Abstract
OBJECTIVES
The aim of this study was to evaluate aortic media changes in bicuspid aortic valve (BAV) patients who underwent aortic valve replacement (AVR) and simultaneous replacement of the proximal aorta for BAV stenosis vs BAV insufficiency.
METHODS
Review of our institutional BAV database identified a subgroup of 79 consecutive BAV patients (mean age 52.3 ± 13 years, 81% men) with BAV stenosis or insufficiency and concomitant proximal aortic dilatation of ≥50 mm who underwent AVR and simultaneous replacement of proximal aorta from 1995 through 2005. All cases of BAV disease and concomitant ascending aortic dilatation of 40–50 mm underwent isolated AVR and therefore were excluded from this analysis. Proximal aortic media elastic fibre loss (EFL) was assessed (graded 0 to 3+) and compared between patients with BAV stenosis (Group I, n = 44) vs BAV insufficiency (Group II, n = 35). Follow-up (690 patient-years) was 100% complete and 9.1 ± 4.6 years long.
RESULTS
Mean aortic media EFL was 1.3 ± 0.7 in Group I vs 2.5 ± 0.8 in Group II (P = 0.03). Moderate/severe EFL (i.e. defined as grade 2+/3+) was found in 13 patients (29%) in Group I vs 28 patients (80%) in Group II (P < 0.001). Logistic regression identified BAV insufficiency as the strongest predictor of moderate/severe EFL (OR 9.3; 95% CI 3.2–29.8, P < 0.001). Valve-related event-free survival was 64 ± 8% in Group I vs 93% ± 5% in Group II at 10 years postoperatively (P = 0.05). A total of 4 patients (5%, 3 from Group I and 1 from Group II) underwent redo aortic root surgery for prosthetic valve endocarditis during follow-up.
CONCLUSIONS
Patients with BAV insufficiency and a proximal aorta of ≥50 mm have a significantly higher rate of moderate/severe EFL as compared to their counterparts with BAV stenosis.
Keywords: Bicuspid aortic valve, Aorta, Aortic complication
INTRODUCTION
The surgical treatment of patients with bicuspid aortic valve (BAV) disease and ascending aortic aneurysm is still controversial [1, 2]. In particular, information on the natural history of proximal aortic disease in BAV patients is lacking [3].
BAV has been shown to be a heterogeneous disorder with distinct forms of associated aortopathy [4, 5]. These distinct forms (i.e. so-called BAV phenotypes) which incorporate specific aortic valve anatomy and coexistent lesions of the proximal aorta may be caused by unique pathogenetic mechanisms and may require specific surgical strategies. Recent studies have provided some insight into the different phenotypes of BAV disease [4–7]. However, there is still a notable paucity of clinical data on different BAV phenotypes. Therefore, there is a lack of clinical evidence to recommend a tailored surgical approach to the different forms of this heterogeneous clinical entity.
The aim of this study was to compare the proximal aortic media changes in patients with BAV stenosis vs BAV insufficiency with a coexistent proximal aortic aneurysm of ≥50 mm who underwent simultaneous replacement of the aortic valve and the proximal aorta.
MATERIALS AND METHODS
We reviewed our institutional database to identify all BAV patients who underwent simultaneous replacement of the aortic valve and the proximal aorta for BAV stenosis or insufficiency and a concomitant proximal aortic aneurysm of ≥50 mm from January 1995 through December 2005. Study approval from our local ethics committee was obtained. Individual patient consent was waived.
A total of 530 consecutive patients with BAV disease (mean age 54 ± 13 years, 77% men) underwent aortic valve replacement (AVR) surgery during the study period. Patients operated on after 2005 were excluded in order to obtain an adequate length of follow-up. No patient with Marfan syndrome was included in this group. All BAV patients with an ascending aorta diameter of <50 mm (n = 443) underwent isolated AVR without replacement of the aorta and were excluded from this analysis. A total of 8 BAV patients with a proximal aortic aneurysm of ≥50 mm had a normally functioning BAV (as assessed by preoperative echocardiography/angiography) and were also excluded. Based on these inclusion criteria, a total of 79 (i.e. 15% of the total BAV population) consecutive BAV patients were identified and served as a focus of the current study. A total of 44 BAV patients had predominant/pure aortic valve stenosis (Group I) and the remaining 35 BAV patients presented with an isolated aortic valve insufficiency (Group II).
Definitions and measurements
The morphology and function of the aortic valve was assessed by preoperative echocardiography in all the patients. BAV was suspected if 2D short-axis imaging of the aortic valve demonstrated the existence of only two commissures delimiting two aortic valve cusps. The final decision regarding the bicuspidality of the aortic valve, however, was made based on the intraoperative description of valve morphology by the surgeon. Surgical reports described 1-raphe in 80% and no-raphe in 9% of study patients. No data regarding exact BAV morphology were found in the remaining 11% of study patients.
The diameter of proximal aorta was measured preoperatively by means of transthoracic echocardiography and aortic angiography (i.e. during cardiac catheterization). All patients with a dilated proximal aorta, as diagnosed in these screening examinations, underwent subsequent preoperative contrast-enhanced computed tomography scan or magnetic resonance angiography for precise quantification of the aortic diameters. Moreover, the maximal diameter of the proximal aorta was routinely measured intraoperatively (i.e. using a calliper) before going on pump. If a proximal aortic aneurysm of >50 mm in maximal diameter was observed, then simultaneous aortic surgery by means of composite graft replacement of the aortic root and ascending aorta was performed. As stated above, patients with a proximal aortic diameter of <50 mm underwent isolated AVR only.
Histological examination
All operatively excised proximal aortic tissue (i.e. aortic root and ascending aorta) was sent for histological examination. The specimens were sent to our pathology unit and evaluated by a leading pathologist with a significant experience in cardiovascular medicine. Simultaneously, all specimens were sent to the reference unit of adjacent university hospital. Essentially, no discordant interpretation of elastic fibre loss (EFL) was found between both institutions (i.e. all cases with moderate/severe EFL were graded similarly).
All sections of histological specimen were performed perpendicular to the aortic wall. Multiple samples were obtained from different sites of explanted aorta and histological sections were prepared using haematoxylin–eosin stain and Van Gieson Elastine–Halcian blue stain. EFL in the aortic media was graded from 0 to 3+: grade 0 represented no/minimal fragmentation of elastic fibres; grade 3+ represented complete loss of elastic fibres. Proximal aorta in 10 necropsy cases served as a reference histology for our study. None of these had aortic valve disease or dilated ascending aortas.
For statistical analysis, patients were divided into two groups: patients with grade 0–1 disease in whom EFL was absent or mild, and patients with grade 2–3 disease who demonstrated moderate/severe EFL.
Study population
A total of 79 consecutive BAV patients (mean age 52.3 ± 13 years, 81% men) with aortic valve disease and a proximal aortic aneurysm of ≥50 mm underwent AVR and simultaneous aortic surgery during the study period. The predominant aortic valve disease was BAV stenosis in 44 (56%) patients (Group I) and isolated BAV insufficiency in the remaining 35 (44%) patients (Group II). BAV patients with mixed lesions were assigned according to the dominant aortic valve pathology (i.e. BAV patient with a severe stenosis and mild-to-moderate insufficiency was assigned to the BAV stenosis subgroup). All the 79 patients underwent a modified Bentall procedure (i.e. composite graft replacement with direct reimplantation of the coronary buttons) through a median sternotomy using standard cardiopulmonary bypass and moderate systemic hypothermia. Standardized surgical and anaesthetic protocols were followed with only minor changes over time. Cross-clamp was placed on the distal ascending aorta, just below the orifice of brachiocephalic trunk. Distal anastomosis was performed on the distal ascending aorta, just 1–2 cm proximal to the aortic clamp.
The aortic root was replaced with a mechanical conduit in 99% of patients, and a biological conduit in the remaining 1% of patients. The labelled conduit prosthesis size was 21 mm in 3% of patients, 23 mm in 11%, 25 mm in 31%, 27 mm in 29%, 29 mm in 25% and 31 mm in 1%.
Comparisons of the most relevant variables in both study groups are displayed in Table 1. Briefly, the patients in Group II were younger, more often male and had a larger aortic valve annulus diameter as evidenced by larger implanted composite graft size. Relevant comorbidities were almost equally distributed in both groups. Particularly, there was no difference in preoperative diameter of the proximal aorta in both groups.
Table 1:
Perioperative characteristics of patients with bicuspid aortic valve (BAV) stenosis (Group I) and BAV insufficiency (Group II)
| Variables | Group I (n = 44) | Group II (n = 35) | P-value |
|---|---|---|---|
| Age (years) | 56 ± 12 | 48 ± 12 | 0.03 |
| Male | 33 (75) | 31 (89) | 0.1 |
| Hypertension | 19 (43) | 17 (49) | 0.6 |
| Smoking | 13 (30) | 8 (23) | 0.5 |
| COLD | 5 (11) | 1 (3) | 0.2 |
| LV ejection fraction (%) | 48 ± 8 | 50 ± 4 | 0.6 |
| Diameter of the proximal aorta (mm) | 55 (50–59.5) | 55 (50–60.5) | 0.6 |
| Conduit size (mm) | 25 (25–27) | 27 (25–29) | 0.3 |
| Hemiarch replacement | 2 (5) | 2 (6) | 0.8 |
Median (IQR 25-75), COLD: chronic obstructive lung disease; LV: left ventricle.
In-hospital mortality was 1/79 (1.3%). One patient in Group II died because of septic multiorgan failure (MOF) after an initially uneventful postoperative course. An autopsy was performed and showed evidence of peritonitis as a sequel of prolonged postoperative paralytic ileus.
The primary endpoint of our study was prevalence of moderate/severe EFL in the proximal aortic media. Secondary endpoints included long-term survival and freedom from valve-related adverse events.
Follow-up
Follow-up consisted of a telephone interview with the patients, their family members and/or the patients' family physicians. All imaging data obtained during the postoperative course were requested from the patients' cardiologists or family physicians. All medical records of patients who died in external hospitals were forwarded on request to our hospital. In all cases of out-of-hospital death, we aimed to confirm or exclude sudden cardiac death. Surgical notes were obtained on 4 patients who underwent redo cardiac surgery. Follow-up was 100% complete and was 9.1 ± 4.6 years long.
Statistical analysis
Standard definitions were used for patient variables and outcomes. Categorical variables are expressed as percentages and continuous variables are expressed as mean ± SD with range throughout the manuscript. All statistical analyses were performed with the IBM SPSS 19.0 software (IBM Corp., New York, NY, USA). Correlation analysis was performed using Pearson correlation coefficient. Survival analyses (i.e. long-term survival and valve-related event-free survival) were performed according to the method of Kaplan–Meier and statistical differences were analysed using the log-rank test. A logistic regression analysis of risk factors for moderate/severe EFL was performed. All P-values of 0.05 or less were considered statistically significant.
RESULTS
Histological grading of EFL (0 to 3+) in the proximal aortic media was available for all the 79 (100%) patients (Table 2). The median (interquartile range) EFL score was 1.0 (2.0–1.0) in Group I vs 2.0 (3.0–2.0) in Group II (P = 0.03). Moreover, moderate or severe EFL (defined as grade 2+/3+ disease) was found only in 13 patients (29%) in Group I vs 28 patients (80%) in Group II (P < 0.001). There was still a significant difference in the incidence of moderate or severe EFL between the groups, after performing age-adjusted and aortic diameter-adjusted comparisons using the analysis of covariance (P < 0.01).
Table 2:
Histological grading of elastic fibre loss in both groups
| EFL grade | Group I (n = 44) | Group II (n = 35) | P-value |
|---|---|---|---|
| 0 | 2 (5%) | 0 (0%) | |
| 1+ | 29 (66%) | 7 (20%) | |
| 2+ | 9 (20%) | 13 (37%) | |
| 3+ | 4 (9%) | 15 (43%) | |
| 2+ or 3+ | 13 (29%) | 28 (80%) | <0.001 |
EFL: elastic fibre loss.
There was a significant correlation between EFL and the macroscopic findings at surgery. Surgical reports described markedly thinned and extremely vulnerable aortic wall in nearly all the patients with BAV insufficiency as opposed to the patients with BAV stenosis (i.e. 90% vs 20%). However, there was no major difference between EFL in the sinuses vs tubular part of the ascending aorta.
Mean diameter of the proximal aorta in Group I was 54 ± 5 mm in 31 patients with no/ mild EFL vs 61 ± 7 mm in 13 patients with moderate/severe EFL (P = 0.01). Moreover, there was a linear correlation between the diameter of the proximal aorta and the degree of EFL in Group I (r = 0.5, P = 0.002). There were a total of 17 patients in Group I with the proximal aortic diameter of <55 mm and only 1/17 (6%) of them had a moderate/severe aortic media EFL.
On the contrary, there was no significant difference in the mean diameter of the proximal aorta between 7 patients with no/mild EFL vs 28 patients with moderate/severe EFL (i.e. 55 ± 5 mm vs 57 ± 11 mm, respectively, P = 0.4) in Group II. There was also no linear correlation between proximal aortic diameter and the severity of EFL in Group II (r = 0.25, P = 0.1). There were a total of 14 patients in Group II with a proximal aortic diameter of <55 mm and 10 of 14 (71%) of them had moderate/severe aortic media EFL. Simultaneously, 3 patients in Group II with a proximal aortic diameter of >55 mm had only mild EFL. Correlation between EFL and proximal aortic diameters in both study groups is shown in Fig. 1.
Figure 1:
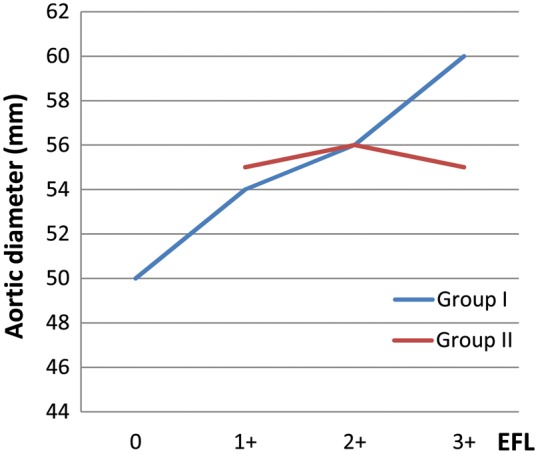
Proximal aortic diameter and elastic fibre loss (EFL) in both groups.
Moderate or severe EFL was found in 7 of 9 (78%) patients with moderate aortic regurgitation (AR), in 18 of 22 (82%) patients with moderate to severe AR and in 3 of 4 (75%) with severe AR in Group II.
We performed a logistic regression analysis in order to identify the risk factors for moderate/severe EFL. A total of four preoperative variables that were found to be significant in the univariate model were included in the logistic regression analysis (Table 3). BAV insufficiency with the coexistent proximal aortic aneurysm of >50 mm was identified as the strongest predictor of moderate/ severe EFL in the aortic media (i.e. OR 9.3; 95% CI 3.2–29.8, P < 0.001).
Table 3:
Predictors of moderate/severe elastic fibre loss (as determined by multiple regression analysis)
| Variables | Odds ratio | P-value | 95% CI |
|
|---|---|---|---|---|
| BAV insufficiency | 9.3 | <0.001 | 3.2 | 29.8 |
| Proximal aorta maximum diametera (mm) | 1.1 | 0.03 | 1.01 | 1.2 |
| Age (years) | 1.0 | 0.9 | 0.9 | 1.1 |
| Hypertension | 1.4 | 0.6 | 0.5 | 4.1 |
BAV: bicuspid aortic valve; CI: confidence interval.
aAs defined by preoperative computed tomography/magnetic resonance tomography.
Follow-up data (a total of 690 patient-years) were obtained on all the 78 hospital survivors (100%). A total of 10 of 44 patients (23%) died in Group I vs 2 of 34 patients (6%) in Group II during follow-up, resulting in an overall 10-year survival of 74 ± 8 vs 93 ± 5% in both groups, respectively (Plog-rank = 0.1) (Fig. 2). The causes of deaths were mostly cardiac-related (67%) and are summarized in Table 4.
Figure 2:
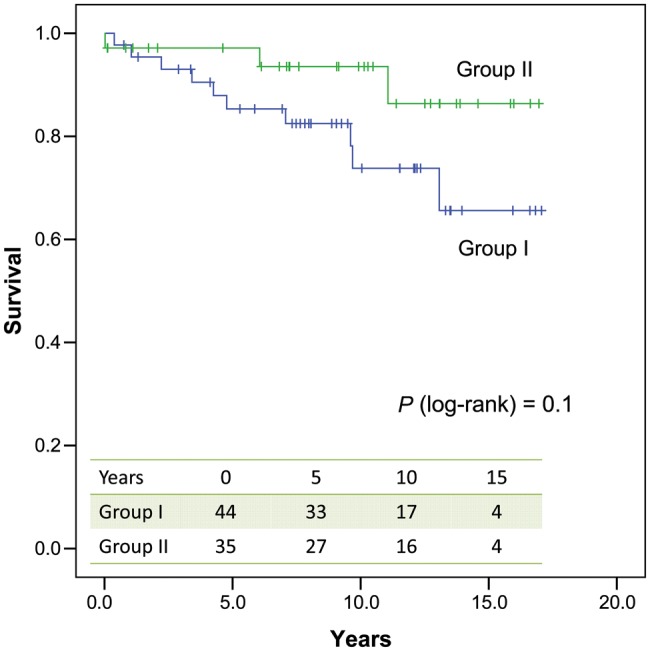
Overall survival in both groups.
Table 4:
Causes of late death in both groups
| Cause of death | Group I | Group II |
|---|---|---|
| Cardiac death | 6 | 2 |
| Myocardial infarction | 1 | 0 |
| Valve-related | ||
| Stroke | 2 | 0 |
| Haemorrhage | 1 | 0 |
| Endocarditis | 2 | 1 |
| Sudden death | 0 | 1 |
| Non-cardiac death | 4 | 0 |
| Malignancy | 1 | 0 |
| Chronic end-stage disease | 2 | 0 |
| Infection | 1 | 0 |
| Total death | 10 | 2 |
Freedom from valve-related adverse events was 76 ± 8% in Group I vs 96 ± 4% in Group II at 10 years postoperatively (Plog-rank = 0.2). Thromboembolic complications were the most common valve-related events which occurred in 5 patients in Group I vs 1 patient in Group II. A total of 4 patients (i.e. 3 in Group I vs 1 in Group II) experienced prosthetic valve endocarditis. One patient in Group I had major cerebral bleeding during follow-up and 1 in Group II experienced sudden cardiac death.
Valve-related event-free survival was 64 ± 8% in Group I vs 93 ± 5% in Group II at 10 years postoperatively (Plog-rank = 0.054) (Fig. 3).
Figure 3:
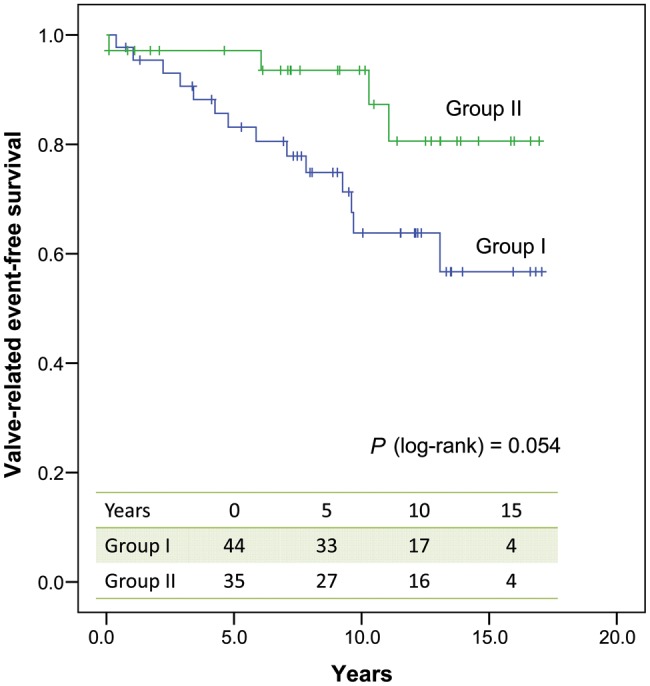
Valve-related event-free survival in both groups.
Redo aortic root surgery was required in 4 (5%) patients (i.e. 3 in Group I and 1 in Group II) after a mean length of time of 8.7 ± 4 years. Indication for redo aortic root surgery was prosthetic valve endocarditis in all patients. No operation was performed for progression of distal aortic disease. A total of 3 of 4 (75%) reoperated patients died in-hospital after redo aortic root surgery. The fourth patient had a prolonged and complicated postoperative course and was transferred to the referring hospital after a total of 2 months intensive care unit stay.
DISCUSSION
Controversy exists regarding the optimal surgical treatment of patients with BAV disease and concomitant dilatation of the proximal aorta [1, 2]. The controversy is potentiated by the marked phenotypic variability of the BAV population, which predisposes to the inconsistency of follow-up data in BAV patients [3]. This heterogeneity of BAV disease has become more widely accepted in the last years as a consequence of identification of different anatomic-clinical forms, so-called BAV phenotypes [4, 5, 8]. Individual BAV phenotypes may be caused by different pathogenetic mechanisms and may require tailored surgical approaches.
It has been well documented that BAV insufficiency and stenosis patients have markedly different clinical and echocardiographic characteristics [9, 10]. BAV insufficiency patients are characterized by a younger age, predominance of male gender and a significantly higher prevalence of aortic annular dilatation compared with their stenotic counterparts [9]. These specific features of BAV insufficiency patients were confirmed in Group II patients in the current study (Table 1). The larger aortic annular diameter in our Group II patients may be observed from the fact that such patients received composite grafts with a larger size (Table 1).
Recent data indicate that BAV insufficiency may be associated with a more malignant form of proximal aortic disease than BAV stenosis [6, 11]. BAV insufficiency (i.e. compared with BAV stenosis) has been identified as an independent risk factor for late adverse aortic events after isolated AVR surgery [11]. Moreover, we were able to identify TGFBR2 gene mutation in affected families with BAV insufficiency, indicating a genetic origin of aortopathy in this specific BAV phenotype [12]. Further indices of the congenital and more malignant nature of aortopathy in patients with the BAV insufficiency compared with the BAV stenosis are presented by the reduction ascending aortoplasty (RAA) studies. A number of published reports identified BAV insufficiency as an independent risk factor for late redilatation of the proximal aorta after RAA, compared with BAV stenosis [13–15]. Moreover, BAV insufficiency patients showed a significantly faster growth of the proximal aortic diameter after RAA compared with those with BAV stenosis [15]. Congenital weakness of aortic wall in BAV insufficiency patients may result in a faster and progressive dilatation of aortic root, in the widening of the aortic annulus and consequently in the occurrence of aortic regurgitation. This hypothesis may be supported by the fact that BAV insufficiency patients were younger, had a larger aortic annulus diameter and got a larger valved conduit in our study. Nonetheless, we may not exclude that a wide range of pulse pressure in the patients with BAV insufficiency may play a role in the progression of microstructural lesions in the aortic wall.
In the current study, we aimed to compare aortic media changes in BAV insufficiency vs BAV stenosis patients who underwent AVR and simultaneous replacement of the proximal aorta. Because of a relatively conservative approach to the proximal aorta in our institution during the study period, only BAV patients with a proximal aortic diameter of ≥50 mm underwent simultaneous aortic surgery and were included in this study. We observed histological changes in the aortic media with regard to loss of elastic fibres, which have been shown to be a good marker of other changes in the aortic media. Although some more detailed histological scoring may better quantify aortic wall lesions [16], the use of multiple criteria is often too complex and not reproducible in the retrospective analysis. Moreover, the examination of elastic fibre structure is easily discernible and highly reproducible with a very low inter- and intraobserver variability [17]. Therefore, we limited our study to the grading of aortic media EFL.
Data of the current study demonstrate clearly that patients with BAV insufficiency and a proximal aorta of ≥50 mm have a significantly higher rate of moderate/severe EFL compared with patients with BAV stenosis (i.e. 80 vs 29%, P < 0.001). Moreover, BAV insufficiency was identified as the strongest predictor of moderate/severe EFL (OR 9.3) in our study. These findings support convincingly the hypothesis that BAV stenosis and BAV insufficiency represent clinically and pathogenetically different forms of BAV-associated aortopathy, which should be considered when making clinical decisions on specific treatment strategies and postoperative patient monitoring.
The high incidence of aortic media EFL and lack of linear correlation between the severity of EFL and proximal aortic diameter in BAV insufficiency patients supports strongly the aggressive surgical treatment strategy of the proximal aorta in this BAV cohort. Composite graft replacement resulted in 93 ± 5% valve-related event-free survival at 10 years postoperatively and no evidence of progression of distal aortic disease in BAV insufficiency cohort. Our previous study [11] showed significantly increased risk of late aortic events in BAV insufficiency patients after an isolated AVR surgery, compared with patients with BAV stenosis (i.e. valve-related event-free survival was 78% in BAV insufficiency patients vs 93% in BAV stenosis (P = 0.01) at 15 years after isolated AVR). All aortic events occurred at the level of the ascending aorta and included acute type A aortic dissection as well as redo surgery for increasing aortic aneurysm. Therefore, based on these data and the high incidence of aortic media EFL in the current study, we would strongly recommend to replace the dilated proximal aorta in BAV insufficiency patients.
In contrast, significantly lower prevalence of EFL in the BAV stenosis patients, especially in the subgroup with the proximal aortic diameter of <55 mm, raises the question about how aggressive surgeons should be with proximal aortic replacement in these patients. Although our conclusions are limited by the small number of included patients, a more conservative surgical approach may be warranted in the subgroup of BAV patients with stenosis, especially when proximal aortic diameter does not exceed 55 mm.
There was a tendency towards worse valve-related event-free survival after composite graft replacement in BAV stenosis subgroup compared with the BAV insufficiency patients. The factors responsible for this finding might be a significantly older patient population with accompanying comorbidities in Group I. This may explain higher rate of non-cardiac deaths in the BAV stenosis subgroup (i.e. 4 vs 0 deaths) during follow-up. Moreover, there was a tendency towards higher incidence of mechanical valve-related events in the elderly Group I patients (i.e. 9 vs 3 events). Therefore, better survival in Group II should be interpreted rather as a function of different baseline characteristics between study subgroups and not as an argument for more benign aortic disease in BAV insufficiency patients.
Redo aortic root surgery after composite graft replacement was associated with an extremely high surgical risk in our study (i.e. in-hospital mortality of 75%). There are several explanations for these dismal results. All these patients underwent redo surgery for the most severe conduit endocarditis. Circumferential aortic root abscess was found intraoperatively in all the 4 patients, which was associated with an extensive involvement of aorto-mitral curtain and adjacent anterior mitral leaflet, and complicated by perforation into the left/right atrium in 2 patients as well as with extension into the left ventricular outflow tract/interventricular septum in 2 further patients. Three of these patients were in MOF caused by recurrent sepsis. As a consequence, all these severely ill patients required a very extensive redo aortic root surgery.
Similar findings have been reported previously by other investigators. Roberts and co-workers were able to demonstrate significant EFL in nearly 50% of patients with pure BAV insufficiency vs 10% of BAV stenosis patients in a cohort of 96 congenitally malformed aortic valve patients who underwent simultaneous AVR and proximal aortic surgery [17]. Lower prevalence of aortic media EFL in both study subgroups compared with our findings may have resulted from inclusion of the patients with smaller diameters of the proximal aorta in their study (i.e. ≥45 mm). In accordance with our findings, presence of BAV insufficiency was associated with a much higher prevalence of significant aortic media EFL compared with BAV stenosis (i.e. OR 8.8; 95% CI 2.9–28.1) in the above mentioned study [17]. Another recent study, which included a significant proportion of BAV insufficiency patients (i.e. 60% of the total study population), showed a high prevalence of moderate/severe histological alterations in the aortic media even in the absence of clinically relevant proximal aortic dilatation [18]. In their multiple regression analysis, these authors demonstrated a significant association between moderate/severe aortic media EFL and diameter of the aortic annulus, which is in turn an indicator of aortic root disease (i.e. root phenotype of BAV disease) [18]. Similarly, Cotrufo and co-workers were able to show significant differences in the expression and spatial distribution of extracellular matrix proteins in the proximal aorta between patients with BAV stenosis vs BAV insufficiency in a series of biomolecular investigations [8].
Study limitations
There are some important limitations of our study. The retrospective design is a clear limitation, which may be overcome only by a randomized controlled trial. The limited number of included patients (i.e. 15% of consecutive BAV patients who underwent AVR during the study period) may be explained by conservative approach to the proximal aorta in our institution during the study period. The third limitation is that we have no data on the serial measurements of the downstream aorta for the whole study population. The available echocardiographic data are of screening value and only sufficient to exclude a clinically relevant progression of distal aortic disease. Therefore, clinically silent progression of downstream aortic disease may not be excluded.
CONCLUSIONS
The current study demonstrates that BAV patients with aortic valve insufficiency and a proximal aorta of ≥50 mm have a significantly higher rate of moderate/severe EFL compared with their counterparts with BAV stenosis.
Conflict of interest: none declared.
REFERENCES
- 1.Bonow RO. Bicuspid aortic valves and dilated aortas: a critical review of the critical review of the ACC/AHA practice guidelines recommendations. Am J Cardiol. 2008;102:111–4. doi: 10.1016/j.amjcard.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Etz CD, Homann TM, Silovitz D, Spielvogel D, Bodian CA, Luehr M, et al. Long-term survival after the Bentall procedure in 206 patients with bicuspid aortic valve. Ann Thorac Surg. 2007;84:1186–94. doi: 10.1016/j.athoracsur.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 3.Girdauskas E, Borger MA, Secknus MA, Girdauskas G, Kuntze T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg. 2011;39:809–14. doi: 10.1016/j.ejcts.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Cotrufo M, Della Corte A. The association of bicuspid aortic valve disease with asymmetric dilatation of the tubular ascending aorta: identification of a definite syndrome. J Cardiovasc Med (Hagerstown) 2009;10:291–7. doi: 10.2459/JCM.0b013e3283217e29. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–8. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 6.Girdauskas E, Disha K, Secknus M, Borger M, Kuntze T. Increased risk of late aortic events after isolated aortic valve replacement in patients with bicuspid aortic valve insufficiency versus stenosis. J Cardiovasc Surg (Torino) 2013;54:653–9. [PubMed] [Google Scholar]
- 7.Hope MD, Hope TA, Meadows AK, Ordovas KG, Urbania TH, Alley MT, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255:53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 8.Della Corte A, Bancone C, Quarto C, Dialetto G, Covino F, Scardone M, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg. 2007;31:397–404. doi: 10.1016/j.ejcts.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2715 additional cases. Mayo Clin Proc. 1999;74:14–26. doi: 10.4065/74.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Hahn RT, Roman MJ, Mogtader AH, Devereux RB. Association of aortic dilation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol. 1992;19:283–8. doi: 10.1016/0735-1097(92)90479-7. [DOI] [PubMed] [Google Scholar]
- 11.Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg. 2012;42:832–7. doi: 10.1093/ejcts/ezs137. [DOI] [PubMed] [Google Scholar]
- 12.Girdauskas E, Schulz S, Borger MA, Mierzwa M, Kuntze T. Transforming growth factor-beta receptor type II mutation in a patient with bicuspid aortic valve disease and intraoperative aortic dissection. Ann Thorac Surg. 2011;91:e70–71. doi: 10.1016/j.athoracsur.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 13.Mueller XM, Tevaearai HT, Genton CY, Hurni M, Ruchat P, Fischer AP, et al. Drawback of aortoplasty for aneurysm of the ascending aorta associated with aortic valve disease. Ann Thorac Surg. 1997;63:762–6. doi: 10.1016/s0003-4975(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 14.Robicsek F, Cook JW, Reames MK, Sr, Skipper ER. Size reduction ascending aortoplasty: is it dead or alive? J Thorac Cardiovasc Surg. 2004;128:562–70. doi: 10.1016/j.jtcvs.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Della Corte A, De Feo M, Bancone C, Provenzano R, Giordano S, Buonocore M, et al. Long-term follow-up of reduction ascending aortoplasty with autologous partial wrapping: for which patient is waistcoat aortoplasty best suited? Interact CardioVasc Thorac Surg. 2012;14:56–63. doi: 10.1093/icvts/ivr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bechtel J, Noack F, Sayk F, Erasmi A, Bartels C, Sievers HH. Histopathological grading of ascending aortic aneurysms: comparison of patients with bicuspid versus tricuspid valves. J Heart Valve Dis. 2003;12:54–61. [PubMed] [Google Scholar]
- 17.Roberts WC, Vowels TJ, Ko JM, Filardo G, Hebeler RF, Jr, Henry AC, et al. Comparison of the structure of the aortic valve and ascending aorta in adults having aortic valve replacement for aortic stenosis versus for pure aortic regurgitation and resection of the ascending aorta for aneurysm. Circulation. 2011;123:896–903. doi: 10.1161/CIRCULATIONAHA.110.972406. [DOI] [PubMed] [Google Scholar]
- 18.Leone O, Biagini E, Pacini D, Zagnoni S, Ferlito M, Graziosi M, et al. The elusive link between aortic wall histology and echocardiographic anatomy in bicuspid aortic valve: implications for prophylactic surgery. Eur J Cardiothorac Surg. 2012;41:322–7. doi: 10.1016/j.ejcts.2011.05.064. [DOI] [PubMed] [Google Scholar]


