Abstract
OBJECTIVES
We investigated whether oxidative stress and the arginine/nitric oxide pathway differ in control subjects and in adult patients who are candidates for the three most common cardiac surgical operations: coronary bypass surgery, aortic valve replacement for calcific non-rheumatic aortic stenosis or mitral valve repair for degenerative mitral insufficiency.
METHODS
In this prospective observational study, we studied 165 consecutive patients undergoing surgery from January to June 2011 (coronary bypass surgery, n = 63; aortic valve replacement for calcific non-rheumatic aortic stenosis, n = 51; mitral valve repair for degenerative mitral insufficiency, n = 51). Thirty-three healthy subjects with cardiovascular risk factors similar to surgery patients were also studied (Controls). Oxidative stress (the ratio of reduced and oxidized glutathione and urinary isoprostane), antioxidants (alpha- and gamma tocopherol) and factors involved in nitric oxide synthesis (arginine, symmetric and asymmetric dimethylarginine) were measured before surgery. Analysis of variance general linear models and principal component analysis were used for statistical analysis.
RESULTS
Surgical patients had increased levels of oxidative stress and decreased levels of antioxidants. Increased levels of nitric oxide inhibitor asymmetric dimethylarginine were detected in surgical candidates, suggesting arginine/nitric oxide pathway impairment. Concerning the differences among surgical procedures, higher oxidative stress and a major imbalance of the ratio between substrate and inhibitors of nitric oxide synthesis were evidenced in patients who were candidates for mitral valve repair with respect to coronary bypass surgery patients and patients with calcific non-rheumatic aortic stenosis.
CONCLUSIONS
Patients undergoing cardiac surgery have increased oxidative stress and a trend towards an impaired arginine/nitric oxide pathway with respect to Controls. Patients affected by mitral valve regurgitation show more pronounced perturbations in these pathways. The clinical implications of these findings need to be investigated.
Keywords: Aortic valve stenosis, Coronary artery disease, Mitral valve regurgitation, Nitric oxide, Oxidative stress
INTRODUCTION
Oxidative stress results from the imbalance between the generation of reactive oxygen species (ROS) and the ability of cells and tissues to readily detoxify them. The increased oxidative stress can impair the synthesis of the main vasoactive mediator, nitric oxide (NO) and inactivate the produced NO by transforming it into peroxynitrate. NO, produced by the oxidation of L-arginine (Arg) by NO synthase (NOS), promotes many beneficial effects in the vasculature, including vasodilatation, enhanced fibrinolysis and inhibition of multiple atherothrombotic processes such as platelet aggregation, leucocyte adhesion and smooth muscle cell proliferation. Some authors have proposed oxidative stress and NO pathway derangements as potential determinants of postoperative adverse events such as cardiopulmonary bypass (CPB)-related complications [1] and vein graft failure [2] or atrial fibrillation [3], since they play a significant role in cardiovascular diseases such as atherosclerosis, coronary artery disease, diabetes and heart failure [4, 5].
In addition, preoperative serum asymmetric dimethylarginine (ADMA) was described to identify paediatric cardiac surgery patients at risk of poor postoperative outcomes following CPB [6].
Much less information is available concerning patients undergoing adult cardiac surgery. A sustained overproduction of ROS was previously demonstrated in coronary artery bypass surgery [7], and evidence was provided of the importance of the NO signalling cascade in aortic valve stenosis and in its progression to sclerosis [8].
Previous studies in patients with valve disease were focused on alterations of valve tissues or on in situ markers of oxidative stress [9, 10], and only limited information is available concerning the circulating levels of the different components of the antioxidant/oxidant balance and the synthetic pathway of NO in coronary vs valve disease and in surgical patients vs healthy controls.
This study was performed to answer two main questions: first, do surgical patients suffering from coronary, aortic and mitral diseases with surgical indication differ with respect to healthy subjects with a similar profile of cardiovascular risk factors, in terms of oxidative stress and NO impairment? Secondly, do the profiles of plasma oxidative and NO precursors/inhibitors differ according to the three surgical pathologies? Thus, in this study, we have assessed the levels of oxidative stress and NO markers in the plasma of healthy controls and patients undergoing isolated coronary bypass surgery, aortic valve replacement for calcific non-rheumatic aortic stenosis or mitral valve repair for degenerative mitral insufficiency, which are the three most common adult cardiac operations.
MATERIALS AND METHODS
Written informed consent to participate to this observational study, which was approved by Centro Cardiologico Monzino Institutional Review Board, was obtained from all patients. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Study design
An observational study comparing three groups of surgical patients and one group of controls with a similar prevalence of risk factors, but free from cardiovascular disease.
Study population
Over a 6-month period (January 2011–June 2011), 321 consecutive patients who were candidates for a cardiac surgical procedure at the Centro Cardiologico Monzino of Milan were screened. Preoperative inclusion criteria were the need for elective, isolated surgical procedure, age between 18 and 80 years, ejection fraction of >30%, normal sinus rhythm and no history of atrial fibrillation. Patients suffering from renal or liver disease or taking antioxidants (e.g. vitamin supplementation) within 30 days prior to surgery were excluded. We enrolled 169 patients who were candidates for the following surgical procedures: coronary artery bypass surgery (CABG, n = 65), aortic valve replacement for calcific non-rheumatic aortic stenosis (Aortic, n = 52) and mitral valve repair for degenerative mitral valve regurgitation (Mitral, n = 52). Two patients (1 CABG and 1 Aortic) were excluded from analyses because they refused to participate in the study, while the other ones (1 CABG and 1 Mitral) underwent multiple interventions. Valve lesions were classified on the basis of echocardiographic and surgical findings.
Healthy subjects (age between 18 and 80 years) with normal sinus rhythm, no electrocardiographic alterations and no history of atrial fibrillation (Controls, n = 33) were screened from those attending the clinic for global control of cardiovascular risk at Centro Cardiologico Monzino, IRCCS. Cardiovascular risk factors present in patients were taken into consideration for the enrollment: in particular, age, gender, family history of coronary artery disease, diabetes mellitus, hypertension, hypercholesterolaemia and history of smoking habits. Subjects suffering from renal or liver disease or taking antioxidants (e.g. vitamin supplementation) within 30 days prior to surgery were excluded. The clinical features of Controls, CABG, Aortic and Mitral patients are listed in Table 1.
Table 1:
Preoperative clinical features of the study populations
| Variable | Controls (N = 33) | CABG (N = 63) | Aortic (N = 51) | Mitral (N = 51) | P-value* |
|---|---|---|---|---|---|
| Age (years) | 57.1 (53.2, 61.0) | 62.6 (60.6, 64.6) | 66.3 (64.2, 68.4) | 58.4 (55.3, 61.4) | <0.0001 |
| Male gender | 21 (63.6%) | 61 (96.8%) | 30 (58.8%) | 31 (60.8%) | <0.0001 |
| Body mass index | 27.3 (25.8, 28.8) | 27.2 (26.4, 28.1) | 26.6 (25.4, 27.9) | 24.8 (23.9, 25.6) | 0.002 |
| Hypertension | 16 (48.5%) | 43 (68.3%) | 31 (60.8%) | 18 (35.3%) | 0.003 |
| Dyslipidaemia | 16 (48.5%) | 51 (81%) | 30 (58.8%) | 27 (52.9%) | 0.002 |
| Triglycerides (mg/dl) | 113 (96.9, 130) | 131 (108, 154) | 104 (86.8, 120) | 98.4 (86.7, 110) | 0.04 |
| High-density lipoprotein cholesterol (mg/dl) | 54.9 (49.2, 60.6) | 43.1 (40.6, 45.6) | 53.3 (49.2, 57.3) | 52.0 (48.1, 55.9) | <0.0001 |
| Low-density lipoprotein cholesterol (mg/dl) | 139 (126, 151) | 114 (104, 123) | 121 (111, 131) | 137 (125, 148) | 0.002 |
| Total cholesterol (mg/dl) | 216 (203, 229) | 182 (172, 192) | 196 (186, 206) | 210 (197, 223) | 0.0002 |
| Creatinine (mg/dl) | 0.83 (0.76, 0.90) | 0.93 (0.89, 0.97) | 0.86 (0.80, 0.92) | 0.88 (0.83, 0.94) | 0.10 |
| Glucose (mg/dl) | 106 (98.5, 113) | 121 (112, 131) | 115 (108, 122) | 107 (102, 112) | 0.01 |
| Current smoker | 6 (18.2%) | 13 (20.6%) | 3 (5.9%) | 7 (13.7%) | 0.15 |
| Echocardiographic ejection fraction | n.a. | 59.4 (56.9, 61.9) | 59.8 (57.2, 62.4) | 63.4 (60.7, 66.1) | 0.063 |
| Previous stroke | 0 (0%) | 2 (3.2%) | 0 (0%) | 2 (3.9%) | 0.38 |
| New York Heart Association class | n.a. | 2.0 (1.8, 2.2) | 2.1 (1.9, 2.4) | 2.0 (1.8, 2.3) | 0.53 |
| Echocardiographic systolic pulmonary artery pressure (mmHg) | n.a. | 27 (25, 30) | 33 (30, 35) | 34 (31, 36) | 0.01 |
| Previous transient ischaemic attack | 0 (0%) | 3 (4.8%) | 1 (2%) | 1 (2%) | 0.52 |
| Drug therapies | |||||
| Antiplatelets | 2 (6.1%) | 43 (68.3%) | 19 (37.3%) | 5 (9.8%) | <0.0001 |
| Angiotensin receptor blockers | 4 (12.1%) | 11 (17.5%) | 16 (31.4%) | 5 (9.8%) | 0.026 |
| Converting enzyme inhibitors | 6 (18.2%) | 22 (34.9%) | 11 (21.6%) | 18 (35.3%) | 0.15 |
| Beta-blockers | 2 (6.1%) | 41 (65.1%) | 17 (33.3%) | 21 (41.2%) | <0.0001 |
| Calcium channel blockers | 3 (9.1%) | 19 (30.2%) | 14 (27.5%) | 2 (3.9%) | 0.0006 |
| Nitrates | 0 (0%) | 19 (30.2%) | 2 (3.9%) | 2 (3.9%) | <0.0001 |
| Statins | 6 (18.2%) | 42 (66.7%) | 17 (33.3%) | 9 (17.6%) | <0.0001 |
Means and 95% CI or number (%).
*P-values refer to the homogeneity among the four groups, as assessed by ANOVA (continuous variables) or by chi-square (categorical variables); significant P values are reported in bold.
All subjects were assessed with detailed medical history, physical examination and electrocardiography. Echocardiography was performed in patients who were candidates for surgery.
Blood sampling and biochemical measurements
In all surgical patients, blood and urine were collected the day before surgery or the day before coronary angiography. When prescribed, the latter was performed during the same admission, within 2 days before surgery. Controls underwent sample collection at a scheduled visit.
Whole blood: a peripheral blood sample was drawn from patients and controls while fasting, collected in tubes containing ethylenediaminetetraacetic acid, (EDTA) disodium salt (9.3 mM; Vacutainer Systems, Becton Dickinson, Franklin Lakes, NJ, USA), kept on ice and immediately precipitated with 10% trichloroacetic acid (Sigma-Aldrich, St Louis, MO, USA) in 1 mM EDTA solution. After centrifugation at ×10 000g for 10 min at 4°C, the supernatant was stored at −80°C until analysis.
Plasma: EDTA-anticoagulated blood was centrifuged at ×3000g for 10 min at 4°C within 30 min after being drawn. Plasma was separated and aliquots were stored at −80°C until analysis.
Urine: An overnight urine collection the night before surgery or the night before the visit was carried out and samples stored at −80°C until analysis.
In this study, we have assessed the following markers:
-
Oxidative stress: whole blood reduced a glutathione (GSH)/disulphide glutathione (GSSG) ratio (GSH/GSSG); urinary 8-iso-prostaglandin F2α (8-iso-PGF2α).
Levels of GSH and GSSG were determined in whole blood by the liquid chromatography–tandem mass spectrometry (LC–MS/MS) method. The separation of analytes was conducted on a Luna PFP analytical column (100 × 2.0 mm, 3 µm, Phenomenex, Torrance, CA, USA) eluted at 35°C under isocratic conditions at 200 µl/min by 1% methanol in ammonium formate 0.75 mM adjusted to pH 3.5 with formic acid. Analysis was performed by an Accela chromatographic system coupled with a triple quadrupole mass spectrometer TSQ Quantum Access (Thermo Fisher Scientific, San Jose, CA, USA) using electrospray ionization source in the positive ion mode. The transitions used in the multiple reaction monitoring were m/z 308.1 → m/z 76.2 + 84.2 + 161.9 for GSH and m/z 613.2 → m/z 230.5 + 234.6 + 354.8 for GSSG. Data were obtained after comparison with calibration curves using GSH and GSSG standard solutions (Sigma-Aldrich). The intra- and inter-coefficients of variation (%) obtained with standard samples were <5% for the both the analytes considered. The limits of detection were 0.031 µmol/l and 0.008 µmol/l for GSH and GSSG, respectively. Levels of GSH and GSSG were corrected for haemoglobin and expressed as µmol/g Hb.
Urinary 8-iso-PGF2á was detected by the LC–MS/MS method according to Cavalca et al. [11]. The urinary concentration was calculated from the area ratio of the ion peaks of the 8-iso-PGF2α over the respective deuterated standard (8-iso-PGF2α-d4). The estimated values were corrected for the urinary creatinine levels and expressed as pg/mg of creatinine.
Antioxidants: plasma vitamin E (α-tocopherol and γ-tocopherol). Plasma α- and γ-tocopherol were measured by high-performance liquid chromatography (ESA Bioscences, Chelmford, MA, USA) equipped with fluorimetric detector FP-1520 (Jasco, Tokyo, Japan), after organic extraction as previously described [12].
Arginine/NO pathway: the substrate L-arginine (Arg) and the endogenous inhibitors of NO synthesis, ADMA and symmetric dimethylarginine (SDMA) were evaluated in plasma.
Their simultaneous determination was performed by LC–MS/MS as previously described [13]. The Arg/ADMA and Arg/SDMA ratios were calculated as indexes of the Arg/NO metabolic pathway status.
Statistical analysis
Continuous variables are summarized as mean ± 95% confidence intervals (95% CIs) if normally distributed; in case, they were not normally distributed, they were log-transformed before analysis. Categorical variables are summarized as frequency and percentage, and compared among groups by χ2. Variables with a sparse distribution (<5 expected counts in one or more cells) were compared by the Fisher's exact test. Plasma biomarkers of oxidative balance and the NO pathway were first compared between Controls and patients who were candidates for surgery (CABG, Aortic, Mitral patients together) by general linear models. As there were major differences in the clinical features in the study groups, three statistical models with different levels of adjustment for baseline clinical features were employed: Model 1, unadjusted; Model 2, adjusted for age and gender; Model 3, adjusted for age, gender, body mass index, hyperglycaemia, dyslipidaemia, hypertension and treatment with anti-platelet, anti-hypertensive drugs, nitrates and statins. The same models were employed to test the differences within the three groups of patient who were candidates for surgery. All the variables that yielded a significant P-value for analysis of variance (ANOVA; three-group comparison) underwent a post hoc analysis to test individual between-group differences.
Our sample size allowed 80% power to deem as significant a difference of ∼50% of a standard deviation in the comparison of patients vs Controls and a difference of ∼60% in the comparison of different patient groups.
We then analysed the overall patterns of oxidative balance and NO pathway markers by including all 10 variables (log GSH/GSSG ratio, log 8-iso PGF2α, α-tocopherol, log γ-tocopherol, GSH, Arg, ADMA, SDMA, Arg/ADMA ratio and Arg/SDMA ratio) in a principal component analysis (PCA). PCA is a statistical dimension-reduction technique widely employed to compress a large set of variables into a few components that convey a large proportion of the information contained in the original data. We used the first two principal components to plot the reciprocal distances of the four groups of subjects, in terms of oxidative balance and NO pathway markers, in a bidimensional graph.
All tests were two-sided and P-values of <0.05 were considered significant. All analyses were performed by SAS v. 9.2.
RESULTS
Patients
Patient groups differed in terms of preoperative clinical features (Table 1), according to the natural history of the coronary or valve diseases. Briefly, patients with mitral valve disease were the youngest and Aortic patients were the oldest; CABG patients were more frequently males, hypertensive and dyslipidaemic. As expected, drug regimens sensibly differed, with CABG patients more frequently receiving statins, anti-platelet and anti-hypertensive drugs, and aortic disease patients receiving more drug therapies than mitral valve patients and controls.
Oxidative stress
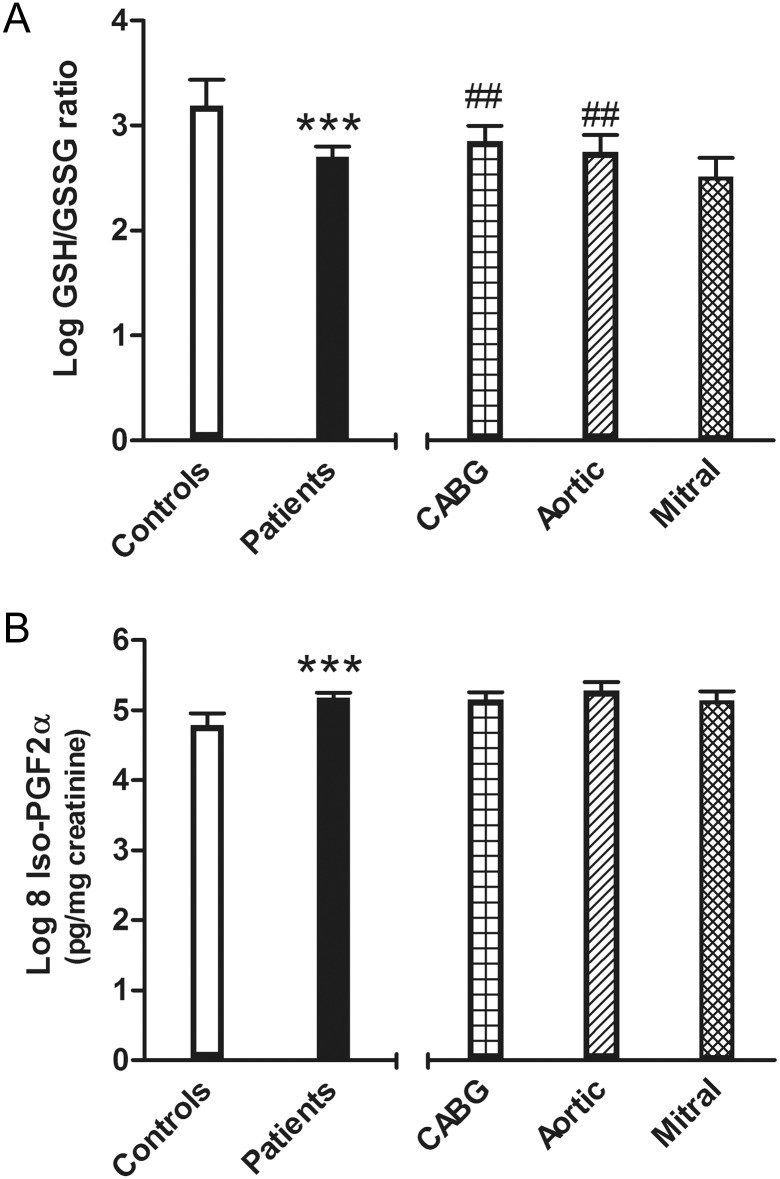
Oxidative stress was significantly greater in patient who were candidates for surgery with respect to controls, regardless of the statistical model employed (Table 2): GSH/GSSG ratios were higher, and urinary 8-iso-PGF2α levels were lower in Controls, compared with surgery patients (Fig. 1A and B).
Table 2:
Oxidative stress markers, antioxidants and nitric oxide pathway
| Variable | Modela | Controls (N = 33) | Patients (N = 165) (all groups) | P-value |
|---|---|---|---|---|
| Log GSH/GSSG ratio | 1 | 3.19 (2.95, 3.43) | 2.70 (2.60, 2.80) | <0.001 |
| 2 | 3.19 (2.95, 3.43) | 2.70 (2.60, 2.80) | <0.001 | |
| 3 | 3.35 (3.09, 3.61) | 2.68 (2.58, 2.78) | <0.001 | |
| Log 8-iso PGF2α (pg/mg creatinine) | 1 | 4.79 (4.63, 4.95) | 5.18 (5.10, 5.26) | <0.001 |
| 2 | 4.80 (4.64, 4.96) | 5.17 (5.09, 5.25) | <0.001 | |
| 3 | 4.80 (4.62, 4.98) | 5.18 (5.10, 5.26) | <0.001 | |
| α-Tocopherol (µg/ml) | 1 | 14.1 (13.1, 15.0) | 11.9 (11.5, 12.3) | <0.001 |
| 2 | 13.9 (13.0, 14.9) | 11.9 (11.5, 12.4) | <0.001 | |
| 3 | 13.5 (12.5, 14.5) | 12.1 (11.6, 12.4) | 0.0068 | |
| Log γ-tocopherol (pg/ml) | 1 | 2.45 (2.33, 2.57) | 2.03 (1.97, 2.09) | <0.001 |
| 2 | 2.43 (2.31, 2.55) | 2.04 (1.98, 2.10) | <0.001 | |
| 3 | 2.40 (2.26, 2.54) | 2.04 (1.98, 2.10) | <0.001 | |
| GSH (µmol/g Hb) | 1 | 7.59 (7.29, 7.89) | 6.96 (6.66, 7.26) | 0.10 |
| 2 | 7.62 (6.90, 8.34) | 6.95 (6.65, 7.25) | 0.090 | |
| 3 | 7.98 (7.22, 8.74) | 6.90 (6.60, 7.20) | 0.011 | |
| Arginine (µmol/l) | 1 | 84.4 (75.7, 93.1) | 83.5 (79.2, 87.9) | 0.86 |
| 2 | 82.3 (72.6, 91.9) | 84.1 (79.6, 88.5) | 0.75 | |
| 3 | 81.7 (70.4, 93.0) | 84.1 (79.5, 88.7) | 0.72 | |
| ADMA (µmol/l) | 1 | 0.43 (0.39, 0.47) | 0.49 (0.4, 0.51) | 0.010 |
| 2 | 0.43 (0.39, 0.47) | 0.49 (0.47, 0.51) | 0.018 | |
| 3 | 0.41 (0.37, 0.45) | 0.49 (0.47, 0.51) | 0.0076 | |
| SDMA (µmol/l) | 1 | 0.38 (0.34, 0.42) | 0.41 (0.39, 0.43) | 0.33 |
| 2 | 0.41 (0.37, 0.45) | 0.40 (0.38, 0.42) | 0.70 | |
| 3 | 0.42 (0.36, 0.48) | 0.40 (0.38, 0.42) | 0.55 | |
| Arg/ADMA ratio | 1 | 202 (181, 223) | 175 (165, 186) | 0.024 |
| 2 | 197 (174, 221) | 176 (166, 187) | 0.12 | |
| 3 | 202 (176, 228) | 175 (165, 186) | 0.075 | |
| Arg/SDMA ratio | 1 | 230 (200, 260) | 215 (200, 230) | 0.37 |
| 2 | 212 (180, 245) | 219 (204, 234) | 0.70 | |
| 3 | 210 (173, 248) | 219 (204, 234) | 0.68 |
Controls vs patient who were candidate for surgery.
Adjusted means and 95% CI.
No missing data except for 7 8-iso PGF2α determinations among patients (3 in the Aortic group and 4 in the Mitral group).
BMI, hyperglycaemia, dyslipidaemia, hypertension and treatment with anti-platelet, anti-hypertensive drugs, nitrates and statins.
ADMA: asymmetric dimethylarginine; Arg: arginine; GSH: glutathione; GSSG: disulphide glutathione; PGF: prostaglandin; SDMA: symmetric dimethylarginine.
aModel 1 = unadjusted; Model 2 = adjusted for age and gender; Model 3 = adjusted for age, gender; significant P values are reported in bold.
Figure 1:
Oxidative stress markers: glutathione/disulphide glutathione ratio (A) and 8-iso-prostaglandin F2α (B) adjusted means and 95% CI. On the left side: controls and surgical patients (***P < 0.001, Model 3); on the right side: patients grouped by cardiac surgical procedures (##P < 0.01, Model 3, coronary artery bypass surgery or Aortic vs Mitral).
The GSH/GSSG ratio was lower in Mitrals than in both CABG and Aortic patients (Fig. 1A and Table 3), whereas no differences according to surgery treatment were found in urinary 8-iso-PGF2α (Fig. 1B and Table 3).
Table 3:
Oxidative stress markers, antioxidants and nitric oxide pathway
| Variable | Model | CABG (N = 63) | Aortic (N = 51) | Mitral (N = 51) | P-value* |
|---|---|---|---|---|---|
| Log GSH/GSSG ratio | 1 | 2.84 (2.68, 3.00)** | 2.73 (2.55, 2.91) | 2.51 (2.33, 2.69) | 0.026 |
| 2 | 2.83 (2.65, 3.01)* | 2.74 (2.54, 2.94) | 2.51 (2.31, 2.71) | 0.058 | |
| 3 | 2.87 (2.67, 3.07)** | 2.80 (2.60, 3.00)** | 2.42 (2.22, 2.62) | 0.008 | |
| Log 8-iso PGF2α (pg/mg creatinine) | 1 | 5.14 (5.02, 5.26) | 5.26 (5.12, 5.40) | 5.13 (4.99, 5.27) | 0.32 |
| 2 | 5.18 (5.04, 5.32) | 5.21 (5.07, 5.35) | 5.13 (4.99, 5.27) | 0.73 | |
| 3 | 5.19 (5.03, 5.35) | 5.21 (5.05, 5.37) | 5.14 (4.96, 5.32) | 0.83 | |
| α-Tocopherol (µg/ml) | 1 | 11.4 (10.7, 12.0) | 12.1 (11.4, 12.9) | 12.3 (11.5, 13.1) | 0.15 |
| 2 | 11.5 (10.8, 12.2) | 12.1 (11.3, 12.9) | 12.1 (11.3, 12.9) | 0.42 | |
| 3 | 11.9 (11.0, 12.7) | 11.9 (11.1, 12.7) | 11.9 (11.1, 12.8) | 1.00 | |
| Log γ-tocopherol (pg/ml) | 1 | 1.99 (1.91, 2.07) | 2.11 (2.01, 2.21) | 2.02 (1.92, 2.12) | 0.18 |
| 2 | 2.01 (1.93, 2.09) | 2.10 (2.00, 2.20) | 2.01 (1.91, 2.11) | 0.34 | |
| 3 | 2.04 (1.94, 2.14) | 2.09 (1.99, 2.19) | 1.98 (1.86, 2.10) | 0.41 | |
| GSH (µmol/g Hb) | 1 | 7.10 (6.58, 7.62) | 6.71 (6.13, 7.29) | 7.01 (6.45, 7.57) | 0.58 |
| 2 | 7.15 (6.61, 7.69) | 6.56 (5.96, 7.16) | 7.10 (6.50, 7.70) | 0.30 | |
| 3 | 7.05 (6.41, 7.69) | 6.74 (6.10, 7.38) | 7.13 (6.47, 7.79) | 0.66 | |
| Arginine (µmol/l) | 1 | 83.8 (76.7, 90.9) | 89.5 (80.6, 98.3) | 78.7 (71.0, 86.5) | 0.19 |
| 2 | 86.5 (78.9, 94.0) | 87.9 (78.7, 97.0) | 76.7 (68.9, 84.5) | 0.10 | |
| 3 | 89.8 (80.2, 99.5) | 87.4 (77.8, 97.0) | 73.1 (63.4, 82.8) | 0.053 | |
| ADMA (µmol/l) | 1 | 0.45 (0.43, 0.47)** | 0.50 (0.46, 0.54) | 0.52 (0.48, 0.56) | 0.005 |
| 2 | 0.46 (0.42, 0.50)** | 0.49 (0.45, 0.53) | 0.52 (0.48, 0.56) | 0.031 | |
| 3 | 0.48 (0.44, 0.52) | 0.49 (0.45, 0.53) | 0.50 (0.46, 0.54) | 0.86 | |
| SDMA (µmol/l) | 1 | 0.39 (0.37, 0.41) | 0.40 (0.36, 0.44) | 0.43 (0.39, 0.47) | 0.35 |
| 2 | 0.38 (0.34, 0.42)* | 0.40 (0.36, 0.44) | 0.43 (0.39, 0.47) | 0.11 | |
| 3 | 0.39 (0.35, 0.43) | 0.40 (0.36, 0.44) | 0.43 (0.39, 0.47) | 0.46 | |
| Arg/ADMA ratio | 1 | 190 (174, 205)** | 180 (160, 200)* | 154 (137, 171) | 0.011 |
| 2 | 192 (175, 208)** | 182 (162, 202)* | 150 (133, 167) | 0.003 | |
| 3 | 192 (171, 212)* | 181 (161, 202)* | 151 (130, 171) | 0.032 | |
| Arg/SDMA ratio | 1 | 225 (201, 249) | 231 (201, 261)* | 191 (165, 217) | 0.077 |
| 2 | 235 (211, 260)** | 227 (197, 257)* | 182 (156, 207) | 0.008 | |
| 3 | 239 (207, 270)* | 226 (194, 258)* | 178 (147, 210) | 0.036 |
Surgical candidates (CABG vs Aortic vs Mitral).
Models description: see Table 2.
No missing data except for 7 8-iso PGF2α determinations among patients (3 in the Aortic group and 4 in the Mitral group).
ADMA: asymmetric dimethylarginine; Arg: arginine; GSH: glutathione; GSSG: disulphide glutathione; PGF: prostaglandin; SDMA: symmetric dimethylarginine.
*ANOVA three-group comparison: *P < 0.05 vs Mitral, **P < 0.01 vs Mitral; significant P values are reported in bold; no statistically significant differences were observed between CABG and Aortic patients in any comparison of any model.
Antioxidants
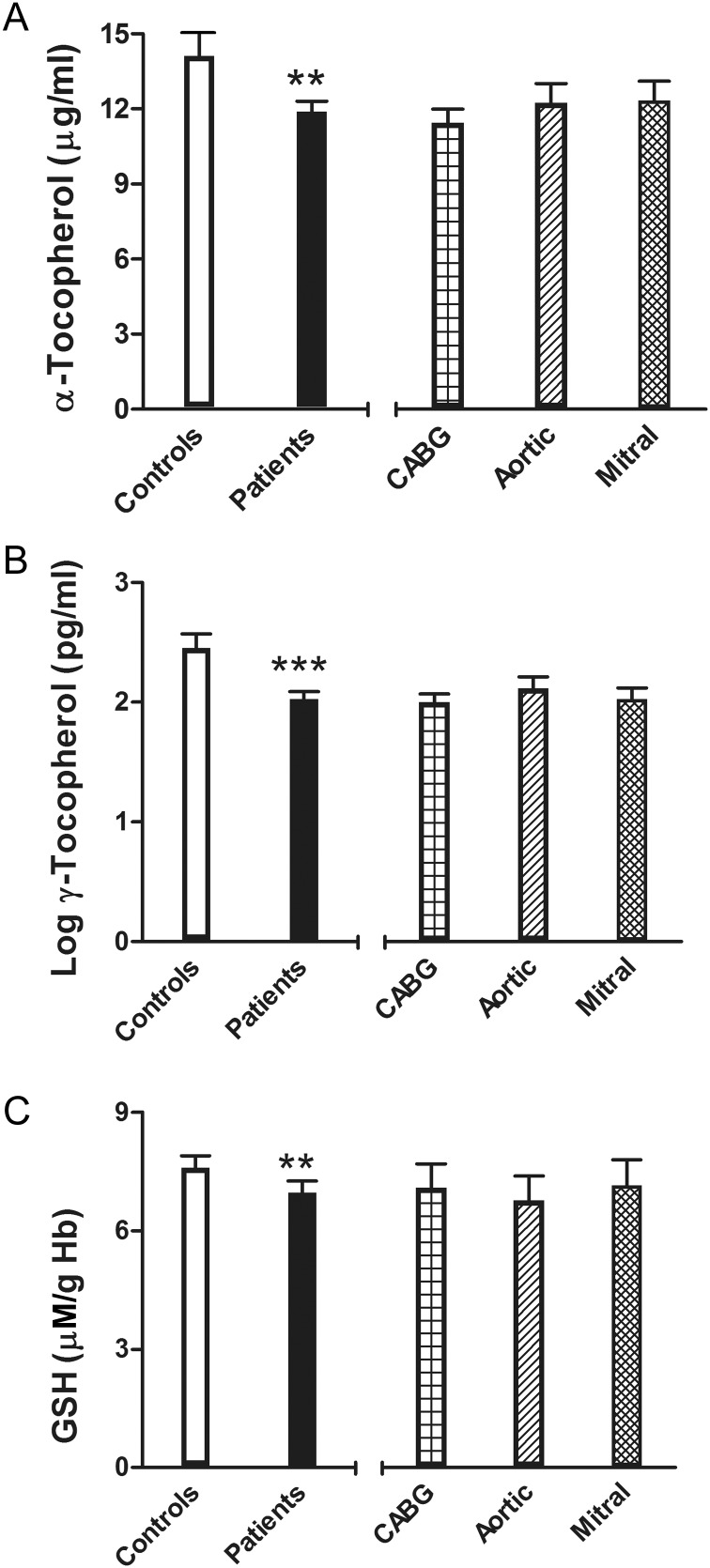
Plasma α- and γ-tocopherol were lower in patients scheduled for surgery with respect to Controls (Table 2); GSH levels also were lower in patients than in Controls, reaching significance after adjusting for all the variables considered (Model 3). No differences were detected in relation to the type of surgery (Table 3 and Fig. 2).
Figure 2:
Antioxidant factors: α-tocopherol (A), γ-tocopherol (B) and glutathione (C) adjusted means and 95% CI. On the left side: controls and surgical patients (***P < 0.001 or **P < 0.01, Model 3); on the right side: patients grouped by cardiac surgical procedures.
Arginine/nitric oxide pathway
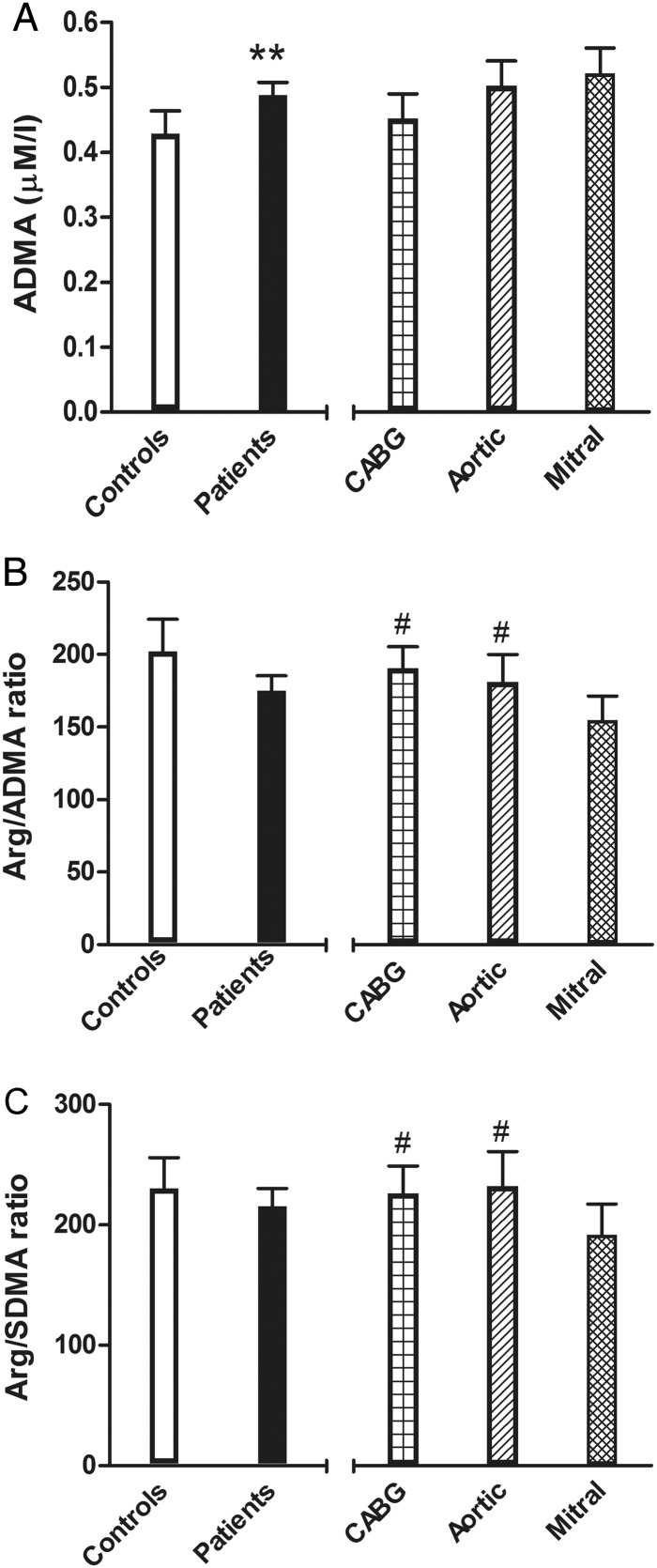
Arginine levels, as well as SDMA levels, did not differ between Controls and patients; ADMA levels were higher in candidates for surgery and there was a trend towards a lower Arg/ADMA ratio (Table 2). Interestingly, both Arg/ADMA and Arg/SDMA ratios were lower in Mitral patients with respect to CABG and Aortic patients; no differences were found between the last two groups (Table 3 and Fig. 3B and C).
Figure 3:
Arginine/nitric oxide (Arg/NO) pathway: endogenous synthesis inhibitor asymmetric dimethylarginine (ADMA) (A), indexes of the Arg/NO metabolic pathway Arg/ADMA ratio (B) and Arg/symmetric dimethylarginine ratio (C) adjusted means and 95% CI. On the left side: controls and surgical patients (**P < 0.01, Model 3); on the right side: patients grouped by cardiac surgical procedures (#P < 0.05, Model 3, coronary artery bypass surgery or Aortic vs Mitral).
Principal component analysis
The PCA takes into consideration all the variables tested, allowing for a global evaluation. The first two principal components accounted for nearly 50% of the total sample variance (28% the first and 18% the second). Table 4 reports the Pearson's correlation coefficients of the first two principal components with the 10 variables included in the analysis. The first principal component was more correlated with the variables involved in oxidative stress (positively with GSH and negatively with urinary isoprostanes) and could be interpreted as an antioxidant defence component; the second component was more correlated with the Arg/NO pathway (negatively with arginine and positively with SDMA) and could be interpreted as an impaired NO pathway component. Figure 4 shows the location of the four groups in the plain defined by the first two principal components. In the bidimensional plot, Mitral patients were located at a greater distance from the controls than CABG and Aortic patients.
Table 4:
Correlation coefficients of the first two principal components with the variables included in the analysis
| Variable | First principal component | Second principal component |
|---|---|---|
| Log GSH/GSSG ratio | 0.64 | 0.26 |
| Log 8-iso PGF2α | −0.31 | −0.06 |
| α-Tocopherol | 0.25 | 0.27 |
| Log γ-tocopherol | 0.60 | 0.48 |
| GSH | 0.33 | 0.09 |
| Arginine | 0.29 | −0.49 |
| ADMA | −0.50 | −0.07 |
| SDMA | −0.29 | 0.42 |
| Arg/ADMA ratio | 0.28 | −0.36 |
| Arg/SDMA ratio | 0.59 | −0.67 |
Coefficients of ≥0.3 are reported in bold.
ADMA: asymmetric dimethylarginine; Arg: arginine; GSH: glutathione; GSSG: disulphide glutathione; PGF: prostaglandin; SDMA: symmetric dimethylarginine.
Figure 4:
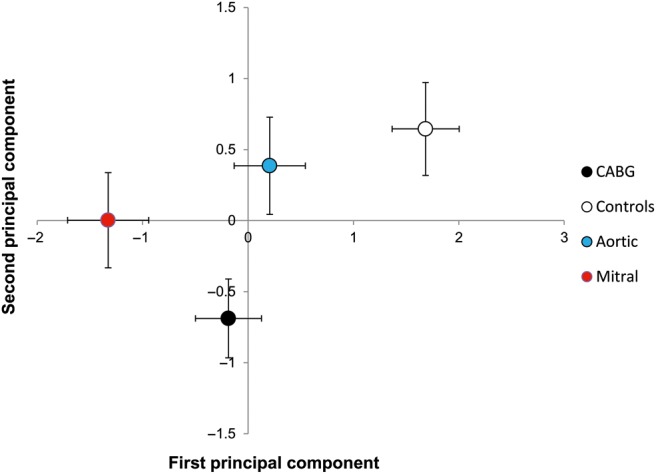
Principal component analysis: location of the four groups (means and SEM) in the plain defined by the two first principal components derived from 10 markers of oxidative balance and the nitric oxide (NO) pathway. The first principal component (horizontal axis) is mainly correlated with antioxidant defences (mostly glutathione), while the second component (vertical axis) is more associated with the arginine (Arg)/NO pathway (mostly Arg and symmetric dimethylarginine).
DISCUSSION
In recent years, the main role of oxidative stress in cardiovascular disease has been underscored. Enhanced production or attenuated degradation of ROS both affect endothelial and vascular functions and may contribute to atherosclerosis progression. NO, released by normal endothelium, is one of the main determinants of normal endothelial and vascular function. It has been shown that cardiovascular risk factors can promote an imbalance between endogenous oxidants and antioxidants resulting in oxidative stress, impaired NO pathway function and, eventually, vascular dysfunction. The combination of oxidative stress and NO pathway impairment may ultimately contribute to developing clinical cardiovascular events. Previous studies have widely addressed the problem of oxidative stress in atherosclerosis and coronary artery disease, suggesting that oxidative stress might even be considered as an unifying mechanism for many cardiovascular risk factors [14], and that a vicious circle between oxidative stress and inflammation can occur not only in the diseased arterial wall, where it also causes loss of antioxidant protection and cell death.
Much less is known concerning the role of oxidative stress and the Arg/NO pathway in patients undergoing cardiac surgery, in particular in those affected by valve diseases. Regarding non-rheumatic calcific aortic stenosis, the most frequent type of valve disease that eventually leads to surgery, several studies have addressed the role of localized oxidative stress (within the diseased aortic valve itself). Calcified areas of stenotic aortic valves have shown elevated levels of oxidative stress [15], suggesting that the presence of ROS promotes the progression of aortic valve calcification [9].
In addition, it has been proposed that an uncoupled NOS may also contribute to and exacerbate oxidative stress [15]. Many of the risk factors involved in coronary artery valve disease and coronary atherosclerosis are associated with endothelial dysfunction. Several studies in multiple species have demonstrated a role of endothelial NO in the pathogenesis of calcific aortic valve disease, but the mechanism by which it affects valvular interstitial cells is still unknown. Regarding aortic valve disease, Schumm et al. [16] demonstrated a direct link between endothelial function and haemodynamic parameters of moderate-to-severe aortic stenosis. This effect might be mediated by NO release, due to turbulent poststenotic blood flow or the rising transvalvular gradient. However, limited evidence is available on systemic circulating markers. Ochoa et al. [17] studied 11 candidates for coronary bypass and 11 for aortic valve surgery, showing that plasma levels of thiobarbituric acid reactive substances, α-tocopherol, coenzyme Q and retinol were similar between the two groups; more recently, Ferrari et al. showed similar levels of ADMA and homocysteine in healthy controls and in patients with calcific aortic stenosis scheduled for surgery [18], but in both cases the sample size of the study populations were somehow limited. Furthermore, Cagirci et al. [19] suggested ADMA as a valuable biomarker to assess the grade of aortic valve stenosis and to monitor disease progression.
If we consider the second most frequent valve disease of the adult, mitral valve regurgitation, data concerning the levels of markers of oxidative stress and the Arg/NO pathway are even more limited: some early animal studies suggested increased oxidative stress both in plasma and in tissues [20], whereas studies done in humans showed increased levels of oxidative stress in the left ventricular myocardium of patients undergoing repair surgery [21]. A recent pilot study assessing a limited number of patients suggested a possible link between serum oxidative stress index, left atrial enlargement and atrial fibrillation [22]. Finally, Ci et al. [23] showed an increase in endothelial microparticles that, in turn, impair endothelial cell function and may accelerate the oxidative damage of the mitral valve. All these results suggest that the L-arginine/NO pathway may play a role in heart-valve pathogenesis.
To our knowledge, there are no studies that have compared oxidative stress markers, antioxidants levels and the molecules involved in the Arg/NO pathway. In this study, we have enrolled all comers for an elective isolated surgical procedure (associated procedures such as CABG plus valve were excluded) who were in sinus rhythm without a previous history of atrial fibrillation at the time of surgery (the possible contribution of atrial fibrillation to increased oxidative stress levels was thus excluded) in order to characterize the different diseases. Our data clearly show that candidates for heart surgery have increased oxidative stress markers and reduced antioxidant levels with respect to controls, and this is paralleled by in increase of ADMA levels, which, in turn, might eventually result in an impairment of NO production.
Even more important, differences and similarities between the different pathologies have been observed, taking into account that our sample size was adequate to asses even minor differences, of the order of one half of a standard deviation, for each variable.
Aortic patients had similar levels of oxidative stress, antioxidants and Arg/NO pathway markers with respect to CABG patients. Very similar levels of these biomarkers were also found when Aortic patients were categorized by the absence (n = 36) or the presence (n = 15) of mild or moderate (<50%) coronary stenoses at coronary angiography (data not shown). This suggests that the similarities observed between coronary disease and aortic valve disease may be determined by their common atherosclerotic origin and may be predominant, rather than the dissimilarities [24], causing a similar impairment of pathways considered in this study whose role in cardiovascular disease progression is still probably overlooked.
Patients with mitral valve disease, instead, had increased plasma oxidative stress markers with respect to CABG and Aortic groups and a more pronounced impairment of the NO synthetic pathway as documented by the lowest ratios between precursors and inhibitors, i.e. Arg/ADMA and Arg/SDMA ratios, probably resulting in a higher degree of endothelial dysfunction. Indeed, this is a rather unexpected finding as the baseline clinical features of Mitral patients, who are younger (notably, their mean age is comparable with that of Controls) and have lower incidence of cardiovascular risk factors with respect to CABG and Aortic groups, did not anticipate this scenario in terms of the factors involved in oxidative stress and NO synthesis. The increased levels of these markers observed in patients with mitral valve disease persisted even after the adjustment for baseline clinical features, confirming the robustness of these data. A possible explanation of this finding is currently lacking; we might speculate that differences in shear stress or in the rheological features inherent to mitral valve regurgitation may contribute to that, as it is known that patients with mitral regurgitation show very striking perturbations of regurgitant flow in the left atrium [25]. This, however, remains a mere hypothesis. The next step in the understanding of these processes is the study of the time course of these variables after surgical correction of the underlying heart disease. In other words, this will allow us to ascertain whether the perturbations of these pathways are due to the disease itself or to the haemodynamic derangements/perturbations in flow patterns due to valve disease. If this latter hypothesis is true, a normalization of these variables over time is expected. Although the increase in oxidative stress markers is well documented in our study, at present it is too early to suggest that the therapeutic use of antioxidants could somehow improve the clinical outcomes in these patients. In fact, although experimental studies indicate that oxidative stress and inflammation interact and conribute to an accelerated atherosclerotic disease process, being very detrimental for patients affected by severe heart disease, the use of antioxidants before surgery has given inconsistent results in clinical trials. Therefore, further studies are needed, particularly with early enrollment of patients before surgery, which could allow the demonstration of whether antioxidant preoperative administration can reverse the pro-oxidant state that we have documented to occur in these patients.
This study has one limitation: the differences in baseline clinical features between the different patient populations. These differences were accepted in order to gather the most reliable information concerning patients who need cardiac surgery for the three most frequent cardiac diseases of the adult: coronaries, aortic and mitral valves. We chose to study all comers (with subsequent analyses performed in order to adjust for different clinical features) instead of performing a cherry pick among candidates. In addition, our results reflect the characteristics of patients attending our specific centre, and more in general, the clinical features of patients who were candidates for the different types of surgery.
CONCLUSIONS
Our study shows that oxidative stress is increased in surgical candidates with respect to controls, and that Mitral patients have increased levels of this stress. In addition, Mitral patients have a more pronounced impairment of the factors involved in NO generation. The possible clinical consequences of these findings have to be addressed in future studies.
Funding
This work was supported by the Italian Ministry of Health (Ricerca Corrente CA 05 2012) to the Centro Cardiologico Monzino, IRCCS.
Conflicts of interest: none declared.
REFERENCES
- 1.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–44. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]
- 2.Weaver H, Shukla N, Ellinsworth D, Jeremy JY. Oxidative stress and vein graft failure: a focus on NADH oxidase, nitric oxide and eicosanoids. Curr Opin Pharmacol. 2012;12:160–5. doi: 10.1016/j.coph.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Harling L, Rasoli S, Vecht JA, Ashrafian H, Kourliouros A, Athanasiou T. Do antioxidant vitamins have an anti-arrhythmic effect following cardiac surgery? A meta-analysis of randomised controlled trials. Heart. 2011;97:1636–42. doi: 10.1136/heartjnl-2011-300245. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Paulus WJ, Vermeulen MA, Richir MC, Davids M, Wisselink W, et al. The role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur J Heart Fail. 2010;12:1274–81. doi: 10.1093/eurjhf/hfq158. [DOI] [PubMed] [Google Scholar]
- 5.Cavalca V, Cighetti G, Bamonti F, Loaldi A, Bortone L, Novembrino C, et al. Oxidative stress and homocysteine in coronary artery disease. Clin Chem. 2001;47:887–92. [PubMed] [Google Scholar]
- 6.Hassinger AB, Wainwright MS, Lane JC, Haymond S, Backer CL, Wald E. Elevated preoperative serum asymmetrical dimethylarginine (ADMA) is associated with poor outcomes after pediatric cardiac surgery. Intensive Care Med. 2012;38:1697–704. doi: 10.1007/s00134-012-2657-2. [DOI] [PubMed] [Google Scholar]
- 7.Cavalca V, Sisillo E, Veglia F, Tremoli E, Cighetti G, Salvi L, et al. Isoprostanes and oxidative stress in off-pump and on-pump coronary bypass surgery. Ann Thorac Surg. 2006;81:562–7. doi: 10.1016/j.athoracsur.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Sverdlov AL, Ngo DT, Chan WP, Chirkov YY, Gersh BJ, McNeil JJ, et al. Determinants of aortic sclerosis progression: implications regarding impairment of nitric oxide signalling and potential therapeutics. Eur Heart J. 2012;33:2419–25. doi: 10.1093/eurheartj/ehs171. [DOI] [PubMed] [Google Scholar]
- 9.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–70. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 10.Rajamannan NM. Bicuspid aortic valve disease: the role of oxidative stress in Lrp5 bone formation. Cardiovasc Pathol. 2011;20:168–76. doi: 10.1016/j.carpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalca V, Minardi F, Scurati S, Guidugli F, Squellerio I, Veglia F, et al. Simultaneous quantification of 8-iso-prostaglandin-F(2alpha) and 11-dehydro thromboxane B(2) in human urine by liquid chromatography-tandem mass spectrometry. Anal Biochem. 2010;397:168–74. doi: 10.1016/j.ab.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Werba JP, Cavalca V, Veglia F, Massironi P, De Franceschi M, Zingaro L, et al. A new compound-specific pleiotropic effect of statins: modification of plasma gamma-tocopherol levels. Atherosclerosis. 2007;193:229–33. doi: 10.1016/j.atherosclerosis.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Squellerio I, Tremoli E, Cavalca V. Quantification of arginine and its metabolites in human erythrocytes using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;412:108–10. doi: 10.1016/j.ab.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 14.De Rosa S, Cirillo P, Paglia A, Sasso L, Di Palma V, Chiariello M. Reactive oxygen species and antioxidants in the pathophysiology of cardiovascular disease: does the actual knowledge justify a clinical approach? Curr Vasc Pharmacol. 2010;8:259–75. doi: 10.2174/157016110790887009. [DOI] [PubMed] [Google Scholar]
- 15.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–50. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumm J, Luetzkendorf S, Rademacher W, Franz M, Schmidt-Winter C, Kiehntopf M, et al. In patients with aortic stenosis increased flow-mediated dilation is independently associated with higher peak jet velocity and lower asymmetric dimethylarginine levels. Am Heart J. 2011;161:893–9. doi: 10.1016/j.ahj.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Ochoa JJ, Vilchez MJ, Mataix J, Ibanez-Quiles S, Palacios MA, Munoz-Hoyos A. Oxidative stress in patients undergoing cardiac surgery: comparative study of revascularization and valve replacement procedures. J Surg Res. 2003;111:248–54. doi: 10.1016/s0022-4804(03)00106-9. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari G, Sainger R, Beckmann E, Keller G, Yu PJ, Monti MC, et al. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J Surg Res. 2010;163:12–7. doi: 10.1016/j.jss.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cagirci G, Cay S, Canga A, Karakurt O, Yazihan N, Kilic H, et al. Association between plasma asymmetrical dimethylarginine activity and severity of aortic valve stenosis. J Cardiovasc Med (Hagerstown) 2011;12:96–101. doi: 10.2459/JCM.0b013e32833cdcea. [DOI] [PubMed] [Google Scholar]
- 20.Olsen LH, Mortensen K, Martinussen T, Larsson LI, Baandrup U, Pedersen HD. Increased NADPH-diaphorase activity in canine myxomatous mitral valve leaflets. J Comp Pathol. 2003;129:120–30. doi: 10.1016/s0021-9975(03)00019-7. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, et al. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60% J Am Coll Cardiol. 2010;55:671–9. doi: 10.1016/j.jacc.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen MC, Chang JP, Liu WH, Yang CH, Chen CJ, Fang CY, et al. Increased serum oxidative stress in patients with severe mitral regurgitation: a new finding and potential mechanism for atrial enlargement. Clin Biochem. 2009;42:943–8. doi: 10.1016/j.clinbiochem.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Ci HB, Ou ZJ, Chang FJ, Liu DH, He GW, Xu Z, et al. Endothelial microparticles increase in mitral valve disease and impair mitral valve endothelial function. Am J Physiol Endocrinol Metabolism. 2013;304:E695–702. doi: 10.1152/ajpendo.00016.2013. [DOI] [PubMed] [Google Scholar]
- 24.Parolari A, Loardi C, Mussoni L, Cavallotti L, Camera M, Biglioli P, et al. Nonrheumatic calcific aortic stenosis: an overview from basic science to pharmacological prevention. Eur J Cardiothorac Surg. 2009;35:493–504. doi: 10.1016/j.ejcts.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Dyverfeldt P, Kvitting J P, Carlhall C J, Boano G, Sigfridsson A, Hermansson U, et al. Hemodynamic aspects of mitral regurgitation assessed by generalized phase-contrast MRI. J Magn Reson Imaging. 2011;33:582–8. doi: 10.1002/jmri.22407. [DOI] [PubMed] [Google Scholar]