Abstract
OBJECTIVES
Surgical ventricular reconstruction has been used to treat ischaemic cardiomyopathy with large akinetic or dyskinetic areas. However, application of this approach requires a sternotomy, cardiopulmonary bypass and a left ventriculotomy. This study assessed the feasibility and efficacy of minimally invasive, off-pump, epicardial catheter-based ventricular reconstruction (ECVR) in an anteroapical aneurysm ovine model.
METHODS
Left ventricular (LV) anteroapical myocardial infarction was induced percutaneously by coil embolization of the left anterior descending coronary artery. Eight weeks after infarction, via mini left thoracotomy and without cardiopulmonary bypass, ECVR was performed in six sheep. The scar was excluded by placing anchor pairs on the LV epicardial anterior wall and the right ventricular side of the interventricular septum under fluoroscopic guidance. LV performance was evaluated before, immediately after device implantation and after 6 weeks by echocardiography. Terminal histopathology was performed.
RESULTS
ECVR was completed expeditiously in all animals without complications. Parameters obtained 6 weeks after device implantation were compared with baseline (pre-device). End-systolic volume was decreased by 38% (25.6 ± 6.1 ml vs baseline 41.2 ± 7.2 ml, P = 0.02) with preservation of stroke volume. Ejection fraction was significantly increased by 13% (48.5 ± 7% vs baseline 35.8 ± 7%, P = 0.02). The circumferential strain in the anterior septum (−7.67 ± 5.12% vs baseline −0.96 ± 2.22%, P = 0.03) and anterior wall (−9.01 ± 3.51% vs baseline −4.15 ± 1.36%, P = 0.01) were significantly improved. The longitudinal strain in apex was reversed (−3.08 ± 1.53% vs baseline 3.09 ± 3.39%, P = 0.01). Histopathology showed full endocardial healing over the anchors with appreciable reduction of the chronic infarct in the LV.
CONCLUSIONS
ECVR without cardiopulmonary bypass is a less invasive alternative to current standard therapies, reverses LV remodelling and improves cardiac performance in an ovine model of anteroapical aneurysm.
Keywords: Congestive heart failure, Ventricular reshaping, Off-pump surgery
INTRODUCTION
Surgical left ventricular reconstruction (SVR) operations have been used for treatment of heart failure patients following myocardial infarction to restore left ventricular (LV) chamber size and geometric shape and reduce myocardial wall stress [1–5]. Currently, application of SVR requires cardiopulmonary bypass and a left ventriculotomy. In this study, we introduce a novel device (PliCath HF, BioVentrix, San Ramon, CA, USA), which consists of an implantable anchor and a tether system and is designed to treat post-MI large anterior wall scars in remodelled ventricle by excluding the non-contractile portion of the circumference of the LV. Anchor pairs are placed on the LV epicardial anterior wall and the right ventricular side of the interventricular septum using a procedure designated as epicardial catheter-based ventricular reconstruction (ECVR). The delivery system and the key procedural steps are illustrated in Fig. 1. ECVR is designed to achieve the same results as those of the traditional Dor procedure, but less invasively and without the necessity for cardiopulmonary bypass. It can be performed as a stand-alone procedure or concomitantly through sternotomy, partial sternotomy or left thoracotomy. This study evaluated feasibility and efficacy of ECVR utilizing the PliCath HF system on an ovine ischaemic heart failure model with anteroapical aneurysm.
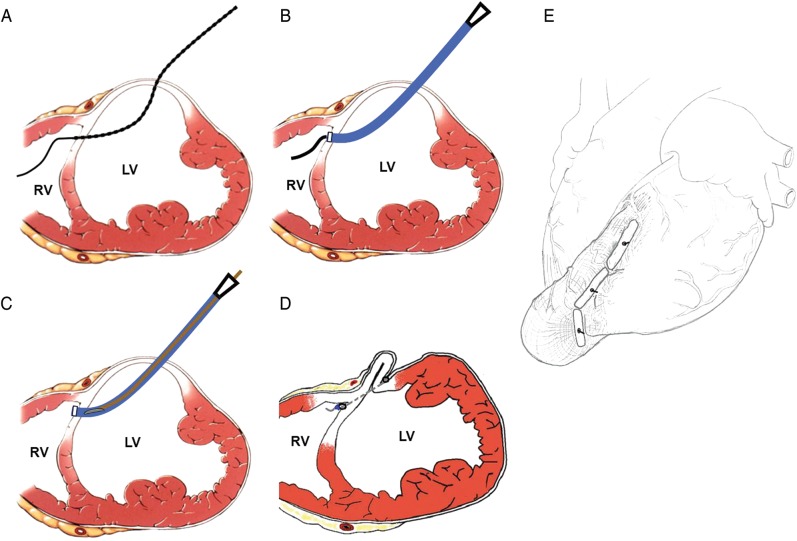
Figure 1:
The PliCath delivery system and the key procedural steps of epicardial catheter-based ventricular reconstruction. (A) A needle pierces the septum from the LV into the RV loaded with a wire. (B) A 14-Fr catheter follows over the wire into the RV. (C) The inner implantable anchor and a tether system are delivered through the sheath. (D) The creation of the geometric reconfiguration of the dilated and failing LV is achieved after anchor deployment. (E) The reconstructed heart after device deployment.
MATERIALS AND METHODS
Study design
The study was conducted in accordance with the Guide for Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of the Jack H. Skirball Center for Cardiovascular Research of the Cardiovascular Research Foundation. All animals received humane care in compliance with current guidelines. LV anterior MI was induced in seven Dorsett hybrid sheep (37 ± 2 kg) by percutaneous coil embolization of the left anterior descending coronary artery (LAD). Two months following MI, ECVR was performed through a left thoracotomy and the devices were implanted under fluoroscopic guidance. LV performance was evaluated by echocardiograms pre-device implantation (baseline), immediately post-device implantation and at 6 weeks’ follow-up.
Myocardial infarction model development
In this study, LV anteroapical MI was induced by LAD coil embolization. All animals that received intramuscular Telazol (4 mg/kg) injections for induction, were then intubated and mechanically ventilated using 1.5–2.5% isoflurane. Heparin (100 units/kg) was injected to maintain an activated clotting time (ACT) of ≥250 s. Under fluoroscopic guidance, the coronary coil (2.0–3.5 mm, Cook, Bloomington, IN, USA) was delivered to the middle LAD, at a point 40–50% of the distance from apex to base, and to the corresponding diagonals to block the coronary blood flow and induce MI. Coronary angiography was performed to confirm complete and persistent occlusion. All catheters and sheaths were removed 2 h after LAD occlusion and animals were recovered.
Surgical procedure (device deployment)
Two months after MI creation, anaesthesia was induced as described above and each sheep was taken to the operating theatre for the device deployment surgery. The heart was exposed through a less invasive left thoracotomy via the fifth intercostal space. The pericardium was opened and retracted with stay sutures and the LV anteroapical and adjacent anterolateral scarred segments were identified. Cardiac surfaces, chamber boundaries and infarct borders were visualized with epicardial echocardiography, while device components were pinpointed with fluoroscopy (non-contrast). By combining these imaging modalities, extremely precise delivery of the device with respect to the infarct border zone and desired postoperative chamber dimensions is possible. After intravenous heparin administration (ACT ≥250 s), ECVR was performed to exclude the acontractile portion of the circumference of the LV. The key operative steps are shown in Fig. 2. The anterolateral wall of the ventricle was brought into a line of apposition with the septum through the deployment of an anchor system, and the intervening segment of the LV wall was excluded from the chamber.
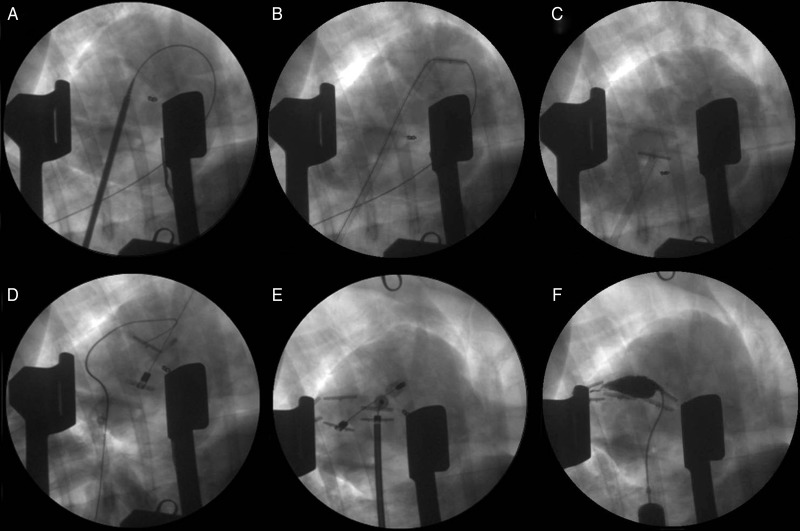
Figure 2:
Angiographic images of key operative steps. (A) After removing the puncture needle, a 14-Fr catheter with dilator is placed over the wire into the RV. (B) The dilator is removed, and an anchor is deployed onto the RV side of the septum. (C) The wire is removed and the anchor is pulled back to the RV septum. (D) An epicardial anchor is placed on the anterior wall of the LV, and additional two to three pairs of anchors are placed as before. (E) The anchors are plicated, and (F) an injection of contrast into the newly formed compartment confirms successful exclusion.
To accomplish this, a custom needle is used to puncture the LV free wall and interventricular septum. The needle was connected to a pressure-transducer (DTX Plus™ DT-XX, BD, Franklin Lakes, NJ, USA) and the ventricular chamber pressures were recorded using the Ponemah Physiology Platform System (LDS Life Science, Valley View, OH, USA), as the needle was passed. Therefore, the position of the needle tip as it crossed the LV chamber and the septum and emerged in the right ventricle was confirmed by both long-axis fluoroscopy and left and right ventricular transition pressures. A flexible wire was advanced into the RV outflow tract and out the pulmonary artery (PA). The needle was then removed, leaving the guidewire in the PA and the 14-Fr catheter with a dilator was placed over the wire into the RV (Fig. 2A). The dilator was removed, and an anchor, which is made of medical grade titanium and covered with knitted polyester hinged attachment to a delivery tether (poly-ether-ether-ketone), was passed through the catheter and deployed onto the RV outflow tract (Fig. 2B). Removal of the wire left the anchor free to pivot into a T configuration against the sheath, and the sheath/T anchor combination was withdrawn producing contact with the septum (Fig. 2C). Complete removal of the sheath left the anchor in place on the RV side of the septum fixed to a tether, which protruded through the LV wall. A second epicardial anchor was placed over the tether on the anterior wall of the LV (Fig. 2D). This external anchor contains a cam-locking device for fixation at any position on the tether, thereby allowing the distance between the inner and external anchors to vary with the thicknesses of the two myocardial walls being apposed.
Additional pairs of anchors (2–3 pairs) were placed, aligned in the long axis, to achieve the desired line of apposition between the lateral LV wall and the septum. The external anchors were serially advanced towards the internal anchors until walls came into contact (Fig. 2E shows advancing of the first anchor pair). A measured compression force of 2 to 4 N, in addition to the force necessary to appose the walls plicated together, was applied. A contrast injection was performed to confirm exclusion of the newly formed compartment (Fig. 2F). A chest tube was placed for drainage and all incisions were then closed using standard methods.
Echocardiography
Echocardiograms were performed in the naive stage, pre-device (baseline), immediately after device implantation and at 6 weeks’ follow-up. Two-dimensional (2D) echocardiographic images were acquired in the right lateral decubitus position using a 5-MHz probe (iE33, Philips Medical Systems, Bothell, WA, USA) from standard parasternal long- and short-axis planes. LV end-diastolic diameter (EDD) and end-systolic diameter (ESD) were determined from short-axis planes; end-diastolic volume (EDV) and end-systolic volume (ESV) were calculated using Simpson's method and LV ejection fraction (EF) was calculated using a standard formula (EF = [(EDV − ESV)/EDV] × 100).
Grayscale images for offline speckle-tracking echocardiography (STE) analysis were acquired at a high frame rate (65–90 frames/s) from standard long-axis and apical four-chamber views. Short-axis images were acquired at three different levels (base, mid and apex) from standard parastemal views. At least two consecutive cardiac cycles were recorded for offline analysis (QLab 6.0 software, Philips, Bothell, WA, USA) [6]. When a cardiac cycle with a good quality image was selected, a region of interest for speckle tracking was defined at end-diastole using a semi-automated border detection method. The locations of the tracking points extending from endocardial to epicardial borders were adjusted and then the segmental myocardial strain curves were automatically generated by the system. For each animal, peak-systolic circumferential and radial strain parameters (six segments: anterior septum, anterior, anterior lateral, inferior lateral, inferior and inferior septum) were averaged from mid and apical short-axis levels. Longitudinal strain in all LV segments (basal septum, basal lateral, mid septum, mid lateral, apical septum, apical lateral and apex) were analysed.
Histopathology
The animals were euthanized at 6 weeks after device deployment. The hearts were explanted and grossly examined through posterior ventriculotomy, then fixed in 10% neutral buffered formalin and submitted for microscopic evaluation and histopathology (Alizée Pathology, Thurmont, MD, USA). In brief, the hearts were trimmed, processed in plastic (Spurr) and prepared to microscopic grade slides by grinding/polishing techniques using an Exakt system. The implant sites were trimmed at regular intervals across the treated ventricular wall (three levels per device, whenever possible, with one level centered on the tether). Resulting slides were stained with haematoxylin and eosin (H&E). In addition to the whole explanted heart, specimens from the lungs, kidneys, liver and brain of all animals were examined grossly and microscopically on all subjects.
Statistics
Statistical analyses were performed using the SAS statistical software (version 9.2; SAS Institute, Inc., NC, USA). The differences in volume and regional strain among three or four time points were detected using repeated measures analysis of variance and differences in volume and regional strain at any two time points were examined using a Bonferroni adjustment. A P-value of <0.05 was considered statistically significant.
RESULTS
Seven animals underwent MI induction; 1 (14%) did not survive the MI induction procedure and six survived to enrolment at 2 months post-MI (baseline). In those six animals, ECVR was generally completed expeditiously with <120 s of fluoroscopy time. Apposition of walls was accomplished in all cases without any adverse haemodynamic consequences. All animals recovered from anaesthesia. No bleeding, cardiac dysfunction, wound complications or arrhythmia were observed. All the six animals survived to sacrifice.
Echocardiography
Echocardiographic images and data are shown in Fig. 3 and Table 1. Large anterior infraction and apical dyskinetic motion was observed in all animals without any mitral regurgitation (MR) 2 months after LAD occlusion. All the animals developed congestive heart failure characterized by reduction in LVEF and dilated LV chamber. Immediately after device implantation and compared with baseline (pre-device), ECVR significantly reduced ESD (P = 0.02) and ESV (P = 0.02) and increased EF from 35.8 ± 6.6 to 50.5 ± 3.3% (P = 0.01). At 6 weeks’ follow-up, ECVR had a significant reduction effect on EDV (by 22%, P = 0.02) and ESV (by 38%, P = 0.002) and improved EF by 13% (P = 0.05). No significant difference in EDV or ESV was observed in the interval between post-device implantation surgery and the 6-week evaluation. The stroke volume was preserved in all the animals (P = 0.68). No mitral or tricuspid valve regurgitation was noted directly after ECVR or at 6 weeks after device implantation.
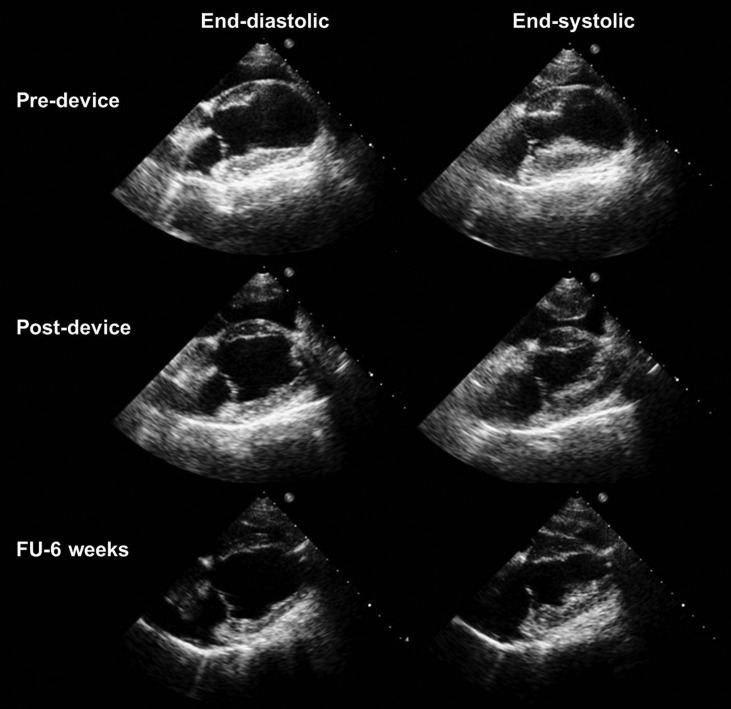
Figure 3:
Echocardiography images before device implantation (8-week post-myocardial infarction), immediately after device implantation and at 6 weeks’ follow-up at end-diastole and end-systole. LV end-diastolic and end-systolic volumes significantly decreased immediately after device implantation and the effect was maintained at 6 weeks’ follow-up.
Table 1:
Echocardiography parameters assessed prior to myocardial infarction (MI), prior to device implantation (baseline: 60-day post-MI) and 6 weeks following device implantation
| Naive (pre-MI) | Baseline (pre-device) | Post-device | 6 weeks’ follow-up | |
|---|---|---|---|---|
| LVEDD (cm) | 3.56 ± 0.22 | 4.22 ± 0.23* | 3.86 ± 0.42 | 4.07 ± 0.30 |
| LVESD (cm) | 2.62 ± 0.23 | 3.33 ± 0.35* | 2.81 ± 0.39** | 2.91 ± 0.33** |
| LVEDV (ml) | 43.5 ± 8.1 | 63.8 ± 6.1* | 49.0 ± 11.4 | 49.5 ± 9.1** |
| LVESV (ml) | 19.4 ± 4.4 | 41.2 ± 7.2* | 24.2 ± 5.3** | 25.6 ± 6.1** |
| SV (ml) | 24.2 ± 4.1 | 22.6 ± 3.3 | 23.9 ± 5.1 | 23.9 ± 5.1 |
| EF (%) | 55.7 ± 3.5 | 35.8 ± 6.6* | 50.5 ± 3.3** | 48.5 ± 6.7** |
Values are mean ± SD.
LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; SV: stroke volume; EF: ejection fraction.
*P < 0.05 vs pre-MI.
**P < 0.05 vs baseline.
All the six animals had adequate image quality and excellent tracking score for long- and short-axis strain analysis. After MI creation, LV peak-systolic longitudinal, circumferential and radial strains in the anterior and septal walls were significantly reduced; and dyskinetic contractility was shown in the apex (Fig. 4). After ECVR, the adverse longitudinal strain in the apex was reversed (−3.08 ± 1.53% vs baseline 3.09 ± 3.39%, P = 0.01), while the longitudinal strains in the remaining segments did not significantly change (Fig. 4A). The circumferential strain in anterior septum (−7.67 ± 5.12% vs baseline −0.96 ± 2.22%, P = 0.03), inferior septum (−11.03 ± 5.20 vs baseline −5.23 ± 3.07%, P = 0.04) and anterior wall (−9.01 ± 3.51 vs baseline −4.15 ± 1.36%, P = 0.01) were significantly improved (Fig. 4B). Anterior lateral radial strain was increased from baseline 15.36 ± 2.84% to 26.3 ± 4.99 (P < 0.001) at 6 weeks’ follow-up (Fig. 4C).
Figure 4:
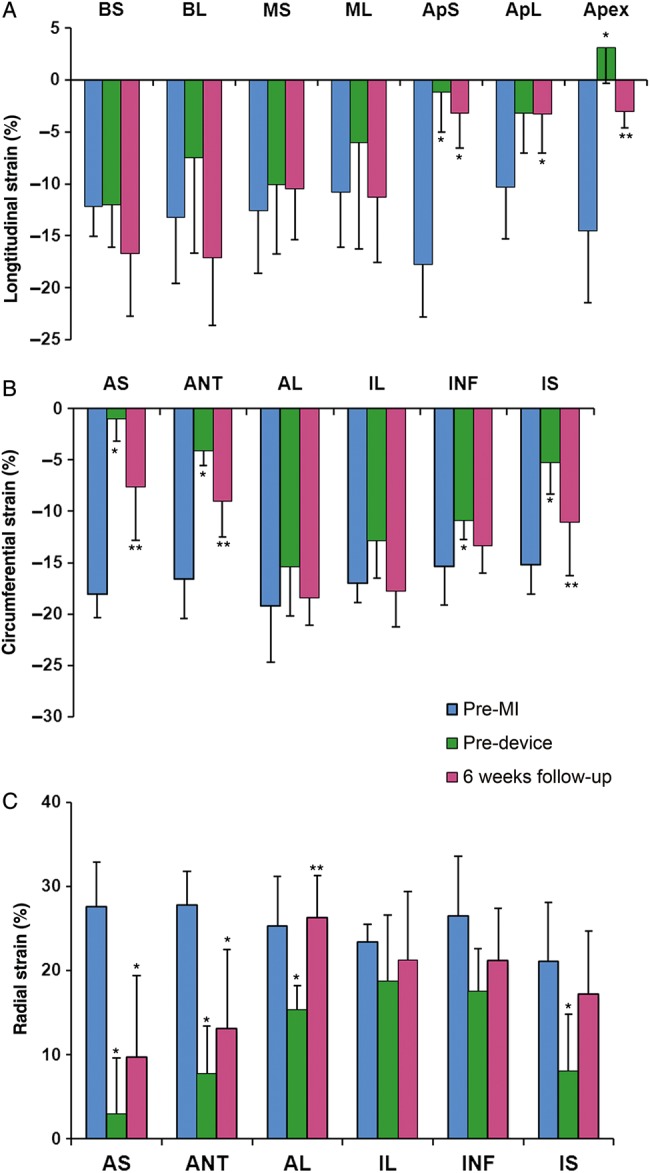
(A) LV peak-systolic longitudinal; (B) circumferential strain and (C) radial strain changes before myocardial infarction (MI), at device implantation, and 6 weeks after device implantation. Values are Mean ± SD. BS: basal septum; BL: basal lateral; MS: mid septum; ML: mid lateral; ApS: apical septum; ApL: apical lateral; AS: anterior septum; ANT: anterior; AL: anterior lateral; IL: inferior lateral; INF: inferior and IS: inferior septum. *P < 0.05 vs Naive, **P < 0.05 vs baseline (pre-device).
Histological evaluation
Gross examination showed that the external anchors had dense fibrous tissue ingrowth and pericardial adhesion. No erosion was seen by the anchors into the heart or damage to surrounding tissue by protruding, cut ends of the tethers. In all the animals, the regular deployment of the anchors along the edges of the infarct scar with generally complete to near-complete reduction of the non-functional ventricular space was shown grossly and microscopically (Fig. 5A). In addition, there was microscopic evidence of fusion of the opposing walls of the scar at all to nearly all implant sites. Fully healing and endothelialization of the endocardium adjacent to and beneath the reduction devices with no mural thrombosis was shown in all cases.
Figure 5:
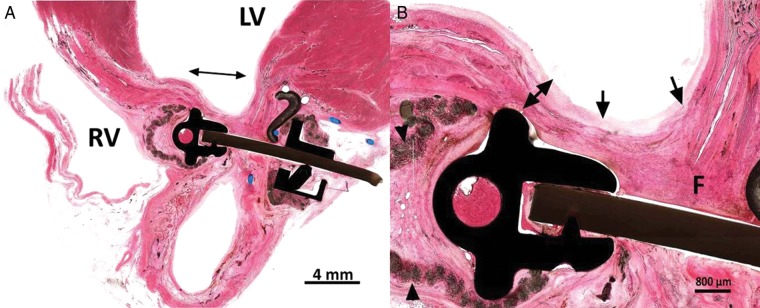
Microscopic tissue examination of the implant sites (H&E Stain, 10x). (A) There is reduction of the infracted area and fusion of the apposed infarct margins (double arrow). (B) There is healing of the fused infarct wall with fibrous tissue healing (F) and coverage of the endocardium by a small amount of neointima (arrows). There is moderate compressive attenuation of the ventricular wall (double arrow). Inflammation around the velour cover is minimal (arrowheads).
Optimal local tolerance of the reduction devices was seen in all the animals. Host tissue response was characterized by a minimal-to-mild foreign body response within the velour mesh (inflammation was primarily comprised of histocytes, foreign body giant cells) and mature fibrous tissue formation around the metal/mesh anchor assembly (Fig. 5B). Responses seen are consistent with a safe, well-tolerated implantable device. Granulocytes (neutrophils or eosinophils) were not seen. Occasional necrosis was very localized and confined to the points of pressure exerted by the anchor on the scar and was primarily associated with trapped adipose tissue. This change was generally accompanied by hyalinization of the surrounding scar tissue and appeared self-limited. Due to its limited extension, it was not regarded as potentially compromising anchorage or the structural integrity of the associated mural scar.
Gross specimens excised from the brain, lung, liver and kidneys were uniformly normal in appearance, with no evidence of congestion, infarction, scarring, thrombus, embolization or microscopic changes.
DISCUSSION
Surgical ventricular reconstruction aims to improve LV function by restoring LV geometry in both akinetic and dyskinetic myocardial conditions. Large-scale clinical studies have demonstrated that the Dor procedure and several modifications thereof are safe and effective in terms of survival benefit and LV functional recovery for patients with ischaemic cardiomyopathy that has developed after large anteroseptal MI [7–9]. SVR has recently been endorsed by the European Task Force on Myocardial Revascularization to be considered a surgical option combined with coronary artery bypass graft surgery (CABG) in selected patients affected by ischaemic heart failure and LV dysfunction, mainly in centres with a high level of surgical expertise [10]. This article presents the feasibility and efficacy of a minimally invasive, off-pump, epicardial ECVR in an anteroapical aneurysm ovine model. Catheter-based LV reconstruction was successfully performed in all the six animals and significant volume reduction and EF improvement was achieved.
A detailed review of current literature shows that comparable animal studies have had mixed results. Ratcliffe et al. [11] report a trend towards redilatation 6 weeks after off-pump anteroapical aneurysm plication in a sheep apical infarct model, which was not seen in the present study. However, studies from the same laboratory have demonstrated that unlike linear repair, patch repair (Dor procedure) significantly reduces LV volume by 29% without redilatation 6-weeks post-procedure [12]. This may be because the linear repair technique cannot exclude the septal scar, and also carries the risk of creating a restrictive residual LV cavity, especially in large aneurysms, leading to diastolic dysfunction and LV failure. In the current study, on the off-pump beating heart, we show that the PliCath HF device successfully achieves ventricular reconstruction akin to patch repair by circumferentially excluding the non-functional scar from both anterior and septal walls through ECVR. Further, ECVR is much less invasive (no ventriculotomy or cardiopulmonary bypass). Significant reductions in LV chamber size and volumes were seen immediately after the device implantation. At 6 weeks’ follow-up, EF was increased by 13% and there was no trend towards redilatation. The PARACHUTE (Cardiokinetix, Inc., Menlo Park, CA, USA) is an in-development percutaneous LV partitioning device that similarly aims to reduce LV volume. While clinical trials are underway, and more information is needed, early feasibility has been demonstrated [13]. It is unclear what thrombus, endocarditis, myocarditis and dislodgement risks may be present with a long-term implantable device in the LV chamber.
A series of large animal studies of SVR using a chronic ovine MI model has been performed with tagged magnetic resonance imaging (MRI) analysis. Zhang et al. [12] found that the Dor procedure not only improves LVEF but also decreases average circumferential wall stress by 23.1% and the systolic circumferential strain in nearly all non-infarcted LV regions at 6 weeks post-procedure. Recent improvements in 2D ultrasound techniques using speckle-tracking imaging have enabled the detection and tracking of tissue pixels, which provides a measurement of ventricle strain that correlates with MRI [14]. In the present study, by using STE analysis, we found that the anterior and septal wall circumferential, radial strains and the apical longitudinal strain was significantly decreased after MI creation and scar maturation. After ECVR, the apex longitudinal strain and the circumferential and radial strains from the border and infarct regions (middle- and apical anterior and septal segments) were significantly improved, while the strains in remote regions did not significantly change. These findings suggest that similar to the traditional SVR reported in the chronic ovine MI model by Zhang et al., ECVR has a positive effect on systolic circumferential strain and wall tension reduction.
Functional MR in patients with ischaemic cardiomyopathy is a secondary phenomenon caused by remodelling of the LV. The presence of MR leads to volume overload that promotes further LV remodelling and carries an excess mortality in postinfarction patients [15, 16]. Combined surgery with SVR and mitral complex reconstruction reduced LV volume in association with improvement of cardiac efficiency in patients with severe heart failure [17]. On the other hand, it is possible that SVR leads to a distortion of the geometry of the LV and subvalvular apparatus, causing presence and an increase in MR [18]. In preclinical and clinical studies [15, 19], MR generally accompanies inferobasal MI, with leaflet tethering by displaced papillary muscles. In the present sheep study, the anteroapical infarction causes LV dilatation and remodelling without MR development. ECVR restored LV shape, reduces LV volume and improves pump function while no MR was observed immediately after the procedure and at 6 weeks’ follow-up.
CONCLUSION
ECVR achieved significant reduction in end-systolic volume, which was durable over a 6-week follow-up. Typical in cases of LV volume reduction, stroke volume was relatively unchanged. EF, global longitudinal strain and systolic circumferential strains from border and infarct regions were improved in the animals after receiving ECVR with the PliCath HF catheter system. This beating heart, epicardial catheter-based approach, therefore generates outcomes, in the animal model, at least, that are consistent with those reported in large human surgical studies; however, it does so without interrupting cardiac function, and therefore, off cardiopulmonary bypass.
Study limitations
The limitations of preclinical animal studies are well known. The model does not perfectly mimic human pathophysiology because the interval between infarction and ECVR does not allow adequate time for the adverse remodelling process to affect the remaining myocardium. Also the scar is very homogeneous, unlike the mosaic distribution most commonly observed in the human patient. For practical reasons, follow-up was limited to 6 weeks, but durability over a greater period would be desirable information. Future investigations in the clinical setting are necessary to determine the clinical benefit of ECVR with the PliCath HF device.
Funding
This work was supported in part by BioVentix (San Ramon, CA, USA).
Conflicts of interest: Kevin Van Bladel and Lon S. Annest are employees of BioVentrix (San Ramon, CA, USA). Andrew S. Wechsler is a consultant of BioVentrix. There are no other financial arrangements or other relationships that could be construed as a conflict of interest.
Acknowledegments
The authors acknowledge Gerard Conditt, Diane Ordanes, and Alyssa Flynn, for their excellent technical assistance and Serge D. Rousselle (Alizée Pathology, LLC, MD, USA), for his assistance with pathology.
REFERENCES
- 1.Lee R, Hoercher KJ, McCarthy PM. Ventricular reconstruction surgery for congestive heart failure. Cardiology. 2004;101:61–71. doi: 10.1159/000075986. [DOI] [PubMed] [Google Scholar]
- 2.Dor V, Saab M, Coste P, Kornazewska M, Montiglio F. Left ventricular aneurysm: new surgical approach. J Thorac Cardiovasc Surg. 1989;37:11–9. doi: 10.1055/s-2007-1013899. [DOI] [PubMed] [Google Scholar]
- 3.Dor V. Left ventricular reconstruction for ischemic cardiomyopathy. J Card Surg. 2002;17:180–7. doi: 10.1111/j.1540-8191.2002.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 4.Klein P, Bax JJ, Shaw LJ, Feringa HH, Versteegh M, Dion RA. Early and late outcome of left ventricular reconstruction surgery in ischemic heart disease. Eur J Cardiovasc Surg. 2008;34:1149–57. doi: 10.1016/j.ejcts.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Dor V, Sabatier M, Di Donato M, Maioli M, Toso A, Montiglio F. Hemodynamic results after left ventricular patch repair associated with coronary grafting in patients with postinfarction akinetic or dyskinetic aneurysm of the left ventricle. J Thorac Cardiovasc Surg. 1995;110:1291–301. doi: 10.1016/S0022-5223(95)70052-8. [DOI] [PubMed] [Google Scholar]
- 6.Koopman LP, Slorach C, Hui W, Manlhiot C, McCrindle BW, Friedberg MK, et al. Comparison between different speckle tracking and color tissue Doppler techniques to measure global and regional myocardial deformation in children. Am Soc Echocardiogr. 2010;23:919–28. doi: 10.1016/j.echo.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Athanasuleas CL, Buckberg GD, Stanley AW, Siler W, Dor V, DiDonato M, et al. Surgical ventricular restoration: the RESTORE group experience. Heart Fail Rev. 2004;9:287–97. doi: 10.1007/s10741-005-6805-4. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo M, Amaducci A, Brunelli F, Dalla Tomba M, Parrella P, Tasca G, et al. Determinants of postinfarction remodeling affect outcome and left ventricular geometry after surgical treatment of ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2004;127:1648–56. doi: 10.1016/j.jtcvs.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 9.Menicanti L, Castelvecchio S, Ranucci M, Frigiola A, Santambrogio C, de Vincentiis C, et al. Surgical therapy for ischemic heart failure: single-center experience with surgical anterior ventricular restoration. J Thorac Cardiovasc Surg. 2007;134:433–41. doi: 10.1016/j.jtcvs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Menicanti L, Castelvecchio S. Left ventricular reconstruction concomitant to coronary artery bypass grafting: when and how? Curr Opin Cardiol. 2011;26:523–7. doi: 10.1097/HCO.0b013e32834ba1cc. [DOI] [PubMed] [Google Scholar]
- 11.Ratcliffe MB, Wallace AW, Salahieh A, Hong J, Ruch S, Hall TS. Ventricular volume, chamber stiffness, and function after anteroapical aneurysm plication in the sheep. J Thorac Cardiovasc Surg. 2000;119:115–24. doi: 10.1016/s0022-5223(00)70225-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, Guccione JM, Nicholas SI, Walker JC, Crawford PC, Shamal A, et al. Endoventricular patch plasty for dyskinetic anteroapical left ventricular aneurysm increases systolic circumferential shortening in sheep. J Thorac Cardiovasc Surg. 2007;134:1017–24. doi: 10.1016/j.jtcvs.2007.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa MA, Pencina M, Nikolic S, Engels T, Templin B, Abraham WT. The PARACHUTE IV trial design and rationale: percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure and dilated left ventricles. Am Heart J. 2013;165:531–6. doi: 10.1016/j.ahj.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Sjøli B, Ørn S, Grenne B, Vartdal T, Smiseth OA, Edvardsen T, et al. Comparison of left ventricular ejection fraction and left ventricular global strain as determinants of infarct size in patients with acute myocardial infarction. J Am Soc Echocardiogr. 2009;22:1232–8. doi: 10.1016/j.echo.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Klein P, Braun J, Holman ER, Versteegh MI, Verwey HF, Dion RA, et al. Management of mitral regurgitation during left ventricular reconstruction for ischemic heart failure. Eur J Cardiothorac Surg. 2012;41:74–80. doi: 10.1016/j.ejcts.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yosefy C, Beeri R, Guerrero JL, Vaturi M, Scherrer-Crosbie M, Handschumacher MD, et al. Mitral regurgitation after anteroapical myocardial infarction: new mechanistic insights. Circulation. 2011;123:1529–36. doi: 10.1161/CIRCULATIONAHA.110.977843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno T, Sakata R, Iguro Y, Yamamoto H, Ueno M, Ueno T, et al. Left ventricular reconstruction with or without mitral annuloplasty. Ann Thorac Cardiovasc Surg. 2009;15:165–70. [PubMed] [Google Scholar]
- 18.Menicante L, Di Donato M, Castelvecchio S, Santambrogia C, Montericcio V, Frigriola A, et al. Functional ischemic mitral regurgitation in anterior ventricular remodeling: results of surgical ventricular restoration with and without mitral repair. Heart Fail Rev. 2004;9:317–27. doi: 10.1007/s10741-005-6808-1. [DOI] [PubMed] [Google Scholar]
- 19.Siefert AW, Rabbah JP, Koomalsingh KJ, Touchton SA, Jr, Saikrishnan N, McGarvey JR, et al. In vitro mitral valve simulator mimics systolic valvular function of chronic ischemic mitral regurgitation ovine model. Ann Thorac Surg. 2013;95:825–30. doi: 10.1016/j.athoracsur.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]