Abstract
OBJECTIVES
Low-dose computed tomography (LDCT) screening improves lung cancer prognosis but also results in diagnostic work-up and surgical treatment in many individuals without cancer. Therefore, we analysed the procedures that screening participants underwent to better understand the extent of overdiagnosis.
METHODS
Between 2009 and 2011, 8649 healthy volunteers aged 50–75 years with a 20 pack-year smoking history underwent LDCT screening, of whom individuals with detected lung nodules had 2 years control. Participants with a nodule >10 mm in diameter or with suspected tumour morphology underwent diagnostic work-up: 283 (6%)/4694 (54%) screened participants had detected lung nodules. One hundred and four individuals underwent surgery, 27 underwent oncological treatment and 152 without a cancer diagnosis underwent further follow-up with LDCT.
RESULTS
In 75% of participants accepted for diagnostic work-up and 25% of surgical patients, the procedures were unnecessary. In 70 (24.7%) participants, a specific diagnosis was obtained mainly due to the low efficacy of fine needle aspiration biopsy [sensitivity, 65.2%; negative predictive value (NPV), 95.9%] and bronchofiberoscopy (sensitivity, 71.4%; NPV, 50%) caused by overinterpretation of LDCT [positive predictive value (PPV), 2%]. Of 104 (36.7%) surgical patients, 43 (41.4%) had a preoperative cancer diagnosis, and 61 (58.6%) underwent surgery without pathological examination. In the latter group, intervention was justified in 35 (57.3%) patients. Complications occurred in 49 (17.3%) participants subjected to diagnostic work-up. In surgical patients, 67 (64.4%) malignant and 37 (35.6%) benign lesions were resected. In the latter group, intervention was justified in only 11 (29.7%) patients. No patient died because of diagnostic or treatment procedures during the study. The complication rate was 14.5% in the malignant and 10.8% in the benign groups. A neoplasm was found in 94 screening participants, of whom 67 (71.3%) underwent surgery; the remaining 27 (28.7%) patients were not surgical candidates. Adenocarcinoma accounted for 49/67 (73%) patients who underwent surgery for non-small-cell lung cancer (NSCLC); 56/67 (84%) patients had stage I NSCLC, and 26/67 (38%) underwent video-assisted thoracoscopic surgery lobectomy.
CONCLUSIONS
Futile diagnostic work-ups and operations must be reduced before LDCT screening can be broadly used. Stage I adenocarcinoma dominated in the NSCLC patients who underwent surgery.
Keywords: Low-dose computed tomography, Lung cancer, Screening, Early detection, Surgery
INTRODUCTION
The poor prognosis associated with lung cancer is generally due to late diagnosis, spurring an intensive search for effective screening tools. The National Lung Screening Trial recently reported that low-dose computed tomography (LDCT) screening of healthy volunteers reduced lung cancer-related mortality by >20% [1]. Furthermore, the International Early Lung Cancer Action Program (I-ELCAP) showed a high prevalence of stage I non-small-cell lung cancer (NSCLC) in screening participants and reported that surgical resection produced a 10-year survival rate of 88%, enhancing the benefit of screening [2].
However, considerable risks must be overcome before LDCT can be broadly used as a preventive tool in populations at risk of developing lung cancer [3–7]. In particular, many individuals undergoing LDCT screening would undergo unnecessary, invasive diagnostic work-up and surgical treatment.
In the present study, we analysed the number of diagnostic procedures and surgical procedures of 8649 volunteers who underwent LDCT screening in Gdansk between 2009 and 2011 to determine the extent and cause of the unnecessary treatment.
PATIENTS AND METHODS
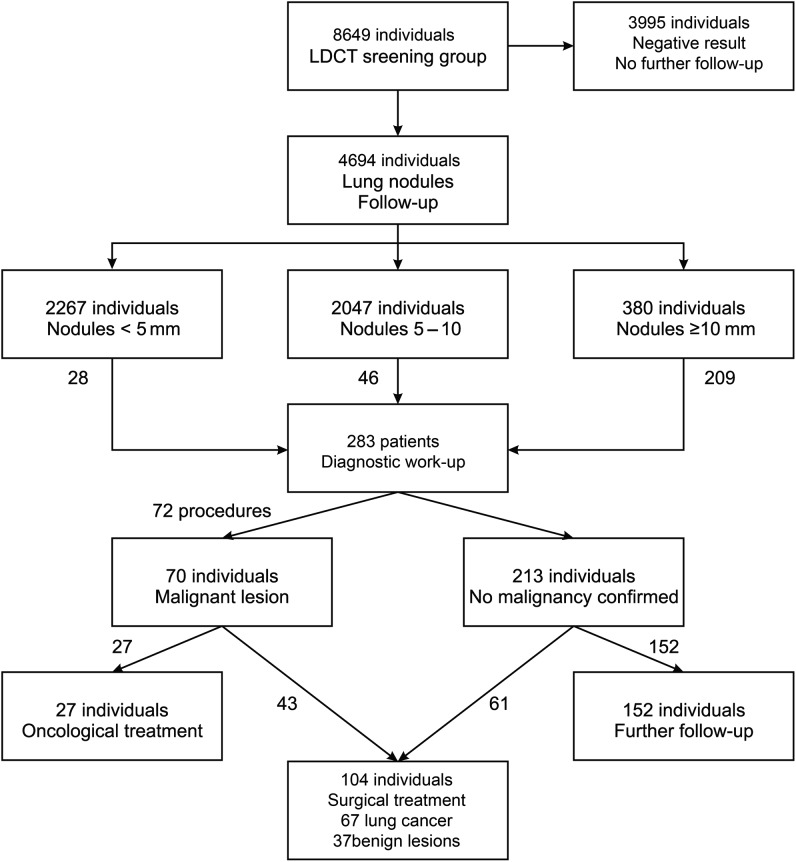
Between February 2009 and April 2011, 8649 healthy volunteers were screened by LDCT through the Pilot Pomeranian Lung Cancer Screening Program after providing informed consent. The study was approved by the institutional review board of Gdansk Medical University (NKEBN/109/2009). This programme included healthy, asymptomatic current or former smokers between 50 and 75 years old with smoking histories of at least 20 pack-years. Among individuals with family histories of lung cancer, the minimum smoking history was 10 pack-years. Participants were recruited through a website and telephone information lines. Their ages were verified using personal documents, and the anamnesis concerning smoking history and the presence of any symptoms suggesting lung cancer was collected. The LDCT screening was carried out at 19 radiological centres in the Pomerania region of Poland. Equipment and methodology conformed to the I-ELCAP radiological protocol, and patients were followed up for 1 year. Patients were categorized into four groups: (i) those with negative LDCT results who did not undergo further observation; (ii) those with nodules <5 mm in diameter who had one follow-up LDCT 12 months after the first round; (iii) those with nodules between 5 and 10 mm who had follow-up LDCT at 3, 6 and 12 months and (iv) those with nodules >10 mm who underwent a diagnostic work-up. The flow chart illustrates the mode of selecting patients for further screening, diagnostic work-up and surgery (Fig. 1). Nodules were evaluated regarding the number, diameter, size, consistency, presence of air, shape, edge pattern and calcification. Additional findings, such as mediastinal and chest wall lesions and liver and suprarenal tumours, were also recorded by each radiologist and consultant using a form available on the program's website.
Figure 1:
The flow chart illustrates the mode of selecting patients for further screening, diagnostic work-up, and surgery.
Three consultants (two radiologists and one thoracic surgeon) reviewed all positive results to determine whether further screening was needed within the programme. All individuals who did not undergo further screening were informed to perform the annual LDCT on their own. The results were entered into the web-based form and collected in the central database. Patients with nodules >10 mm and nodules <10 mm with typical radiological findings suggesting malignancy underwent diagnostic work-up at the Department of Thoracic Surgery of the Medical University of Gdansk comprising bronchoscopy or autofluorescence bronchoscopy when indicated, fine needle aspiration biopsy (FNAB), and spirometry tests/exercise tests. The latter two procedures were performed to assess the eligibility for resection in surgical candidates. Two hundred and twenty-seven participants (80%) were referred for diagnostic work-up after baseline LDCT, and 56 (20%) after the second LDCT. Most of these patients (210/283) underwent the standard diagnostic procedures described above, but 73 underwent selected procedures only. All patients accepted for surgery underwent the procedure at the same institution.
Statistics
For statistical analysis, descriptive data were summarized using raw numbers, means and percentages. Sensitivity and positive predictive values (PPVs) were calculated for lung cancer detection in patients with lung nodules in LDCT. Sensitivity and negative predictive value (NPV) were calculated for all diagnostic procedures in lung cancer patients and separately for FNAB and bronchoscopy.
RESULTS
Diagnostic work-up
In 94 screening participants (1.08%), a neoplasm was found (lung cancer, n=90; other malignancies, n=4; Table 1). The sensitivity of the diagnostic procedures in patients with a cancer diagnosis was 90%, and the NPV was 94.7%.
Table 1:
All malignant neoplasms diagnosed in the pilot pomeranian lung cancer screening program
| n | % | |
|---|---|---|
| All malignancies | 94 | 100% |
| Lung cancer | 90 | 95.7% |
| Squamous cell carcinoma | 20 | 21.3% |
| Adenocarcinoma | 44 | 46.8% |
| Bronchoalveolar carcinoma | 14 | 14.9% |
| Carcinoid | 3 | 3.2% |
| Small-cell carcinoma | 5 | 5.3% |
| Mixed (NSCLC + SCLC) | 2 | 2.1% |
| NOS | 2 | 2.1% |
| Stage | ||
| Ia | 48 | 53.3% |
| Ib | 9 | 10.0% |
| IIa | 2 | 2.2% |
| IIb | 3 | 3.3% |
| IIIa | 21 | 23.3% |
| IIIb | 2 | 2.2% |
| IV | 5 | 5.7% |
| Other malignancies | 4 | |
| Lymphoma | 2 | 2.1% |
| Renal cell carcinoma | 1 | 1.1% |
| Hepatocellular carcinoma | 1 | 1.1% |
NSCLC: non-small-cell lung cancer; SCLC: small-cell lung cancer; NOS: not otherwise specified.
Two hundred and eighty-three of 4694 individuals with lung nodules (6%) underwent diagnostic work-up at the Department of Thoracic Surgery. Only in 70 (24.7%) participants was a specific diagnosis obtained. That was mainly due to the low efficacy of FNAB (sensitivity, 65.2%; NPV, 95.9%) and bronchofiberoscopy that revealed intrabronchial lesions only in 18 (6.4%) patients and NSCLC in 10 patients (sensitivity, 71.4%; NPV, 50%). The low sensitivity of diagnostic procedures was induced by overdiagnosis in the radiological interpretation (PPV, 2%).
A total of 104 (36.7%) participants who underwent diagnostic procedures were accepted for surgery. Forty-three patients (41.4%) had a preoperative cancer diagnosis, and 61 (58.6%) underwent operations without pathological diagnosis at the patient's request or because the clinical picture suggested the malignant nature of the nodule. The remaining 179 individuals who were not accepted for surgery were sent for further annual observation with LDCT in the thoracic outpatient clinic (152) or to non-surgical treatment (27).
Surgery
Of the patients accepted for surgery, 67 malignant tumours (64.4%) and 37 benign lesions (35.6%) were resected. No death was registered in the postoperative course during the study period. Detailed information concerning the stage, pathology, type of procedure, and complications is given in Tables 2 and 3. The mean hospital stay was 9 days (range, 5–21 days) for NSCLC patients and 6 days (range, 3–10 days) for those with benign lesions.
Table 2:
Lung cancer patients from the pilot pomeranian lung cancer screening program
| Operated NSCLC patients (n) | 67 |
| Mean age (years) | 62 |
| Females/males | 28/39 |
| Current/former smokers | 50/17 |
| Mean pack-years | 41.39 |
| Mean tumour diameter (mm) | 18.23 |
| Procedures | |
| Lobectomy | 59 (88%) |
| Bilobectomy | 4 (6%) |
| Pneumonectomy | 2 (3%) |
| Segmentectomy | 1 (1.5%) |
| Wedge resection | 1 (1.5%) |
| VATS procedures/thoracotomy | 26 (39%)/41 (61%) |
| Complete resection | 66 (98.5%) |
| Histology | |
| Squamous cell carcinoma | 12 (18%) |
| Adenocarcinoma | 35 (52%) |
| Bronchoalveolar carcinoma | 14 (21%) |
| Carcinoid | 3 (4.5%) |
| SCLC | 2 (3%) |
| Mixed (NSCLC + SCLC) | 1 (1.5%) |
| Tumour stage | |
| IA | 47 (70%) |
| IB | 9 (13.5%) |
| IIA | 2 (3%) |
| IIB | 3 (4.5%) |
| IIIA | 6 (9%) |
| IIIB and IV | 0 (0%) |
| Complications | |
| Death | 0 (0%) |
| Major complications | 0 (0%) |
| Minor complications | 9 (14.5%) |
NSCLC: non-small-cell lung cancer; SCLC: small-cell lung cancer.
Table 3:
Characteristics of patients with non-malignant lesions who underwent surgical treatment
| Patients (n) | 37 |
| Mean age (years) | 61 |
| Females/males | 20/17 |
| Current/former smokers | 23/12 |
| Non-smokers | 2 |
| Mean pack-years | 36 |
| Mean tumour diameter (mm) | 15.4 |
| Procedures | |
| Lobectomy | 2 |
| Segmentectomy | 2 |
| Wedge resection | 27 |
| Mediastinal tumour resection | 4 |
| Chest wall tumour resection | 1 |
| Explorative thoracotomy | 1 |
| VATS procedures/thoracotomy | 13/18 |
| Histology | |
| Tuberculoma | 7 |
| Hamartoma | 6 |
| Sarcoid tumour | 1 |
| Focal fibrosis and atelectasis | 16 |
| Mediastinal cyst | 3 |
| Chest wall lipoma | 1 |
| Neurofibroma | 1 |
| Subpleural lymphnode | 1 |
| Complications | |
| Pleural haematoma | 2 |
| Atrial fibrillation | 1 |
| Prolonged air leak | 1 |
VATS: video-assisted thoroscopic surgery.
Most NSCLC patients (84%) who underwent surgical treatment had stage I disease. Adenocarcinoma was the most common malignancy (73%), and video-assisted thoracoscopic surgery (VATS) lobectomy (38%) was the most frequently performed operation. Minor complications occurred in 10 patients (15%). Complete resection was achieved in 66 (98.5%) NSCLC patients. In 1 patient, after left upper lobectomy, R1 status was confirmed. That patient received adjuvant chemotherapy due to N2 disease (stage IIIA) and died 8 months after the operation.
Twenty-four of 61 patients accepted for surgery without a preoperative cancer diagnosis had a final diagnosis of NSCLC. The remaining 37 patients had benign lesions. Most of the patients with benign tumours who underwent surgical treatment had fibrotic nodules or focal fibroatelectatic consolidations (43%), 7 (19%) had tuberculomas and 6 (16%) had hamartomas. In 11 patients with non-malignant lesions, we found ex post indications for surgery: 7 tuberculomas, 3 thymic cysts and 1 sarcoid tumour. In the remaining 24 patients, surgery showed no benefit to the patient and was recognized as a futile procedure. Minor complications such as prolonged air leak, pleural haematoma or atrial fibrillation occurred in 4 patients (11%). Detailed information regarding pathology, type of procedure and complications is given in Table 3.
DISCUSSION
We report the results of the largest single-institution experience regarding the diagnosis and treatment of patients from a CT screening cohort of 8649 ex- and current heavy smokers. Several publications exist concerning this topic, but only studies involving small groups of patients were reported [8–11]. Wilson et al. [12] reported a considerable number of patients with a detailed presentation of the diagnostic events and treatment results. Of 3642 screened individuals, they found nodules in 40.6%, performed repeat CT in 22.5% and identified 80 subjects (2.2%) with lung cancer, most of whom had stage I disease (85%). They also reported 36 individuals (1%) who had undergone a major surgical procedure with a noncancerous final diagnosis.
In 75% of the participants accepted for diagnostic work-up and 25% of participants receiving surgery, the procedures were futile. In the present study, 6% of the screening participants with detected lung nodules underwent diagnostic procedures that were justified in 25% of these individuals mainly due to the low effectiveness of FNAB and bronchoscopy. FNAB was an effective diagnostic method only for 26% of all patients undergoing work-up. Bronchoscopy was effective for only 4.5% of the patients. Thus, we do not recommend bronchoscopy as a standard diagnostic procedure in the screening cohort.
There are several factors that could have influenced such a high percentage of futile diagnostic procedures in our series. First, our experience in performing screening was limited before implementing the programme both in terms of planning as well as in the management of this specific group of patients. Presently, in most patients accepted for diagnostic work-up, we would advise observation considering that we suspected many nodules to have a fibrotic or atelectatic nature. In this effort, we find the algorithm concerning the final summary nodule interpretation proposed by Wilson et al. [12] to be very useful. Another way to reduce futile procedures is to change the protocol. However, if we had set this limit at >15 mm, as reported by several other studies [1, 2, 11, 13–18], futile diagnostic work-ups and operations would have been significantly reduced. In such a scenario, we would have sent 95 patients to diagnostic work-up and 45 to surgery instead of 283 and 104, respectively. However, we would have omitted 34 patients with a diagnosis of NSCLC from therapeutic treatment.
Second, positron emission tomography (PET) would have been planned for patients with a lesion with malignant morphology without histological confirmation.
In the future, surgical treatment for benign tumours could be reduced by developing molecular screening tests and implementing effective imaging technologies, including those proposed by Yung et al. [19]. These techniques may enhance the effectiveness of lung cancer screening with LDCT. With such an attempt, the percentage of futile procedures could have been reduced significantly within the surgical group.
Most patients with benign lesions were accepted for surgery at their own request because of the threat of cancer development. We would change several issues in our study; however, one issue seems to be of utmost importance. If we had started such a programme again, psychological support for participants would have been planned much more extensively due to the high expectations of individuals with diagnosed lung nodules (primarily those with suspected lesions in whom observation is proposed) that they would receive such an intervention.
Although there are several pitfalls regarding the concept of lung cancer screening with LDCT, and those related to diagnosis and surgery are stated above, it should be emphasized that all procedures are safe and minimally invasive. No patient died as a result of treatment during the study period. Many of these patients underwent VATS procedures with few complications both in the malignant and benign groups. As expected, the NSCLC stage distribution differed between the screening programme and daily practice, with more early-stage cancers in the screening group (stage I cancer, 84%). Because stage IA lung cancers were common, we could perform VATS lobectomy in many of the NSCLC patients. In our study, complete resection was achieved in 98.5% of operated cases.
Low-dose CT screening is becoming more popular for the diagnosis of early lung cancer. Thoracic surgeons must support these diagnostic efforts and continue to adopt all available diagnostic tools as well as minimally invasive surgical techniques for the early-stage tumours detected by screening.
Growing experience in the interpretation of screening results will likely lead to fewer patients being subjected to intervention. To date, screening programmes have reported that 6–34% of all operated patients with benign lesions have undergone surgical treatment [2, 11, 12, 14, 20–25]. This variability is due primarily to the different protocols used and the experience of the screeners. We believe that only experienced multidisciplinary teams can ensure the lowest possible rate of futile procedures. Thoracic surgeons should play a key role in such a multidisciplinary team.
Funding
Financial support was received from the European Economic Area Financial Mechanism and the Marshall of Pomerania County.
Conflict of interest: none declared.
Acknowledgements
We thank Ewa Schmidt and Magdalena Ernst-Dega for their administrative work and support during the project's implementation.
REFERENCES
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–71. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 3.Van't Westeinde SC, van Klaveren RJ. Screening and early detection of lung cancer. Cancer J. 2011;17:3–10. doi: 10.1097/PPO.0b013e3182099319. [DOI] [PubMed] [Google Scholar]
- 4.Frame PS. Routine screening for lung cancer?: Maybe someday, but not yet. JAMA. 2000;284:1980–3. doi: 10.1001/jama.284.15.1980. [DOI] [PubMed] [Google Scholar]
- 5.Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and lung cancer outcomes. JAMA. 2007;297:953–61. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 6.Manser RL, Irving LB, de Campo MP, Abramson MJ, Stone CA, Pedersen KE, et al. Overview of observational studies of low-dose helical computed tomography screening for lung cancer. Respirology. 2005;10:97–104. doi: 10.1111/j.1440-1843.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 7.Pastorino U. Lung cancer screening. Br J Cancer. 2010;102:1681–6. doi: 10.1038/sj.bjc.6605660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacRedmond R, McVey G, Lee M, Costello RW, Kenny D, Foley C, et al. Screening for lung cancer using low dose CT scanning: results of 2 year follow up. Thorax. 2006;61:54–6. doi: 10.1136/thx.2004.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novello S, Fava C, Borasio P, Dogliotti L, Cortese G, Crida B, et al. Three-year findings of an early lung cancer detection feasibility study with low-dose spiral computed tomography in heavy smokers. Ann Oncol. 2005;16:1662–6. doi: 10.1093/annonc/mdi314. [DOI] [PubMed] [Google Scholar]
- 10.Sone S, Nakayama T, Honda T, Tsushima K, Li F, Haniuda M, et al. Long-term follow-up study of a population-based 1996–1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer. 2007;58:329–41. doi: 10.1016/j.lungcan.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235:259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;178:956–61. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menezes RJ, Roberts HC, Paul NS, McGregor M, Chung TB, Patsios D, et al. Lung cancer screening using low-dose computed tomography in at-risk individuals: the Toronto experience. Lung Cancer. 2010;67:177–83. doi: 10.1016/j.lungcan.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Pastorino U, Bellomi M, Landoni C, De Fiori E, Arnaldi P, Picchio M, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003;362:593–7. doi: 10.1016/S0140-6736(03)14188-8. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen JH, Ashraf H, Dirksen A, Bach K, Hansen H, Toennesen P, et al. The Danish randomized lung cancer CT screening trial—overall design and results of the prevalence round. J Thorac Oncol. 2009;4:608–14. doi: 10.1097/JTO.0b013e3181a0d98f. [DOI] [PubMed] [Google Scholar]
- 16.Ashraf H, Tonnesen P, Holst Pedersen J, Dirksen A, Thorsen H, Dossing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST) Thorax. 2009;64:388–92. doi: 10.1136/thx.2008.102475. [DOI] [PubMed] [Google Scholar]
- 17.Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102:1771–9. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark KW, Gierada DS, Marquez G, Moore SM, Maffitt DR, Moulton JD, et al. Collecting 48,000 CT exams for the lung screening study of the National Lung Screening Trial. J Digit Imaging. 2009;22:667–80. doi: 10.1007/s10278-008-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sone S, Li F, Yang ZG, Honda T, Maruyama Y, Takashima S, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer. 2001;84:25–32. doi: 10.1054/bjoc.2000.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veronesi G, Bellomi M, Mulshine JL, Pelosi G, Scanagatta P, Paganelli G, et al. Lung cancer screening with low-dose computed tomography: a non-invasive diagnostic protocol for baseline lung nodules. Lung Cancer. 2008;61:340–9. doi: 10.1016/j.lungcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Lopes Pegna A, Picozzi G, Mascalchi M, Maria Carozzi F, Carrozzi L, Comin C, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer. 2009;64:34–40. doi: 10.1016/j.lungcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Infante M, Chiesa G, Solomon D, Morenghi E, Passera E, Lutman FR, et al. Surgical procedures in the DANTE trial, a randomized study of lung cancer early detection with spiral computed tomography: comparative analysis in the screening and control arm. J Thorac Oncol. 2011;6:327–35. doi: 10.1097/JTO.0b013e318200f523. [DOI] [PubMed] [Google Scholar]
- 23.Diederich S, Thomas M, Semik M, Lenzen H, Roos N, Weber A, et al. Screening for early lung cancer with low-dose spiral computed tomography: results of annual follow-up examinations in asymptomatic smokers. Eur Radiol. 2004;14:691–702. doi: 10.1007/s00330-003-2200-5. [DOI] [PubMed] [Google Scholar]
- 24.van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON) Int J Cancer. 2007;120:868–74. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 25.Yung RC, Zeng MY, Stoddard GJ, Garff M, Callahan K. Transcutaneous computed bioconductance measurement in lung cancer: a treatment enabling technology useful for adjunctive risk stratification in the evaluation of suspicious pulmonary lesions. J Thorac Oncol. 2012;7:681–9. doi: 10.1097/JTO.0b013e31824a8dcd. [DOI] [PubMed] [Google Scholar]