Abstract
A 60-year old male patient with multiple risk factors and two previous interventions over the mitral valve was admitted to the emergency unit with symptoms of cardiac failure. Initial examination revealed a competent mitral bioprosthesis with severe perivalvular mitral insufficiency. Based on previous experiences with transapical procedures, a transapical transcatheter closure of the perivalvular leak was performed. The apex was punctured with a 7 French introducer sheath, and a hydrophilic guidewire was advanced with the aid of a right Judkins catheter and positioned across the defect in the left atrium. Fluoroscopic and tridimensional transoesophageal echocardiography was used to guide the manoeuvre. Next, a long introducer sheath was advanced through the guidewire and positioned inside the left atrium. Two Amplatzer Vascular Plug II (St Jude Medical) were deployed, resulting in a significant reduction in the perivalvular leak. The procedure was considered to be successful. The patient regained consciousness, and the orotracheal tube was removed in the operating theatre. No neurological deficits were detected, and the patient was transferred to the intensive care unit. The patient recovered well and was transferred to the ward after 1 day. Discharge was accomplished after 4 days.
Keywords: Cardiac catheterization/intervention, Heart valve, Transapical, Percutaneous, Mitral valve
CASE REPORT
A 60-year old male patient with hypertension, chronic obstructive lung disease, atrial fibrillation, pulmonary hypertension, rheumatic fever, and two previous interventions over the mitral valve (mitral replacement in 2001 and mitral replacement in 2009) was admitted to emergency unit with symptoms of cardiac failure (New York Heart Association Class IV).
Initial clinical and echocardiographic examination revealed a competent mitral bioprosthesis with severe perivalvular mitral insufficiency. Tridimensional echocardiographic evaluation revealed a 0.67 cm × 2.05 cm defect close to the left atrial appendage (left atrium size 68 mm), left ventricular ejection fraction of 50%, and elevated pulmonary artery pressure. Previous echocardiographic evaluation showed no periprosthetic leak, and no evidence of endocarditis was found.
The Logistic Euroscore predicted a 19.2% mortality and Society of Thoracic Surgery score of 9.2%. Based on previous experiences with the aortic and mitral transapical procedure, a transapical transcatheter closure of the perivalvular leak was performed. Informed consent was obtained.
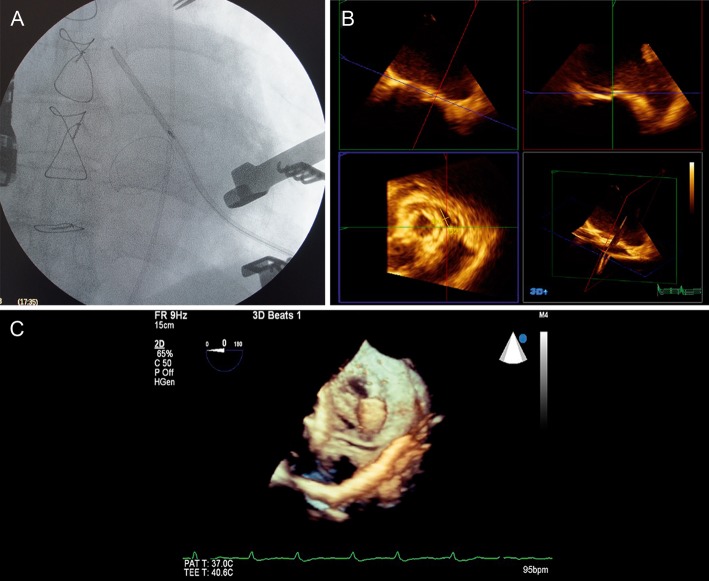
Using general anaesthesia in a hybrid operating theatre, the left ventricular apex was exposed through a minithoracotomy. A purse-string suture with Teflon pledgets was placed to control bleeding. Heparin (2 mg/kg) was administered before puncture of the apex. The apex was punctured with a 7 French introducer sheath, and a hydrophilic guidewire was advanced with the aid of a right Judkins catheter and positioned across the defect in the left atrium. Fluoroscopic and tridimensional transoesophageal echocardiography was used to guide the manoeuvre (Fig. 1). The duration of fluoroscopy required was 3 min. The dimensions of the defect (0.67 cm × 2.05 cm) were measured with tridimensional echocardiography.
Figure 1:
(A) Fluoroscopic view of the guidewire crossing the paravalvular defect. (B) Transoesophageal tridimensional echocardiography, showing defect identification. (C) Transoesophageal tridimensional echocardiography, showing the guidewire crossing the paravalvular defect and initial deployment of the Amplatzer Vascular Plug II.
Next, a long introducer sheath was advanced through the guidewire and positioned inside the left atrium across the defect. An Amplatzer Vascular Plug II (St Jude Medical) measuring 12 mm was used, attempting to leave a 2 mm rim in order to avoid prosthesis dislodgement and embolization.
After first deployment, transoesophageal echocardiography confirmed a reduction in regurgitation, but there was still a moderate leak. A second device was then used following the same procedure described above, but the crossing time was 2 min because the position of the first prosthesis helped guidance. A one-shot closure was not anticipated based on the dimensions of the defect. A second Amplatzer Vascular Plug II, measuring 14 mm, was deployed and resulted in a significant reduction in the perivalvular leak, which was then graded as mild (Fig. 2). The procedure was considered to be successful; the wires and catheters were removed, and the apex was closed. A small chest tube was positioned in the left pleural space.
Figure 2:
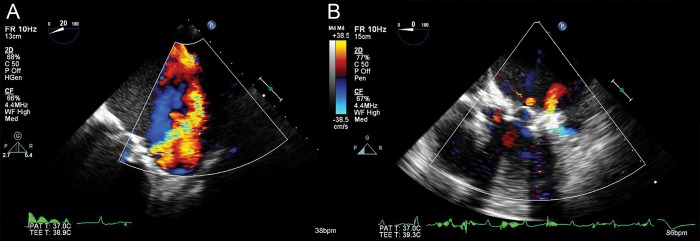
Transoesophageal echocardiography. (A) Preoperative paravalvular mitral regurgitation. (B) Postoperative paravalvular mitral regurgitation.
The patient regained consciousness, and the orotracheal tube was removed in the operating theatre. No neurological deficits were detected, and the patient was transferred to the intensive care unit without inotropic support. No blood products were used.
The patient recovered well and was transferred to the ward after 1 day. Discharge was accomplished after 4 days. The patient was maintained on coumadin therapy.
A follow-up echocardiogram at 2 months postoperatively showed a mild perivalvular leak, no signs of haemolysis, and no prosthesis embolization. The patient recovered functional class to New York Heart Association Class II.
DISCUSSION
Transcatheter valvular techniques have emerged as a safe alternative to conventional surgery in high-risk patients [1]. The transapical approach has been used for aortic and mitral valve implantations, and has several potencial advantages, including easier access to the valves and less manipulation of the arterial system [2, 3].
Prosthetic paravalvular leaks are a relative common complication after valve replacement. Severe degrees of regurgitation combined with symptoms of cardiac failure demand surgical correction, sometimes with high risk due to multiple previous reoperations and baseline patients conditions [1].
The possibility of treating this type of valvular complication in a minimally invasive manner has recently come into focus after the spreading of transcatheter techniques. Usually, trans-septal techniques are used in order to deploy Amplatzer plugs in paravalvular defects, aiming to reduce the degree of regurgitation or abolish it [1, 4]. Unfortunately, in most situations the trans-septal approach can be incredibly challenging and prosthesis deployment is imprecise for many reasons, such as the inability to cross the defect and the need for multiple prosthesis deployment with risk of dislodgement [1].
The transapical approach has emerged as an alternative access site with multiple benefits, including easier defect crossing, easier device manipulation, and the avoidance of trans-septal puncture and its associated complications. Some groups have reported positive results using this technique [4, 5]. Some drawbacks also have to be reported, such as apical closure. Fortunately, the small introducer sheath required for device placement avoids most of the problems associated with apical closure that have been reported for transcatheter valve implantation. It is possible that closure devices could be used, and closed chest techniques could also be considered in these situations.
In the reported case, the time from apical puncture to defect crossing was low, possibly reflecting the ease of the access. In addition, deployment of the second device was also easy, being aided by tridimensional transoesophageal echocardiography, which is, in our opinion, fundamental for correct assessment of the guidewire position and defect measurement.
The final result and follow-up echocardiogram confirmed the sustained result and usefulness of the technique in allowing high-risk reoperated patients to be treated in a less invasive manner. Multiple other combination techniques could also be accomplished, such as simultaneous transcatheter valve-in-valve mitral implantation and perivalvular leak closure, further extending the range of possibilities for transcatheter techniques.
Certainly, the devices have to be improved for this kind of procedure. A custom-designed prosthesis based on preoperative imaging studies using computed tomography and tridimensional echocardiography might possibly improve results and reduce the need for multiple devices. In addition, a custom-designed device would probably reduce valve inflow interference and the risk of dislodgement. An ideal device would probably have an oval-shaped disc, allowing a single device to occlude the perivalvular leak completely.
This case also demonstrates that crossing times can be dramatically reduced using an apical approach, decreasing radiation exposure and total procedure time, especially compared with the usual trans-septal approach. There was no need for considerable experience with the technique in order to achieve a satisfactory result in a relatively simple procedure.
Conflict of interest: none declared.
REFERENCES
- 1.Binder RK, Webb JG. Percutaneous mitral and aortic paravalvular leak repair: indications, current application, and future directions. Curr Cardiol Rep. 2013;15:342. doi: 10.1007/s11886-012-0342-2. doi:10.1007/s11886-012-0342-2. [DOI] [PubMed] [Google Scholar]
- 2.Webb JG, Wood DA, Ye J, Gurvitch R, Masson JB, Rodes-Cabau J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848–57. doi: 10.1161/CIRCULATIONAHA.109.924613. doi:10.1161/CIRCULATIONAHA.109.924613. [DOI] [PubMed] [Google Scholar]
- 3.Gaia DF, Palma JH, de Souza JA, Ferreira CB, Macedo MT, Gimenes MV, et al. Transapical mitral valve-in-valve implant: an alternative for high risk and multiple reoperative rheumatic patients. Int J Cardiol. 2012;154:e6–7. doi: 10.1016/j.ijcard.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Guler A, Tavlasoglu M, Kadan M, Barcin C. Transapical closure of mitral paravalvular leakage. Eur J Cardiothorac Surg. 2013;43:861–3. doi: 10.1093/ejcts/ezs608. [DOI] [PubMed] [Google Scholar]
- 5.Thourani VH, Smith CM, Guyton RA, Block P, Liff D, Willis P, et al. Repair of prosthetic mitral valve paravalvular leak using an off-pump transapical approach. Ann Thorac Surg. 2012;94:275–8. doi: 10.1016/j.athoracsur.2011.12.035. doi:10.1016/j.athoracsur.2011.12.035. [DOI] [PubMed] [Google Scholar]