flow distal to CTO competes with retrograde native flow through collateral feeding arteries. In contrast to stenosis, the severity of which does not change after CABG, the extent of retrograde native collateral perfusion can decline over the course of time after revascularization. In this study, preoperatively detected retrograde collateral feeding arteries had indeed disappeared in all of the postoperative coronary angiographies. Similar to the present results, several reports regarding percutaneous coronary interventions for CTO in the LAD have demonstrated that collateral perfusion distal to CTO regresses soon after successful coronary intervention as demonstrated by coronary angiography [16, 17]. All of these findings show that retrograde collateral perfusion tends to be surmounted by ITA graft flow and disappears over time, since collateral channels are supposedly much smaller, providing greater resistance, than ITA graft. Furthermore, as collateral perfusion declines, ITA graft flow can increase over time, since ITA graft can autoregulate its flow depending on the demand and resistance of myocardial territories [4–6]. Further studies are warranted to elucidate the long-term graft patency in patients with CTO especially in association with the preoperative degree of collateral development.
This study also clarifies that a Rentrop grade showing angiographic collateral circulation certainly reflects the degree of haemodynamic ‘retrograde’ collateral perfusion and inversely reflects the extent of myocardial ischaemia distal to CTO in the LAD. To select optimal treatment strategies for ischaemic heart disease, it is essential to diagnose the extent of myocardial ischaemia. Researchers have recently demonstrated that evaluation of the significance of a stenotic lesion and assessment of whether revascularization is necessary for it are assisted by measuring perfusion pressures over the stenosis to determine the fractional flow reserve [8, 18]. In CABG, preoperative measurement of fractional flow reserve can help to predict graft flow competition distal to a mild stenosis [8]. However, in CTO, fractional flow reserve cannot be used to measure the extent of distal perfusion because access is not possible through completely occluded lesions. Thus, the Rentrop grade could be a useful alternative means of assessing the degree of myocardial ischaemia distal to CTO and determining the optimal timing of treatment.
Probably because it can communicate with the LAD through septal branches, the RCA, rather than the LCX, seems to be the major collateral source for the territory distal to CTO of the LAD. In the patients of Groups M and R in whom a collateral source was detectable, collateral perfusion was most often from the RCA. Furthermore, the LCX did not provide rich (Rentrop grade 3) collateral perfusion in any patient. Takami et al. [16] reported that even after successful CABG, well-developed septal branches are still observable in some patients with CTO in the LAD. According to several reports regarding percutaneous coronary interventions, well-developed septal branches can be used to access CTO in a retrograde fashion [19]. During preoperative evaluation of patients with CTO in the LAD, we should pay particular attention to well-developed septal branches of the RCA that may persist in supplying perfusion distal to CTO even after revascularization.
The phenomenon of graft flow competition distal to CTO could be more accurately assessed if we were able to examine the dynamic flow interference between graft flow distal to CTO and perfusion of the collateral territory in each patient. Verhoye et al. [2] revealed the phenomenon of graft flow competition by clamping another graft to other territories in patients with triple vessel disease including CTO in the RCA and measuring the alterations of MGF distal to CTO in the RCA. The same method, namely measuring MGF and PI before and after clamping grafts to other territories, could be used to evaluate graft flow competition in patients with CTO in the LAD. This technique would be expected to accurately demonstrate graft flow competition distal to CTO in the LAD.
Researchers have recently discussed whether the degree of coronary collateral development could affect clinical outcomes of patients with coronary artery disease [13, 14]. Well-developed coronary collaterals have been reported to protect against perioperative myocardial damage and cognitive decline after operations [13, 14]. With regard to cardiac event-free rates, the superior results in Group R compared with those in the other two groups may have resulted from the well-developed collateral circulation. Therefore, the present results regarding clinical outcomes could have been influenced by the extent of coronary collateral development in each group, and it is unclear how the currently demonstrated flow difference of ITA graft affects the long-term outcomes. Further studies are warranted to elucidate the association of coronary collateral development with the clinical outcomes in patients with CTO and then evaluate their operative outcomes appropriately.
The present study has several limitations. First, it was a retrospective investigation with a relatively small number of patients, possibly too few to accurately compare graft patency rates and long-term clinical outcomes. Furthermore, the small sample size precluded correlation of the findings with the presence and severity of coronary stenoses in other territories. These factors may have influenced the present results. Secondly, because this indicator was not reported in the early stages of this relatively long-term study, we did not measure intraoperative backward graft flow, which is reportedly another means of assessing transit time flow together with MGF and PI [11, 12]. Thirdly, we did not evaluate the extent of ischaemia distal to CTO by other means such as myocardial scintigraphy, which would be useful for further appropriate demonstration of a correlation between the Rentrop grade and degree of ischaemia. Fourthly, the quality of ITA graft itself was not assessed, although it could influence the graft flow pattern. Fifthly, the early postoperative examinations of coronary angiography might be not enough to elucidate the possible graft failure related to the graft flow competition, as only long-term angiographic evaluation could clarify the actual influence of the competition on graft patency.
In conclusion, this study demonstrates that there is potential competition between well-developed native collateral flow (high Rentrop grade) distal to CTO in the LAD and distally anastomosed left ITA graft flow, although retrograde collateral circulation could regress over time after revascularization and the competition itself might not become clinically serious in the long-term. With regard to long-term outcomes after CABG in patients with CTO, further studies are warranted to elucidate how the extent of coronary collateral development by itself affects the overall clinical outcomes. The current findings are informative to precisely understand the physiology of coronary collateral circulation especially distal to CTO. They also clarify that a Rentrop grade showing angiographic collateral circulation certainly correlates with the degree of haemodynamic collateral perfusion and inversely with the degree of ischaemia distal to CTO. Thus, we should thoroughly consider the preoperative degree of coronary collateral perfusion distal to CTO (Rentrop grade) to deliberate optimal timing of CABG and especially evaluate intraoperative graft flow appropriately in association with the resultant possibility of graft flow competition.
Conflict of interest: none declared.
REFERENCES
- 1.Tsirikos Karapanos N, Suddendorf SH, Li Z, Huebner M, Joyce LD, Park SJ. The impact of competitive flow on distal coronary flow and on graft flow during coronary artery bypass surgery. Interact CardioVasc Thorac Surg. 2011;12:993–7. doi: 10.1510/icvts.2010.255398. doi:10.1510/icvts.2010.255398. [DOI] [PubMed] [Google Scholar]
- 2.Verhoye JP, Abouliatim I, Drochon A, de Latour B, Leclercq C, Leguerrier A, et al. Collateral blood flow between left coronary artery bypass grafts and chronically occluded right coronary circulation in patients with triple vessel disease. Observations during complete revascularisation of beating hearts. Eur J Cardiothorac Surg. 2007;31:49–54. doi: 10.1016/j.ejcts.2006.09.033. doi:10.1016/j.ejcts.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Nordgaard H, Nordhaug D, Kirkeby-Garstad I, Løvstakken L, Vitale N, Haaverstad R. Different graft flow patterns due to competitive flow or stenosis in the coronary anastomosis assessed by transit-time flowmetry in a porcine model. Eur J Cardiothorac Surg. 2009;36:137–42. doi: 10.1016/j.ejcts.2009.02.036. doi:10.1016/j.ejcts.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Sabik JF, III, Lytle BW, Blackstone EH, Khan M, Houghtaling PL, Cosgrove DM. Does competitive flow reduce internal thoracic artery graft patency? Ann Thorac Surg. 2003;76:1490–7. doi: 10.1016/s0003-4975(03)01022-1. doi:10.1016/S0003-4975(03)01022-1. [DOI] [PubMed] [Google Scholar]
- 5.Aris A, Borrás X, Ramió J. Patency of internal mammary artery grafts in no-flow situations. J Thorac Cardiovasc Surg. 1987;93:62–4. [PubMed] [Google Scholar]
- 6.Hartman J, Kelder H, Ackerstaff R, van Swieten H, Vermeulen F, Bogers A. Preserved hyperaemic response in (distal) string sign left internal mammary artery grafts. Eur J Cardiothorac Surg. 2007;31:283–9. doi: 10.1016/j.ejcts.2006.11.016. doi:10.1016/j.ejcts.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol. 2005;95:1088–91. doi: 10.1016/j.amjcard.2004.12.065. doi:10.1016/j.amjcard.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 8.Botman CJ, Schonberger J, Koolen S, Penn O, Botman H, Dib N, et al. Does stenosis severity of native vessels influence bypass graft patency? A prospective fractional flow reserve-guided study. Ann Thorac Surg. 2007;83:2093–7. doi: 10.1016/j.athoracsur.2007.01.027. doi:10.1016/j.athoracsur.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Jokinen JJ, Werkkala K, Vainikka T, Peräkylä T, Simpanen J, Ihlberg L. Clinical value of intra-operative transit-time flow measurement for coronary artery bypass grafting: a prospective angiography-controlled study. Eur J Cardiothorac Surg. 2011;39:918–23. doi: 10.1016/j.ejcts.2010.10.006. doi:10.1016/j.ejcts.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Kieser TM, Rose S, Kowalewski R, Belenkie I. Transit-time flow predicts outcomes in coronary artery bypass graft patients: a series of 1000 consecutive arterial grafts. Eur J Cardiothorac Surg. 2010;38:155–62. doi: 10.1016/j.ejcts.2010.01.026. doi:10.1016/j.ejcts.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Tokuda Y, Song MH, Ueda Y, Usui A, Akita T. Predicting early coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg. 2007;84:1928–33. doi: 10.1016/j.athoracsur.2007.07.040. doi:10.1016/j.athoracsur.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 12.Di Giammarco G, Rabozzi R. Can transit-time flow measurement improve graft patency and clinical outcome in patients undergoing coronary artery bypass grafting? Interact CardioVasc Thorac Surg. 2010;11:635–40. doi: 10.1510/icvts.2010.235663. doi:10.1510/icvts.2010.235663. [DOI] [PubMed] [Google Scholar]
- 13.Dieleman J, Sauër AM, Klijn C, Nathoe H, Moons K, Kalkman C, et al. Presence of coronary collaterals is associated with a decreased incidence of cognitive decline after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2009;35:48–53. doi: 10.1016/j.ejcts.2008.10.004. doi:10.1016/j.ejcts.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Caputo M, Anis RR, Rogers CA, Ahmad N, Rizvi SI, Baumbach A, et al. Coronary collateral circulation: effect on early and midterm outcomes after off-pump coronary artery bypass surgery. Ann Thorac Surg. 2008;85:71–9. doi: 10.1016/j.athoracsur.2007.08.026. doi:10.1016/j.athoracsur.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–92. doi: 10.1016/s0735-1097(85)80380-6. doi:10.1016/S0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 16.Takami Y, Matsumoto H. Angiographic fate of collateral vessels after surgical revascularization of the totally occluded left anterior descending artery. Ann Thorac Surg. 2007;83:120–5. doi: 10.1016/j.athoracsur.2006.08.033. doi:10.1016/j.athoracsur.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Zimarino M, Ausiello A, Contegiacomo G, Riccardi I, Renda G, Di Iorio C, et al. Rapid decline of collateral circulation increases susceptibility to myocardial ischemia: the trade-off of successful percutaneous recanalization of chronic total occlusions. J Am Coll Cardiol. 2006;48:59–65. doi: 10.1016/j.jacc.2005.12.079. doi:10.1016/j.jacc.2005.12.079. [DOI] [PubMed] [Google Scholar]
- 18.Pijls NH, Sels JW. Functional measurement of coronary stenosis. J Am Coll Cardiol. 2012;59:1045–57. doi: 10.1016/j.jacc.2011.09.077. doi:10.1016/j.jacc.2011.09.077. [DOI] [PubMed] [Google Scholar]
- 19.Utsunomiya M, Mukohara N, Hirami R, Nakamura S. Percutaneous coronary intervention for chronic total occlusive lesion of a left anterior descending artery using the retrograde approach via a septal-septal channel. Cardiovasc Revasc Med. 2010;11:34–40. doi: 10.1016/j.carrev.2009.03.001. doi:10.1016/j.carrev.2009.03.001. [DOI] [PubMed] [Google Scholar]


