Abstract
OBJECTIVES
The purpose of this study was to compare the efficacy of 320-detector row computed tomography (CT) with that of 64-detector row CT for three-dimensional assessment of pulmonary vasculature of candidates for pulmonary segmentectomy.
METHODS
We included 32 patients who underwent both 320- and 64-detector CT before pulmonary segmentectomy, which was performed by cutting the pulmonary artery and bronchi of the affected segment followed by dissection of the intersegmental plane along the intersegmental vein. Before the operation, three-dimensional pulmonary vasculature images were obtained for each patient, and the arteries and intersegmental veins of the affected segments were identified. Two thoracic surgeons independently assessed the vessels with visual scoring systems, and kappa analysis was used to determine interobserver agreement. The Wilcoxon signed-rank test was used to compare the visual scores for the assessment of the visualization capabilities of the two methods. In addition, the final determination of pulmonary vasculature at a given site was made by consensus from thoracic surgeons during operation, and receiver operating characteristic analysis was performed to compare their efficacy of pulmonary vasculature assessment. Sensitivity, specificity and accuracy of either method were also compared by means of McNemar's test.
RESULTS
Of the 32 cases, there were no operative complications, but 1 patient died of postoperative idiopathic interstitial pneumonia. Visualization scores for the pulmonary vessels were significantly higher for 320- than those for 64-detector CT (P < 0.0001 for the affected arteries and P < 0.0001 for the intersegmental veins). As for pulmonary vasculature assessment, the areas under the curve showed no statistically significant differences in between the two methods, while the specificity and accuracy of intersegemental vein assessment were significantly better for 320- than those for 64-detector row CT (P < 0.05). Interobserver agreement for the assessment yielded by either method was almost perfect for all cases.
CONCLUSIONS
Three hundred and twenty-detector row CT is more useful than conventional 64-detector row CT for preoperative three-dimensional assessment of pulmonary vasculature, especially when we identify the intersegmental veins, in candidates for pulmonary segmentectomy.
Keywords: Computed tomography, Surgery, Pulmonary artery, Pulmonary vein, Segmentectomy
INTRODUCTION
Complete surgical resection is considered the treatment of choice for individuals with Stage I–II non-small-cell lung carcinoma (NSCLC) and plays a part in the multimodality treatment of resectable Stage IIIA disease [1]. Much supportive evidence has been reported for surgical treatments of NSCLC patients, including segmentectomy, wedge resection and lobectomy. Moreover, the number of varieties of procedures that can be performed using minimally invasive surgical approaches is currently expanding.
However, lobectomy or more extended anatomical procedures may not be feasible for some NSCLC patients due to limited cardiopulmonary functional reserve or extensive comorbidities that may preclude more aggressive surgical resection. Moreover, with an increase in early detection of ever smaller non-small-cell lung cancers through the development of better image diagnostic technology, segmentectomy has come into clinical use for NSCLC with primary tumours <20 mm in diameter and appears to be gaining interest [2]. In anatomical segmentectomy, identification of the intersegmental veins is essential for accurate dissection. It has previously been proposed that segmentectomy can be performed safely with the aid of three-dimensional (3D) multidetector computed tomography (MDCT) simulation for localization of the intersegmental veins [3], and we have been using this technique with satisfactory results.
Recently, however, significant advances have been made in the form of the latest generation 320-multidetector row CT, also known as area detector CT (ADCT), resulting in high visualization due to almost isotropic volume data acquisition and a reduced radiation dose compared with that for a helical scan for commercially available multidetector row CT (MDCT). While it has been shown in several studies that ADCT is effective for the assessment of brain images, pulmonary nodules, lung parenchyma, coronary artery and small abdominal vasculature [4–16], there have been no reports about assessment of lung vasculature in lung cancer patients with regard to surgical procedures.
We hypothesized that ADCT could assess pulmonary vasculature more clearly and accurately than can be attained with 64-detector row CT. The purpose of this study was, therefore, to prospectively and directly compare the utility of 320-detector row CT for preoperative 3D pulmonary vasculature assessment with that of 64-detector row CT for candidates for pulmonary segmentectomy.
MATERIALS AND METHODS
Subjects
This study was approved by the local Ethics Committee of our institution, and written informed consent was obtained from all participating patients.
Between April 2009 and March 2012, a total of 32 patients (24 males and 8 females; mean age, 67 years) suspected of having lung cancer or with metastatic tumours detected on chest radiographs and/or with CT at a nearby hospital and admitted to our clinic prospectively underwent contrast-enhanced chest CT using ADCT and 64-detector row CT for preoperative assessment of pulmonary vasculature in candidates for segmentectomy (Table 1). The median time between ADCT and MDCT examination was 31 (range 17–38) days.
Table 1:
Patient characteristics (N = 32)
| Age in years (range) | 67 ± 9 (48–85) |
| Sex (male/female) | 24/8 |
| Body mass index | 23.0 ± 3.6 |
| Forced vital capacity (l) | 3.23 ± 0.75 |
| Forced expiratory volume in 1 s (l) | 2.28 ± 0.60 |
| Tumour size (cm) | 1.9 ± 0.6 |
| Diagnosis | |
| Lung cancer | 27 |
| Metastatic tumour | 2 |
| Inflammation | 3 |
| Hamartoma | 1 |
Results are expressed as mean ± standard deviation.
Contrast-enhanced CT examinations
For preoperative contrast-enhanced CT examination of all subjects, ADCT (Aquillion One, Toshiba Medical System, Ohtawara, Japan) and two 64-detector row CTs (Aquillion 64; Toshiba Medical System) were used. For each ADCT examination, a wide volume scan was used with the following parameters: 200–320 × 0.5 mm detector collimation, 0.5 s/gantry rotation, 120 kVp, 270 mA and 3–5 rotations. For each MDCT examination, a helical scan was used with the following parameters: 64 × 0.5 mm detector collimation, beam pitch 0.83, 0.5 s/gantry rotation, 120 kVp and 270 mA. A dual-head power injector (Dual Shot GX; Nemoto Kyorindo, Tokyo, Japan) was used for the bolus administration of 20–40 ml (0.2 ml per kg of body weight) of contrast media via a cubital vein at a rate of 0.5 ml/s, which was followed by the administration of 20 ml of saline solution at the same rate, and scanning at 30 s. All contrast-enhanced CT data were reconstructed with the aid of standard kernels for image analyses. The estimated volume CT dose index (CTDIvol; i.e. pitch) displayed on the CT scanner console was recorded for each patient. This value was based on the weighted CT dose index (e.g. tube voltage or tube current). The CTDIvol for each ADCT and MDCT studies were 18.4 and 19.7 mGy, respectively. The estimated radiation doses per length for each study, calculated as CTDIvol multiplied by scanning length, were determined to be 682.8 and 698.3 mGy cm, respectively.
Image reconstruction for 3D assessment of pulmonary vasculature
From the ADCT and MDCT data for each of the subjects, the 3D CT images for pulmonary vasculature assessment were reconstructed by a board-certified thoracic surgeon (S.T.) with 8 years’ experience by using 3D volume rendering software (Aquarius net; TeraRecon, San Mateo, CA, USA). The window level and width were adjusted to identify differences in contrast agent density to help distinguish between arteries and veins (window width: 1700 and window level: 600). All reconstructed images were generated for identification of pulmonary artery and veins, which were relevant to segmentectomy. If the affected segment shared two borders with adjacent segments, it was assessed as having two intersegmental veins. The average time for the 3D image construction was within 10 min.
Operative procedure
We performed segmentectomy with the aid of the previously mentioned simulated images. The lungs were collapsed on the operating side to anesthetize the patients under differential ventilation. We first ligated and resected the segmental artery and detached the bronchus to allow it to be dissected with a stapler after inflation of the lung on the operating side. The inflation–deflation line became gradually clear when the lung was recollapsed. The intersegmental veins were identified by reference to the preoperative simulation and the inflation–deflation line, and the pulmonary parenchyma was dissected from hilum towards the periphery with an ultrasonically activated device, while the intersegmental veins were preserved (Fig. 1). All pulmonary vasculature anatomies were then recorded, and the final determination of pulmonary vasculature at a given site was made by consensus from thoracic surgeons. That is, one of the three thoracic surgeons (Y.M., W.N. and M.Y.) with 23, 25 and, 31 years of experiences took part in the operation and watching the operation movie, visual confirmation was made by all of three surgeons after surgery. The operating time, blood loss volume and the duration of chest tube drainage were also recorded.
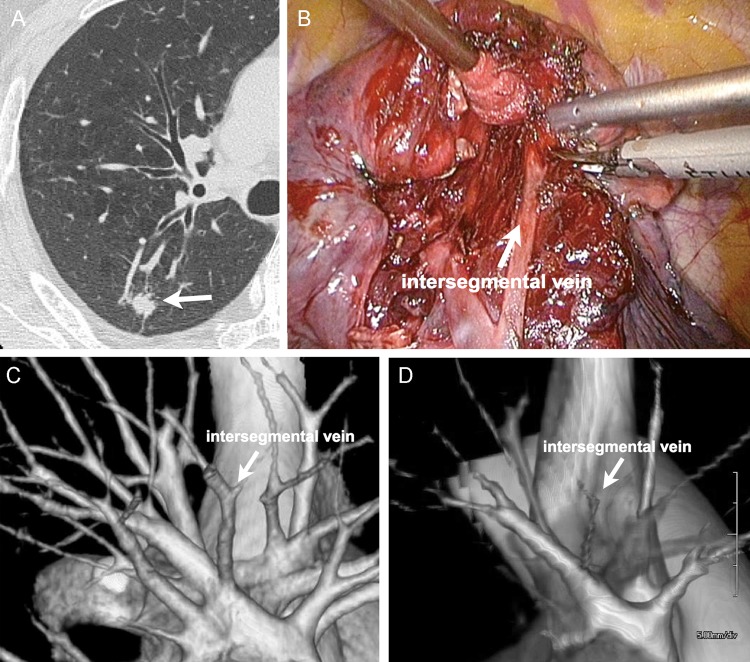
Figure 1:
S.2 segmentectomy of the right lobe of a 56-year old male. (A) CT image shows a 12-mm primary lung tumour (adenocarcinoma) located in S.2. (B) Interoperative view. The intersegmental plane was dissected while preserving the intersegmental vein between S.2 and S.3. (C) 3D vasculature image from area detector CT (ADCT) data clearly shows the intersegmental vein between two segments. This image was scored as 5. (D) 3D vasculature image from multidetector CT (MDCT) data clearly shows the intersegmental vein between two segments. This image was scored as 3.
Image analysis
To assess pulmonary vasculature for segmentectomy, 3D CT images for each of the subjects were interpreted with a picture archiving and communication system (PACS; Centricity; General Electric Healthcare, Milwaukee, WI, USA). Two experienced thoracic surgeons with 8 years’ experience (S.T. and D.H.) independently assessed the visualization of the pulmonary artery and intersegmental veins, which were involved in the segment to be resected. They were also blind to the operating surgeons.
To determine the capability for visualization of the pulmonary artery and intersegmental veins in each of the candidates for segmentectomy, the vasculature as visualized on ADCT and MDCT were assessed by means of a five-point visual scoring system as follows: 1, absolutely untraceable; 2, incompletely traceable; 3, clearly traceable, but the subsegmental vessel was unclear; 4, the subsegmental vessel was clearly traceable and 5, the entire vessel was clearly traceable. The final scores were then determined by consensus of the two readers.
To compare capability for pulmonary vasculature assessment relevant to segmentectomy based on 3D CT images obtained with ADCT and MDCT, identification of pulmonary artery and intersegmental veins relevant to segmentectomy was independently evaluated with a five-point visual scoring system: 1, definitely undetermined; 2, probably undetermined; 3, equivocal; 4, probably determined and 5, definitely determined. Final scores were then determined by consensus of the two readers.
Statistical analysis
Kappa values, which were used to evaluate reproducibility for several observers, were used to determine interobserver agreement on a per-patient basis for the assessment of visualization of pulmonary arteries and intersegmental veins. Interobserver agreement was considered to be slight for κ < 0.21, fair for κ = 0.21–0.40, moderate for κ = 0.41–0.60, substantial for κ = 0.61–0.80 and perfect for κ = 0.81–1.00 [17].
For a comparative analysis of the visualization capabilities of ADCT and MDCT for pulmonary vasculature, visualization scores for pulmonary arteries and intersegmental veins were statistically compared by means of the Wilcoxon signed-rank test.
For a comparison of the capabilities of ADCT and MDCT for pulmonary vasculature assessment relevant to segmentectomy, receiver operating characteristic (ROC) analysis for pulmonary arteries and intersegmental veins was performed on a per-patient basis. Sensitivity, specificity and accuracy for pulmonary artery and intersegmental vein assessments were then directly compared by means of McNemar's test.
A P-value of <0.05 was considered statistically significant.
RESULTS
Operating result
A representative case is shown in Fig. 1. All examinations were successfully performed. There was only 1 hospital death—the patient died of idiopathic interstitial pneumonia on Day 32 after surgery. The location and number of the resected segments were: right segment (S.) 1:1, S.2:4, S.3:1, S.6: 3, S.7+8:2, S.8 + 9 + 10:1, left S.1 + 2 + 3:9, S.1 + 2:4, S.3:1, S.4 + 5:2, S.6:3, S.9 + 10:1. One patient underwent simultaneous segmentectomy of the ipsilateral S. upper division and S. superius for synchronous lung cancer. Ten cases underwent open thoracotomy, and the remaining 22 cases complete thoracoscopic surgery. The surgical results were: operating time, 207 min (range 120–272); volume of haemorrhage, median 100 ml (range 0–575); duration of chest tube insertion, median 2 days (mean 2.6 ± 2.1, range 2–13).
Capability for pulmonary vasculature visualization
Interobserver agreements for the visualization of pulmonary arteries and intersegmental veins are summarized in Table 2. Interobserver agreements for pulmonary arteries and veins were rated almost perfect, with κ = 0.92 for pulmonary arteries on ADCT, κ = 0.85 for pulmonary arteries on MDCT, κ = 0.88 for intersegmental veins on ADCT and κ = 0.82 for intersegmental veins on MDCT.
Table 2:
Overall interobserver agreement for visualization scores on a per-patient basis for area detector CT (ADCT) and multidetector CT (MDCT)
| Observer |
Visualization score |
κ-value | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| ADCT | Reader 1 for the affected arteries | 0 | 2 | 1 | 5 | 24 | 0.92 |
| Reader 2 for the affected arteries | 0 | 2 | 2 | 4 | 24 | ||
| MDCT | Reader 1 for the affected arteries | 1 | 2 | 9 | 18 | 2 | 0.85 |
| Reader 2 for the affected arteries | 1 | 2 | 10 | 15 | 4 | ||
| ADCT | Reader 1 for the intersegmental veins | 1 | 1 | 4 | 12 | 24 | 0.88 |
| Reader 2 for the intersegmental veins | 1 | 2 | 2 | 12 | 25 | ||
| MDCT | Reader 1 for the intersegmental veins | 2 | 1 | 9 | 19 | 11 | 0.82 |
| Reader 2 for the intersegmental veins | 2 | 1 | 9 | 20 | 10 | ||
Comparison of visualization capabilities of ADCT and MDCT for pulmonary vasculatures showed that mean scores for pulmonary artery and intersegmental vein assessment on ADCT (pulmonary arteries: 4.6 ± 0.8 and intersegmental veins: 4.4 ± 0.7) were significantly higher than those on MDCT (pulmonary arteries: 3.6 ± 0.8; P < 0.0001 and intersegmental veins: 3.9 ± 0.7; P < 0.0001).
Capability of pulmonary vasculature assessment for segmentectomy
Interobserver agreements for capability of pulmonary vasculature assessment for segmentectomy are summarized in Table 3. Interobserver agreements for pulmonary arteries and veins were rated as substantial or almost perfect, with κ = 0.91 for pulmonary arteries on ADCT, κ = 0.75 for pulmonary arteries on MDCT, κ = 0.80 for intersegmental veins on ADCT and κ = 0.79 for intersegmental vein on MDCT.
Table 3:
Overall interobserver agreement for vasculature assessment capability scores on a per-patient basis for area detector CT (ADCT) and multidetector CT (MDCT)
| Observer |
Probability score |
κ-value | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| ADCT | Reader 1 for the affected arteries | 1 | 3 | 5 | 9 | 14 | 0.91 |
| Reader 2 for the affected arteries | 2 | 2 | 5 | 8 | 15 | ||
| MDCT | Reader 1 for the affected arteries | 1 | 5 | 6 | 10 | 11 | 0.75 |
| Reader 2 for the affected arteries | 1 | 5 | 6 | 8 | 13 | ||
| ADCT | Reader 1 for the intersegmental veins | 1 | 7 | 2 | 8 | 24 | 0.80 |
| Reader 2 for the intersegmental veins | 2 | 7 | 2 | 8 | 23 | ||
| MDCT | Reader 1 for the intersegmental veins | 1 | 2 | 11 | 18 | 10 | 0.79 |
| Reader 2 for the intersegmental veins | 1 | 3 | 9 | 21 | 8 | ||
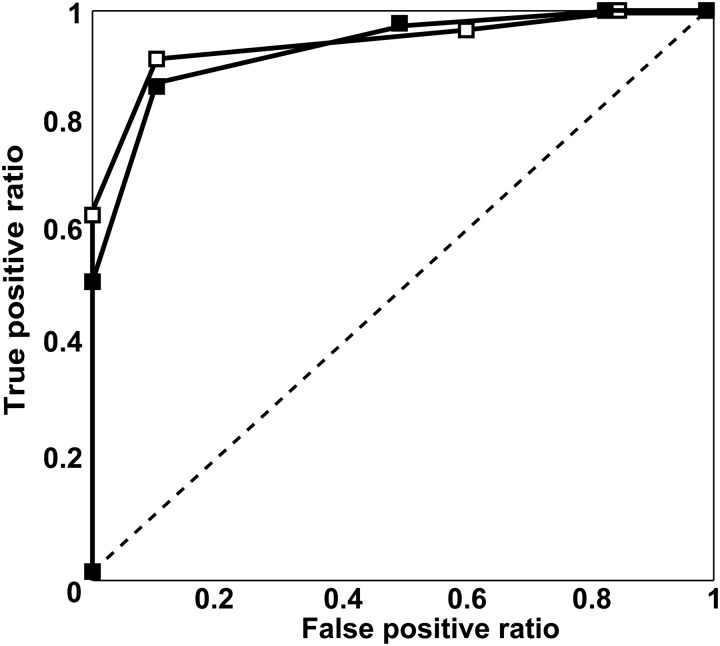
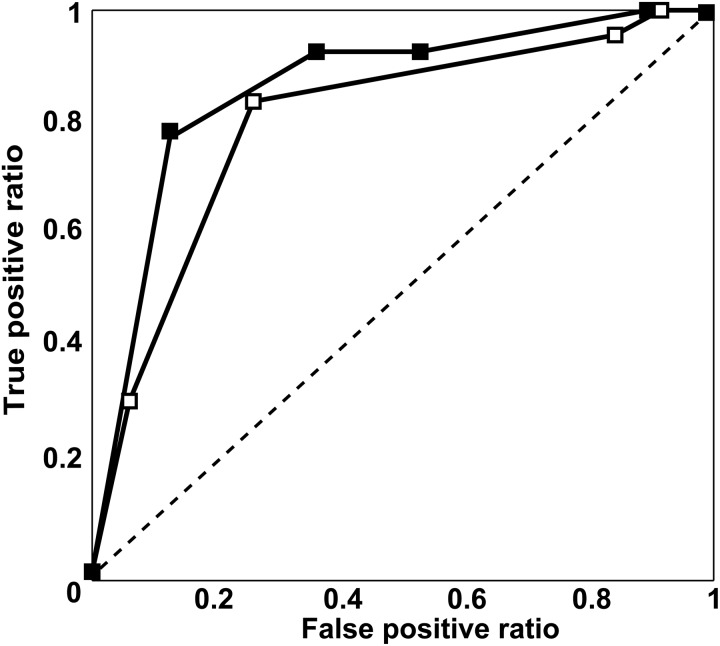
Results of ROC analyses for pulmonary vasculature assessment for segmentectomy are shown in Figs 2 and 3. Areas under the curve (Az) for ADCT (pulmonary arteries: Az = 0.93 and intersegmental veins: Az = 0.85) showed no significant differences from those for MDCT (pulmonary arteries: Az = 0.92, P > 0.05 and intersegmental veins: Az = 0.79; P > 0.05). The feasible threshold values for the two methods were: pulmonary arteries on ADCT: 4, pulmonary arteries on MDCT: 4, intersegmental veins on ADCT: 4 and intersegmental veins on MDCT: 3.
Figure 2:
Results of ROC analysis of segmental pulmonary arterial assessment based on area detector CT (ADCT) and multidetector CT (MDCT) (filled square: ADCT; open square: MDCT). Az of ADCT (pulmonary artery: Az = 0.93) did not differ significantly from those of MDCT (pulmonary artery: Az = 0.92; P > 0.05)
Figure 3:
Results of ROC analysis of intersegmental pulmonary venous assessment based on area detector CT (ADCT) and multidetector CT (MDCT) (filled square: ADCT; open square: MDCT). Az of ADCT (Az = 0.85) did not differ significantly from those of MDCT (Az = 0.79, P > 0.05)
Capabilities for pulmonary vasculature assessment for segmentectomy using ADCT and MDCT are presented in Table 4. The specificity and accuracy of ADCT (50 and 79%) for the assessment of intersegmental veins were significantly better than those of MDCT (21%, P = 0.03 and 70%, P = 0.03, respectively).
Table 4:
Comparison on a per-patient basis of the capabilities of area detector CT (ADCT) and multidetector CT (MDCT) for pulmonary vasculature assessment
| Feasible threshold value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|
| Affected artery | ||||||
| ADCT | 4 | 100 (22/22) | 91 (10/11) | 96 (22/23) | 100 (10/10) | 97 (32/33) |
| MDCT | 4 | 91 (20/22) | 91 (10/11) | 95 (20/21) | 83 (10/12) | 91 (30/33) |
| Intersegmental vein | ||||||
| ADCT | 4 | 92 (26/28) | 50 * (7/14) | 79 (26/33) | 78 (7/9) | 79 * (33/42) |
| MDCT | 3 | 96 (27/28) | 21 * (3/14) | 71 (27/38) | 75 (3/4) | 70 * (30/42) |
PPV: positive predictive value; NPV: negative predictive value.
*Significant difference (P < 0.05) between ADCT and MDCT.
DISCUSSION
Our results demonstrate that the capability of ADCT for pulmonary vasculature assessment in NSCLC scheduled for segmentectomy is equal to or better than that of MDCT.
With advances in diagnostic imaging techniques, small-sized lung cancers are now detected much more often, and even for such small lesions, lobectomy has been the standard surgical procedure for decades [18]. On the other hand, minimized resection procedures, such as segmentectomy, may be used as alternative surgery options for patients with small peripheral lung cancers, for example bronchioloalveolar carcinoma, that may have more indolent biological behaviour [19]. For such procedures, accurate assessment of the vasculature involved is essential, but to the best of our knowledge, no prospective and direct comparison has been made of the capabilities of MDCT and ADCT for pulmonary vasculature assessment.
The kappa values for the assessment of visualization of pulmonary arteries and intersegmental veins ranged from 0.82 to 0.92, indicating that agreement on assessment for visualization was virtually perfect, so that our evaluation of pulmonary vascular visualization capability can be considered reliable.
The comparison of capabilities for pulmonary vasculature visualization showed that mean scores for pulmonary artery and intersegmental vein assessment by ADCT were significantly higher than those by MDCT. It has been suggested in the last few years that the latest generation ADCT system has significant advantages [4–16]. It enables the acquisition of all dynamic data within a 16-cm area every 2 s without helical scanning and makes it possible to obtain images of the pulmonary vessels involved in a single rotation. As for the cardiovascular region, it has been reported that ADCT can provide coronary CT angiography images of excellent quality even for patients with atrial fibrillation, because ADCT enables sufficient craniocaudal coverage in a single rotation and allows imaging of the entire coronary tree within a single beat [4, 7, 12, 16]. In addition, electrocardiograph (ECG)-gated ADCT scans, which provide ECG-gated volumetric scan data of the whole lung without any additional radiation exposure, feature excellent lung image quality [20]. However, published information on ADCT scans of the lung is still very limited [20–22]. While our findings can be considered compatible with previously reported results, ADCT with our proposed protocol cannot be used for the assessment of pulmonary vasculature within the whole lung. However, it can assess the relationship between pulmonary arteries or veins that affect surgical treatment and the main trunks of pulmonary arteries or veins for the acquisition of isotropic volumetric data within 160 mm, and also clearly display the pulmonary vasculature with fewer measurement errors caused by contrast material administration and motion artefacts. Our results, therefore, suggest that it would be better to use ADCT for better visualization of pulmonary vasculatures than can be obtained with MDCT in this context.
For pulmonary vasculature assessment for segmentectomy, interobserver agreement for pulmonary arteries and veins on ADCT and MDCT were either almost perfect or substantial. In addition, kappa values for ADCT were better than those for MDCT. Our results thus indicate that ADCT is better for pulmonary vasculature assessment for segmentectomy in routine clinical practice.
ROC analyses of capability of assessment of pulmonary vasculature for segmentectomy revealed that ADCT could be considered to be almost equal to MDCT. In addition, the application of feasible threshold values showed that specificity and accuracy of ADCT for the assessment of intersegmental veins were significantly higher than those of MDCT. In anatomical segmentectomy, the factor that most influences postoperative morbidity, such as that caused by prolonged air leakage, is identification of the correct intersegment [23], because we usually first identify the intersegmental veins and then dissect the lung parenchyma along them from the hilum to the periphery. The inflation–deflation line is also used as a reference for the intersegmental line, but detection of the line is sometimes hampered in cases of severely emphysematous lung. Preoperative assessment of intersegmental veins thus makes it possible to identify them accurately.
Our results also indicate that there was no significant difference in pulmonary artery assessment capabilities between the two CT systems. This suggests that the actual assessment capability of either system is sometimes masked by false positive/negative results, because the crossing of pulmonary vessels in the hilum makes it difficult to distinguish pulmonary arteries from veins. This anatomical problem can, therefore, not be overcome by using the ADCT system. On the other hand, the intersegmental veins were difficult to identify unless they were visualized to the periphery, so that the ADCT protocol, with its improved visualization of the peripheral vessels such as the intersegmental veins, proved to be a more effective tool for use with candidates for segmentectomy. In fact, although the cutting plain of the segmentectomy could have led to prolonged postoperative air leakage, none of the cases enrolled in our study suffered from complications because we could accurately dissect the parenchyma along the intersegmental veins with the aid of 3D CT simulation. One hospital death occurred, but it was caused by postoperative idiopathic interstitial pneumonia. In support of our finding, Oizumi et al. [3] reported that the use of 3D CT angiography for the intersegmental veins was advantageous for pulmonary segmentectomy. Ours is the first study, however, to show that ADCT has better capability than MDCT for preoperative assessment of pulmonary vessels in candidates for pulmonary segmentectomy. This means that our results are compatible with those of the above-mentioned previous studies [4–16], and that ADCT is better for pulmonary vasculature assessment.
There are a few limitations to this study. First, when compared with our usual surgical procedures with 64-detedctor row CT, that with ADCT tended to shorten the operating time and reduce blood loss volume in case of segmentectomy. Although we did not directly compare those issues between two CT scanners, we assume that ADCT could make the surgery easier in the candidates for segmentectomy. Therefore, in future study, we hope to assess these issues at a randomized control trial in a large prospective cohort. Secondly, we only assessed a relatively small number of vessels, so that further studies with larger populations are needed to validate our results. Thirdly, we did not use ECG-gated ADCT in this study, even though a previous study found that its lung image quality was better than that of non-ECG-gated MDCT [20]. However, ECG-gated MDCT usually requires additional radiation exposure and does not provide volumetric scan data of the entire lung when prospective ECG gating is used for step-and-shoot scanning [8, 24, 25]. Nevertheless, we could have obtained better outcomes if we had used ECG-gated ADCT rather than our protocol. Fourthly, a slight lack of contiguousness was occasionally observed as a unique image artefact at junctional points of the three gantry rotations used for ADCT. This artefact was not carefully examined in this study, although no obvious influences on the results were observed. In addition, frequencies of pulmonary arteries and veins can vary, while the size or number of branches of pulmonary arteries and veins that affect surgical treatment depends on the surgical procedures. These factors may well have influenced our study results. Moreover, several recommendations for preoperative evaluation of NSCLC patients do not specify the need for pulmonary vasculature variation assessment on CT. It is advisable for thoracic surgeons to be familiar with variations in pulmonary vasculature, because such preoperative information can set the surgeon's mind at ease during the operation. However, it is not clear whether the information assessed in this study is clinically relevant, for example, to surgical procedures and hospitalization time, clinical outcomes and cost-effectiveness. Further investigations are thus warranted to clarify this point.
In conclusion, 320-area detector row CT is more useful than conventional MDCT for preoperative 3D assessment of pulmonary vasculature, especially when we identify the intersegmental vein, in candidates for pulmonary segmentectomy. This stool could make the surgical treatment of the pulmonary vessels easier and the operation safer in pulmonary segmentectomy.
Funding
Yoshiharu Ohno obtained research grants from Toshiba Medical Systems and Bayer Pharma.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr E. Bishay (Birmingham, UK): Can you just explain to me (my understanding about a segmentectomy is based on the airway) why does it matter that I identify these veins? Am I doing something wrong in my practice?
Dr Tane: The inflation and deflation line is also important, but if the airway has emphysematous changes, we use both intersegmental veins and inflation and deflation line.
Dr T. Marjanski (Gdansk, Poland): Your study was smartly presented; the conclusions are indisputable. I have got a question, because I don't understand. You performed CT twice, once a 64 row and second time a 320 row, yes?
Dr Tane: No. Before surgery I performed two CT's.
Dr Marjanski: Two angiographies?
Dr Tane: Yes.
Dr Marjanski: So you placed the contrast through the patient twice, yes?
Dr Tane: No. MDCT is usually performed for preoperative staging and at the time of admission ADCT is performed, because the duration between admission and staging is long.
Dr Marjanski: I'm very sorry but I think that this study raises some ethical concerns. I think it is not justified to perform two angiographies instead of routine chest CT, just for research purposes. I think that in the light of the risk of postoperative renal failure, or postoperative hyperthyrosis, it raises some concerns. We are running a similar study but basing it on routinely performed simple CT with IV contrast, not angiography, and definitely not angiography performed twice. Nevertheless, I think the topic is important, as the software provides us with excellent visualization of vasculature.
REFERENCES
- 1.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:234–42. doi: 10.1378/chest.07-1378. doi:10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- 2.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132:769–75. doi: 10.1016/j.jtcvs.2006.02.063. doi:10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 3.Oizumi H, Kanauchi N, Kato H, Endoh M, Suzuki J, Fukaya K, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg. 2011;141:678–82. doi: 10.1016/j.jtcvs.2010.08.027. doi:10.1016/j.jtcvs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Rybicki FJ, Otero HJ, Steigner ML, Vorobiof G, Nallamshetty L, Mitsouras D, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–46. doi: 10.1007/s10554-008-9308-2. doi:10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 5.Salomon EJ, Barfett J, Willems PW, Geibprasert S, Bacigaluppi S, Krings T. Dynamic CT angiography and CT perfusion employing a 320-detector row CT: protocol and current clinical applications. Klin Neuroradiol. 2009;19:187–96. doi: 10.1007/s00062-009-9019-7. doi:10.1007/s00062-009-9019-7. [DOI] [PubMed] [Google Scholar]
- 6.Siebert E, Bohner G, Dewey M, Masuhr F, Hoffmann KT, Mews J, et al. 320-slice CT neuroimaging: initial clinical experience and image quality evaluation. Br J Radiol. 2009;82:561–70. doi: 10.1259/bjr/27721218. doi:10.1259/bjr/27721218. [DOI] [PubMed] [Google Scholar]
- 7.Steigner ML, Otero HJ, Cai T, Mitsouras D, Nallamshetty L, Whitmore AG, et al. Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int J Cardiovasc Imaging. 2009;25:85–90. doi: 10.1007/s10554-008-9347-8. doi:10.1007/s10554-008-9347-8. [DOI] [PubMed] [Google Scholar]
- 8.Hoe J, Toh KH. First experience with 320-row multidetector CT coronary angiography scanning with prospective electrocardiogram gating to reduce radiation dose. J Cardiovasc Comput Tomogr. 2009;3:257–61. doi: 10.1016/j.jcct.2009.05.013. doi:10.1016/j.jcct.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Dewey M, Zimmermann E, Deissenrieder F, Laule M, Dubel HP, Schlattmann P, et al. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation. 2009;120:867–75. doi: 10.1161/CIRCULATIONAHA.109.859280. doi:10.1161/CIRCULATIONAHA.109.859280. [DOI] [PubMed] [Google Scholar]
- 10.Diekmann S, Siebert E, Juran R, Roll M, Deeg W, Bauknecht HC, et al. Dose exposure of patients undergoing comprehensive stroke imaging by multidetector-row CT: comparison of 320-detector row and 64-detector row CT scanners. AJNR Am J Neuroradiol. 2010;31:1003–9. doi: 10.3174/ajnr.A1971. doi:10.3174/ajnr.A1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einstein AJ, Elliston CD, Arai AE, Chen MY, Mather R, Pearson GD, et al. Radiation dose from single-heartbeat coronary CT angiography performed with a 320-detector row volume scanner. Radiology. 2010;254:698–706. doi: 10.1148/radiol.09090779. doi:10.1148/radiol.09090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasis A, Leung MC, Antonis PR, Cameron JD, Lehman SJ, Hope SA, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol. 2010;106:1429–35. doi: 10.1016/j.amjcard.2010.06.073. doi:10.1016/j.amjcard.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 13.Sugihara R, Kitajima K, Maeda T, Yoshikawa T, Konishi M, Kanata N, et al. Comparison of capability of abdominal 320-detector row CT and of 16-detector row CT for small vasculature assessment. Kobe J Med Sci. 2011;56:154–61. [PubMed] [Google Scholar]
- 14.Kitajima K, Maeda T, Ohno Y, Yoshikawa T, Konishi M, Kanata N, et al. Capability of abdominal 320-detector row CT for small vasculature assessment compared with that of 64-detector row CT. Eur J Radiol. 2011;80:219–23. doi: 10.1016/j.ejrad.2010.05.014. doi:10.1016/j.ejrad.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Ohno Y, Koyama H, Matsumoto K, Onishi Y, Takenaka D, Fujisawa Y, et al. Differentiation of malignant and benign pulmonary nodules with quantitative first-pass 320-detector row perfusion CT versus FDG PET/CT. Radiology. 2011;258:599–609. doi: 10.1148/radiol.10100245. doi:10.1148/radiol.10100245. [DOI] [PubMed] [Google Scholar]
- 16.Takagi Y, Akita K, Kondo H, Ishida M, Kaneko K, Sato M, et al. Non-invasive evaluation of internal thoracic artery anastomosed to the left anterior descending artery with 320-detector row computed tomography and adenosine thallium-201 myocardial perfusion scintigraphy. Ann Thorac Cardiovasc Surg. 2012;18:24–30. doi: 10.5761/atcs.oa.11.01684. doi:10.5761/atcs.oa.11.01684. [DOI] [PubMed] [Google Scholar]
- 17.Svanholm H, Starklint H, Gundersen HJ, Fabricus J, Barlebo H, Olsen S. Reproducibility of histomorphologic diagnoses with special reference to the kappa statistic. APMIS. 1989;97:689–98. doi: 10.1111/j.1699-0463.1989.tb00464.x. doi:10.1111/j.1699-0463.1989.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-u. doi:10.1016/0003-4975(95)00537-U. [DOI] [PubMed] [Google Scholar]
- 19.Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg. 2010;89:2096–7. doi: 10.1016/j.athoracsur.2010.03.040. doi:10.1016/j.athoracsur.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Yamashiro T, Miyara T, Takahashi M, Kikuyama A, Kamiya H, Koyama H, et al. Lung image quality with 320-row wide-volume CT scans: the effect of prospective ECG-gating and comparisons with 64-row helical CT scans. Acad Radiol. 2012;19:380–8. doi: 10.1016/j.acra.2011.12.001. doi:10.1016/j.acra.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Kroft LJ, Roelofs JJ, Geleijns J. Scan time and patient dose for thoracic imaging in neonates and small children using axial volumetric 320-detector row CT compared to helical 64-, 32-, and 16- detector row CT acquisitions. Pediatr Radiol. 2010;40:294–300. doi: 10.1007/s00247-009-1436-x. doi:10.1007/s00247-009-1436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagnetz U, Roberts HC, Chung T, Patsios D, Chapman KR, Paul NS, et al. Dynamic airway evaluation with volume CT: initial experience. Can Assoc Radiol J. 2010;61:90–7. doi: 10.1016/j.carj.2009.11.007. doi:10.1016/j.carj.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Jones DR, Stiles BM, Denlinger CE, Antippa P, Daniel TM. Pulmonary segmentectomy: results and complications. Ann Thorac Surg. 2003;76:343–8. doi: 10.1016/s0003-4975(03)00437-5. doi:10.1016/S0003-4975(03)00437-5. [DOI] [PubMed] [Google Scholar]
- 24.Schertler T, Wildermuth S, Willmann JK, Alkadhi H, Marincek B, Boehm T. Effects of ECG gating and postprocessing techniques on 3D MDCT of the bronchial tree. AJR Am J Roentgenol. 2004;183:83–9. doi: 10.2214/ajr.183.1.1830083. doi:10.2214/ajr.183.1.1830083. [DOI] [PubMed] [Google Scholar]
- 25.Schertler T, Glucker T, Wildermuth S, Jungius KP, Marincek B, Boehm T. Comparison of retrospectively ECG-gated and nongated MDCT of the chest in an emergency setting regarding workflow, image quality, and diagnostic certainty. Emerg Radiol. 2005;12:19–29. doi: 10.1007/s10140-005-0435-y. doi:10.1007/s10140-005-0435-y. [DOI] [PubMed] [Google Scholar]