Abstract
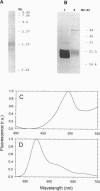
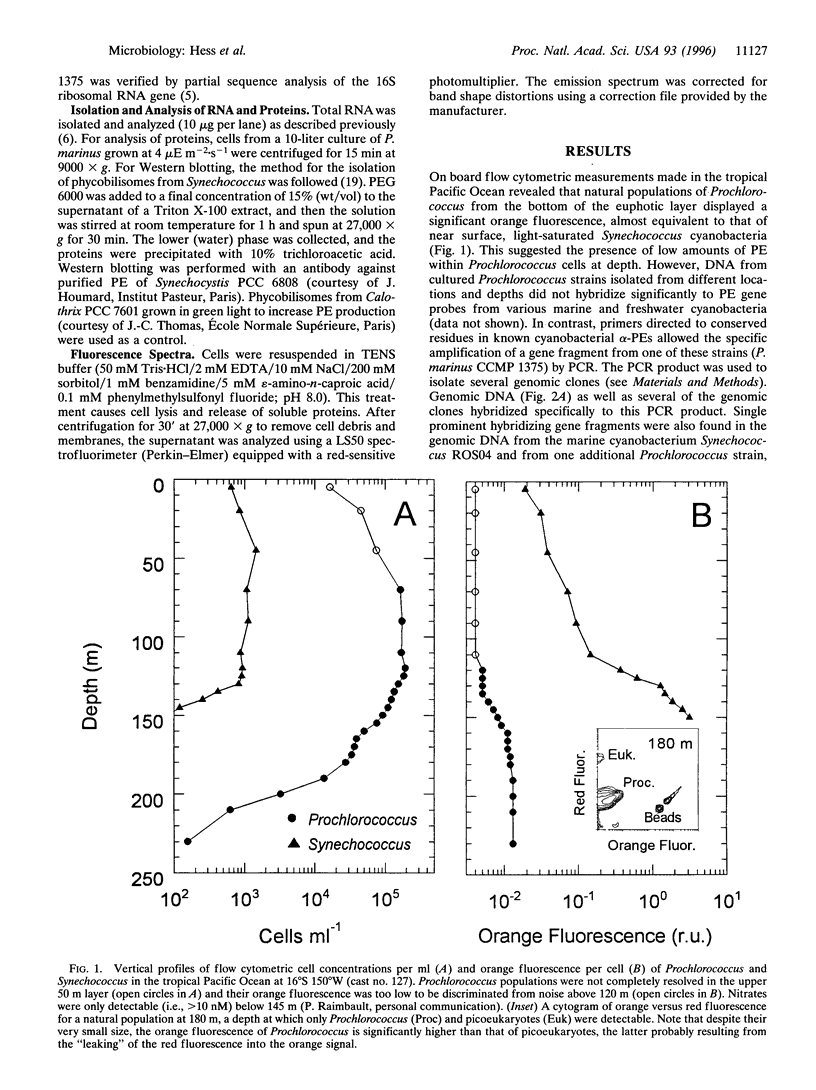
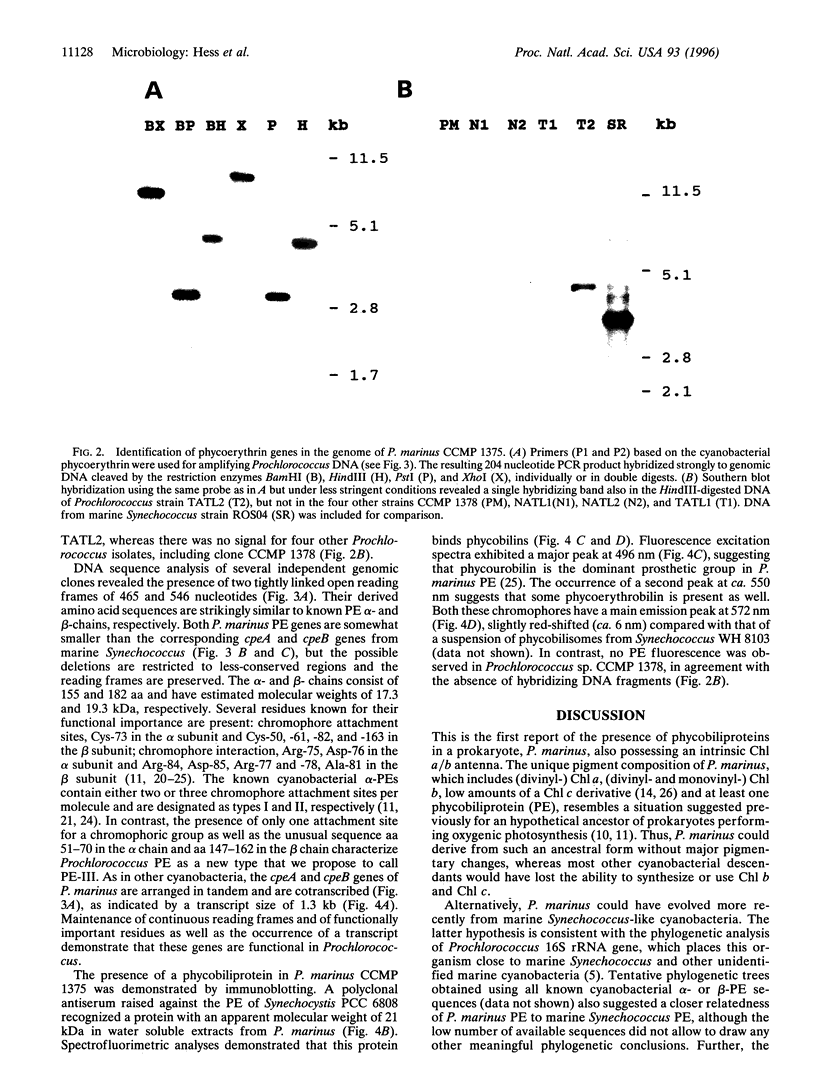
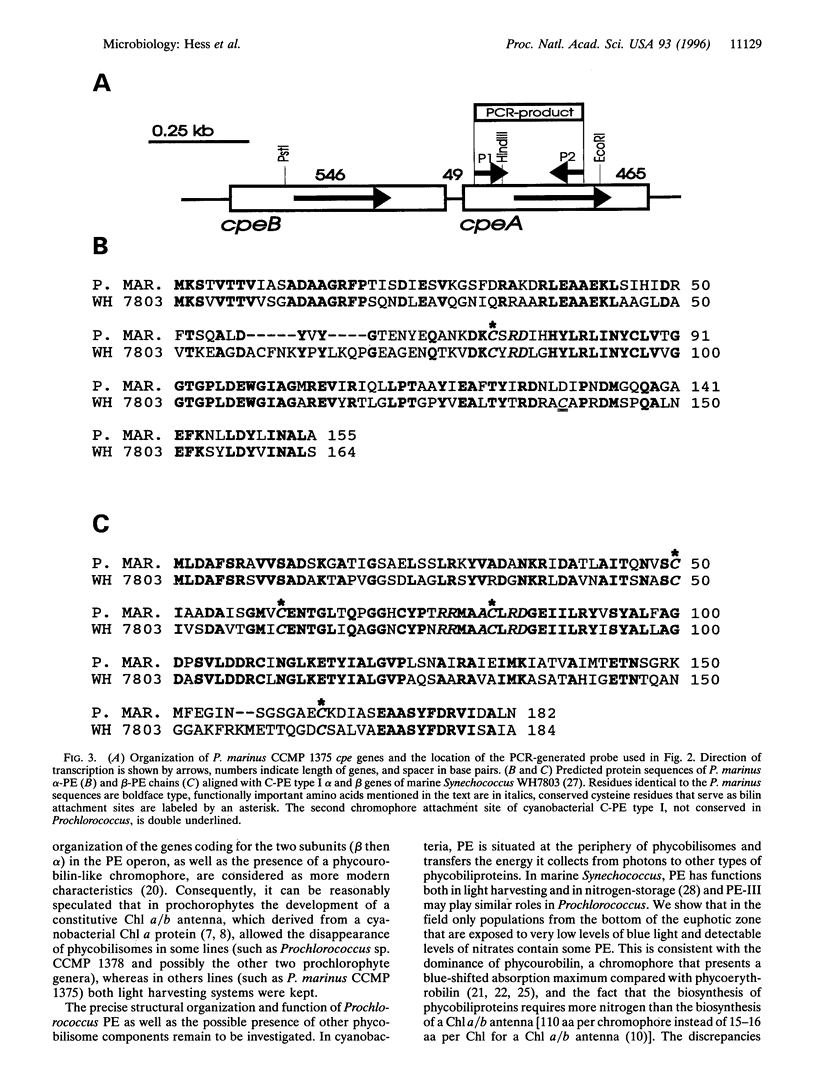
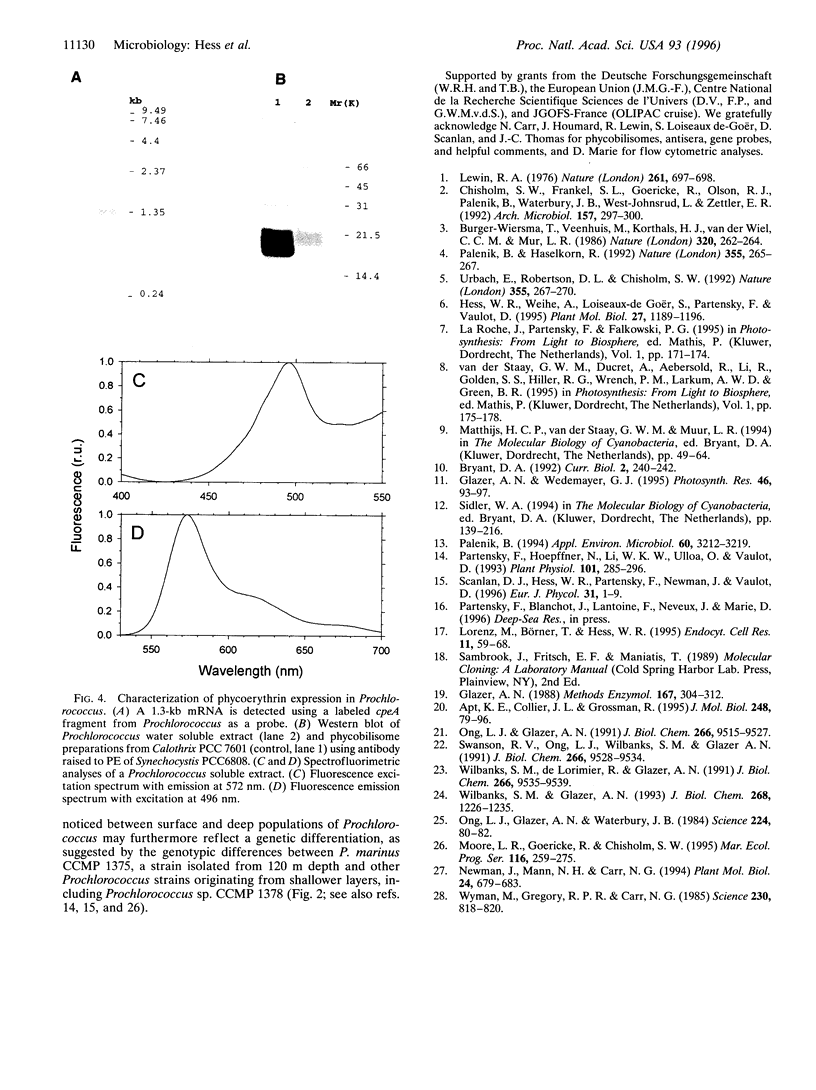
Prochlorococcus marinus CCMP 1375, a ubiquitous and ecologically important marine prochlorophyte, was bound to possess functional genes coding for the alpha and beta subunits of a phycobiliprotein. The latter is similar to phycoerythrins (PE) from marine Synechococcus cyanobacteria and bind a phycourobilin-like pigment as the major chromophore. However, differences in the sequences of the alpha and beta chains compared with known PE subunits and the presence of a single bilin attachment site on the alpha subunit designate it as a novel PE type, which we propose naming PE-III. P. marinus is the sole prokaryotic organisms known so far that contains chlorophylls a and b as well as phycobilins. These data strongly suggest that the common ancestor of prochlorophytes and the Synechococcus cyanobacteria contained phycobilins. Flow cytometric data from the tropical Pacific Ocean provide evidence that deep populations of Prochlorococcus possess low amounts of a PE-like pigment, which could serve either in light harvesting or nitrogen storage or both.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apt K. E., Collier J. L., Grossman A. R. Evolution of the phycobiliproteins. J Mol Biol. 1995 Apr 21;248(1):79–96. doi: 10.1006/jmbi.1995.0203. [DOI] [PubMed] [Google Scholar]
- Bryant D. A. Puzzles of chloroplast ancestry. Curr Biol. 1992 May;2(5):240–242. doi: 10.1016/0960-9822(92)90361-d. [DOI] [PubMed] [Google Scholar]
- Hess W. R., Weihe A., Loiseaux-de Goër S., Partensky F., Vaulot D. Characterization of the single psbA gene of Prochlorococcus marinus CCMP 1375 (Prochlorophyta). Plant Mol Biol. 1995 Mar;27(6):1189–1196. doi: 10.1007/BF00020892. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. Prochlorophyta as a proposed new division of algae. Nature. 1976 Jun 24;261(5562):697–698. doi: 10.1038/261697b0. [DOI] [PubMed] [Google Scholar]
- Newman J., Mann N. H., Carr N. G. Organization and transcription of the class I phycoerythrin genes of the marine cyanobacterium Synechococcus sp. WH7803. Plant Mol Biol. 1994 Feb;24(4):679–683. doi: 10.1007/BF00023564. [DOI] [PubMed] [Google Scholar]
- Ong L. J., Glazer A. N. Phycoerythrins of marine unicellular cyanobacteria. I. Bilin types and locations and energy transfer pathways in Synechococcus spp. phycoerythrins. J Biol Chem. 1991 May 25;266(15):9515–9527. [PubMed] [Google Scholar]
- Ong L. J., Glazer A. N., Waterbury J. B. An unusual phycoerythrin from a marine cyanobacterium. Science. 1984 Apr 6;224(4644):80–83. doi: 10.1126/science.224.4644.80. [DOI] [PubMed] [Google Scholar]
- Palenik B. Cyanobacterial community structure as seen from RNA polymerase gene sequence analysis. Appl Environ Microbiol. 1994 Sep;60(9):3212–3219. doi: 10.1128/aem.60.9.3212-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenik B., Haselkorn R. Multiple evolutionary origins of prochlorophytes, the chlorophyll b-containing prokaryotes. Nature. 1992 Jan 16;355(6357):265–267. doi: 10.1038/355265a0. [DOI] [PubMed] [Google Scholar]
- Partensky F., Hoepffner N., Li WKW., Ulloa O., Vaulot D. Photoacclimation of Prochlorococcus sp. (Prochlorophyta) Strains Isolated from the North Atlantic and the Mediterranean Sea. Plant Physiol. 1993 Jan;101(1):285–296. doi: 10.1104/pp.101.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R. V., Ong L. J., Wilbanks S. M., Glazer A. N. Phycoerythrins of marine unicellular cyanobacteria. II. Characterization of phycobiliproteins with unusually high phycourobilin content. J Biol Chem. 1991 May 25;266(15):9528–9534. [PubMed] [Google Scholar]
- Urbach E., Robertson D. L., Chisholm S. W. Multiple evolutionary origins of prochlorophytes within the cyanobacterial radiation. Nature. 1992 Jan 16;355(6357):267–270. doi: 10.1038/355267a0. [DOI] [PubMed] [Google Scholar]
- Wilbanks S. M., Glazer A. N. Rod structure of a phycoerythrin II-containing phycobilisome. I. Organization and sequence of the gene cluster encoding the major phycobiliprotein rod components in the genome of marine Synechococcus sp. WH8020. J Biol Chem. 1993 Jan 15;268(2):1226–1235. [PubMed] [Google Scholar]
- Wilbanks S. M., de Lorimier R., Glazer A. N. Phycoerythrins of marine unicellular cyanobacteria. III. Sequence of a class II phycoerythrin. J Biol Chem. 1991 May 25;266(15):9535–9539. [PubMed] [Google Scholar]
- Wyman M., Gregory R. P., Carr N. G. Novel Role for Phycoerythrin in a Marine Cyanobacterium, Synechococcus Strain DC2. Science. 1985 Nov 15;230(4727):818–820. doi: 10.1126/science.230.4727.818. [DOI] [PubMed] [Google Scholar]