Abstract
For many liver diseases, including viral and autoimmune hepatitis, immune cells play an important role in the development and progression of liver injury. Concanavalin A (Con A) administration to rodents has been used as a model of immune-mediated liver injury resembling human autoimmune hepatitis. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) has been demonstrated to alter the development of immune-mediated diseases. Mice pretreated with TCDD developed exacerbated liver injury in response to administration of a mild dose (6mg/kg) of Con A. In the present study, we tested the hypothesis that TCDD pretreatment exacerbates Con A-induced liver injury by enhancing the activation and recruitment of accessory cell types including neutrophils, macrophages, and natural killer (NK) cells. Mice were treated with 0, 0.3, 3, or 30 μg/kg TCDD and 4 days later with Con A or saline. TCDD pretreatment with doses of 3 and 30 μg/kg significantly increased liver injury from Con A administration. The plasma concentrations of neutrophil chemokines were significantly increased in TCDD-pretreated mice after Con A administration. NKT cell-deficient (CD1d KO) mice were used to examine whether NKT cells were required for TCDD/Con A-induced liver injury. CD1d KO mice were completely protected from liver injury induced by treatment with Con A alone, whereas the injury from TCDD/Con A treatment was reduced but not eliminated. However, T-cell deficient (RAG1 KO) mice were protected from liver injury induced by Con A irrespective of pretreatment with TCDD. TCDD/Con A treatment increased the percentage of NK cells expressing the activation marker CD69. Depletion of NK cells prior to treatment resulted in significant reductions in plasma interferon-γ and liver injury from TCDD/Con A treatment. In summary, exposure to TCDD exacerbated the immune-mediated liver injury induced by Con A, and our findings suggest that NK cells play a critical role in this response.
Key Words: dioxin, autoimmune, inflammation, chemokines, liver.
Despite increasing prevalence in the population, the etiology of autoimmune liver diseases is still not thoroughly understood (Feld and Heathcote, 2003). A number of factors appear to confer susceptibility to these diseases. In addition to known genetic risk factors, exposure to environmental xenobiotics is associated with increased incidence of autoimmune disease (Czaja and Manns, 2010; Gilbert, 2010; Longhi et al., 2010). A role for the aryl hydrocarbon receptor (AhR) in the development of these diseases has also been hypothesized. In support of this hypothesis, exposure of synovial tissue from patients with rheumatoid arthritis to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) led to increased production of inflammatory mediators (Kobayashi et al., 2008). TCDD and other AhR ligands also altered the onset and severity of injury in experimental animal models of autoimmune disease; however, the nature of the effect of AhR stimulation on pathogenesis is complex, with reports of both suppression and exacerbation of responses. For example, prenatal treatment of mice with TCDD increased signs of autoimmunity and enhanced kidney damage in a murine model of autoimmune lupus (Holladay et al., 2011; Kobayashi et al., 2008; Mustafa et al., 2009). On the other hand, in experimental autoimmune encephalomyelitis, deterioration of spinal cord neurons was reduced in TCDD-treated mice (Quintana et al., 2008). TCDD also suppressed the development of autoimmune type 1 diabetes in nonobese diabetic mice (Kerkvliet et al., 2009). Thus, exposure to TCDD can have diverse effects on immune-mediated pathogenesis.
The administration of the polyclonal T-cell mitogen concanavalin A (Con A) to mice has been used to produce a model of immune-mediated liver injury that resembles the pathophysiology of autoimmune hepatitis (Tiegs et al., 1992). In this model, errant immune cell activation and destruction of hepatic parenchymal tissue is similar to that which occurs in patients with autoimmune hepatitis (Peters, 2002; Wang et al., 2012). We have previously reported that pretreatment with TCDD sensitized mice to liver injury induced by Con A (Fullerton et al., 2013). The exacerbated response was associated with increased production of interferon-γ (IFNγ) and enhanced activation of natural killer T (NKT) cells (Fullerton et al., 2013), but the effect of pretreatment with TCDD on other immune cell types in this model is unknown.
In the model of hepatitis induced by Con A alone, activation of a number of hepatic immune cell types contributes to liver injury. The primary effector cells appear to be NKT cells, which are required for the development of injury (Kaneko et al., 2000; Takeda et al., 2000). However, conventional CD4+ T cells also contribute to Con A-mediated liver injury, and other hepatic immune cells appear to play accessory roles in the development of liver damage (Tiegs and Gantner, 1996). Con A administration caused a significant increase in the number of neutrophils in the liver, and neutrophil depletion reduced Con A hepatotoxicity (Bonder et al., 2004; Hatada et al., 2005). In addition, hepatic macrophages contribute to injury through the release of inflammatory mediators including tumor necrosis alpha (TNF-α), interleukin (IL)-18, and IL-12, which act directly on parenchymal cells or on lymphocytes to increase production of other cytokines and augment direct cytolytic activity (Faggioni et al., 2000; Nicoletti et al., 2000; Schümann et al., 2000). It is apparent that Con A-induced hepatitis results from the activity of a variety of immune cell types and that the altered response of any one type has the potential to affect the development and severity of injury.
Similarly, TCDD exposure is known to alter the activity of many types of immune cells. Suppressed immune cell function has been reported (Kerkvliet et al., 2002; Sulentic and Kaminski, 2011); however, TCDD can increase the production of inflammatory mediators, particularly from innate immune cells. This is primarily through the activation of AhR signaling (Esser et al., 2009; Kerkvliet, 1995, 2009). In a mouse model of influenza infection, TCDD exposure increased neutrophil recruitment to the lung, resulting in exacerbated tissue injury (Teske et al., 2005). Enhanced accumulation of neutrophils in the peritoneal cavity and increased inflammatory cytokine production were observed after administration of sheep red blood cells to TCDD-treated mice (Moos et al., 1994). Furthermore, TCDD treatment augmented the response of macrophages to inflammatory stimuli such as lipopolysaccharide (Moos et al., 1997). Mice exposed to TCDD had increased mRNA expression of monocyte chemoattractant protein-1 (MCP-1) and keratinocyte chemoattractant (KC) in spleen, kidney and liver tissues, and increased expression of these chemokines was associated with enhanced accumulation of macrophages in those tissues (Vogel et al., 2007).
Given the effects of TCDD on neutrophils and macrophages in other models of inflammatory injury (Teske et al., 2005; Wu et al., 2011) and the contribution of these cell types to the development of liver injury after Con A administration, it was of interest to determine whether these accessory cells play a role in the increased sensitivity of TCDD-pretreated mice to Con A-induced liver injury.
MATERIALS AND METHODS
Materials.
All materials were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise stated. TCDD was purchased from Accustandard (New Haven, CT) dissolved in dimethyl sulfoxide and diluted in olive oil to a working concentration of 0.2 μg/ml.
Mice.
Male C57Bl/6J, B6.129S6-Cd1d1/Cd1d2tm1Spb/J and B6.129S7-Rag1 tm1Mom/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and acclimated for at least 1 week in a 12-h light/dark cycle with access to Global Rodent diet 2018 (Harlan Teklad, Madison, WI) and bottled spring water ad libitum. Mice were used at 10–12 weeks of age, and all procedures were carried out with the approval of the Michigan State University Institutional Animal Care and Use Committee. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the United States National Institutes of Health.
Experimental protocols.
A single administration of 0.3, 3, or 30 μg/kg TCDD or olive oil (vehicle) was given by oral gavage on day 0, followed on day 4, 7, or 10 by IV administration of saline or 6mg/kg Con A (Lot 096 K7011). During the course of each experiment, TCDD-treated mice were housed in separate cages from vehicle-treated mice. The activity of alanine aminotransferase (ALT) in plasma was measured spectrophotometrically using Infinity ALT reagent (Thermo Fischer Scientific, Waltham, MA). Depletion of NK cells was performed using rabbit anti-mouse/rat asialoGM1 polyclonal antibody (Cedar Lane, Burlington, Ontario, Canada) according to the manufacturer’s instructions. Briefly, 50 uL of reconstituted asialoGM1 antibody (Lot DBJ5790) was administered IV to mice in a volume of 150 μL normal rabbit serum. AsialoGM1 antibody or control rabbit serum was given 18h before Con A administration. We have previously demonstrated that this treatment is sufficient to deplete hepatic NK cells (Dugan et al., 2011).
Histopathology.
For neutrophil identification, paraffin-embedded liver sections were stained with rabbit anti-mouse neutrophil antibodies by the Michigan State University Investigative Histopathology Laboratory as described previously (Yee et al., 2003). For each mouse liver section, stained neutrophils were counted in 10 randomly chosen, 40× fields, and the mean count for all fields was used as the value for 1 independent replicate.
Cytokine analysis.
Plasma concentration of IL-12 was measured using an OptEIA ELISA kit purchased from BD (Franklin Lakes, NJ). A bead-based Milliplex MAP immunodetection array (Millipore, Billerica, MA) was used to measure plasma concentrations of KC, MCP-1, and macrophage inflammatory protein 2 (MIP-2) on a Bio-plex instrument (Bio-Rad Laboratories, Hercules, CA).
RNA isolation and Real Time-PCR analysis.
Liver samples were homogenized in TRI reagent (Molecular Research Center, Cincinnati, OH), and isolation of total RNA was performed according to the manufacturer’s instructions. The quantity and quality of isolated RNA was determined using a nanodrop spectrophotometer (Thermo Scientific, Waltham, MA). Complementary DNA (cDNA) was prepared from 1 μg of RNA using iscript reverse transcription supermix for Real Time-qPCR (Bio-Rad Laboratories). Expression levels of target genes were determined on a Step-one real-time PCR system (Applied Biosystems, Foster City CA) utilizing specific DNA oligos and SYBR green PCR master mix (Applied Biosystems). Copy number was determined by comparison with standard curves for each respective gene generated from pooled cDNA. The target gene expression levels were standardized to the geometric mean of expression levels of glyceraldehyde-3-phosphate dehydrogenase (Gapdh), beta-actin (Actb), and hypoxanthine guanine phosphoribosyl transferase (Hprt). To evaluate the expression of target genes, the following PCR primers were used: Gapdh (115bp), 5′-TCAACAGCAACTCCCACTCTTCCA-3′ (forward), 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′ (reverse); Actb (140bp), 5′-TGTGATGGTGGGAATGGGTCAGAA-3′ (forward), 5′-TGTGGTGCCAG ATCTTCTCCATGT-3′ (reverse); Hprt (133bp), 5′-GGAGTCCTG-TTGA TGTTGCCAGTA-3′ (forward), 5′-GGGACGCAGCAACTGACATTTCTA-3′ (reve rse); IL-12p40, Il12b (101bp), 5′-AAAGCTGTCTTCTGCTTGGTTGG C-3′ (forward), 5′-CTGGCTCTGCGGGCATTTAACATT-3′ (reverse); IL-27, Il27 (107bp), 5′-GTGACAGGAGACCTTGGCTG-3′ (forward), 5′-AGCT CTTGAAGGCTCAGGG-3′ (reverse). mRNA expression data are reported as fold change of standardized treatment over standardized vehicle/saline treatment at time zero.
Flow cytometry.
Hepatic leukocytes were isolated from mice and prepared for flow cytometry analysis as follows. Mouse livers were washed with PBS without calcium and magnesium. Livers were placed in RPMI medium supplemented with 5% fetal bovine serum (FBS) and 1% penicillin/streptomycin and then passed through a nylon mesh, and the resulting cell suspension was centrifuged at a speed of 50 × g for 5min at 4°C. The supernatant was removed, and the pelleted hepatocytes were discarded. The supernatant was centrifuged at 450 × g for 5min to pellet leukocytes. The pellet was then incubated for 4min with red blood cell lysis buffer (BioLegend, San Diego, CA) followed by 2 washes with PBS containing 5% FBS. Lympholyte-M (Cedar Lane) was used according to manufacturer’s instructions to further purify leukocytes. For ex vivo stimulation experiments, hepatic leukocytes were cultured in RPMI medium supplemented with 5% FBS and 1% penicillin/streptomycin with or without 7.5 μg/ml Con A for 5h. In all other instances, hepatic leukocytes were stained and prepared immediately for flow cytometric analysis.
Hepatic leukocytes were first incubated with TruStain FcX (anti-mouse CD16/CD32) to minimize nonspecific binding of staining antibodies to Fcγ receptors. Antibodies used for staining of NK, NKT, and T cells included fluorescein isothiocyanate or phycoerythrin-conjugated anti-NK1.1 (PK136) and allophycocyanin-cyanine dye 7-conjugated anti-CD3epsilon (145- 2c11), as well as pacific blue-conjugated anti-CD69 (H1.2F3) and allophycocyanin-conjugated anti-NKG2d (CX5). Staining of hepatic macrophages and neutrophils was performed using fluorescein isothiocyanate or allophycocyanin-conjugated anti-F4/80 (BM8), phycoerythrin-conjugated anti-CD11b (M1/70), and phycoerythrin-cyanine dye 7-conjugated anti-Gr-1 (RB6-8C5). Appropriate fluorescent-conjugated isotype controls were utilized to establish positive and negative gating parameters for each antibody. Unless otherwise stated, all reagents and antibodies for flow cytometric staining were purchased from BioLegend (San Diego, CA). Hepatic leukocyte staining was performed according to manufacturer’s directions, and stained samples were analyzed on a BD FACSCanto II with subsequent data analysis performed using Kaluza software (Beckman Coulter, Brea, CA).
Statistical analysis.
Experimental results are expressed as mean ± SEM. Arcsine transformation was performed on percentile data. In some instances, a Box-Cox transformation was utilized to normalize data for further analysis. Statistical analysis of data was performed using either student’s t-test or 2-way ANOVA followed by pairwise multiple comparisons using Student–Newman–Keuls or Tukey’s method where appropriate. Analysis of nonparametric data was performed using Kruskal–Wallis 1-way ANOVA on ranks followed by pairwise multiple comparisons using Tukey’s or Dunn’s method where appropriate. The criterion for statistical significance was p < 0.05. For all experiments, the term “independent replicates” refers to biological samples collected from separate mice in each treatment group.
RESULTS
TCDD Sensitization to Con A: Dose response
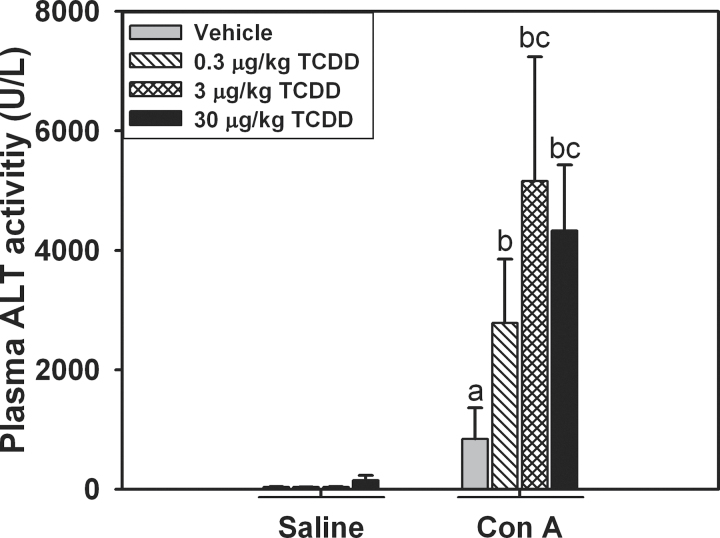
We have previously demonstrated that pretreatment with a nonhepatotoxic dose of 30 μg/kg TCDD exacerbates the inflammatory liver injury induced by a dose of 6mg/kg Con A administered 4 days later (Fullerton et al., 2013). This study was undertaken to determine if smaller doses of TCDD sensitize mice to Con A-induced liver injury. When saline was administered on day 4, no liver injury developed in vehicle-pretreated mice or in TCDD-pretreated mice regardless of dose, as determined by measurements of ALT activity in plasma (Fig. 1A). When Con A was administered on day 4, an increase in ALT activity in the plasma was detected in vehicle-pretreated mice, indicating moderate hepatotoxicity. Compared to pretreatment with vehicle, mice pretreated with 3 or 30 μg/kg TCDD had significantly increased ALT activity in plasma 8h after Con A administration.
Fig. 1.
Dose-dependent exacerbation of Con A-induced liver injury by TCDD. Mice were treated on day 0 with 0, 0.3, 3, or 30 μg/kg of TCDD. After 4 days, they were given 6mg/kg Con A or saline. ALT activity in plasma was measured 8h after Con A or saline administration. a, p < 0.05 vehicle/Con A versus vehicle/saline. b, p < 0.05 TCDD/Con A versus the same TCDD dose with saline treatment. c, p < 0.05 TCDD/Con A versus vehicle/Con A. Data represent the mean ± SE of 3–6 independent replicates per treatment group.
To evaluate the influence of the interval between administration of TCDD and Con A, mice were given Con A 4, 7, or 10 days after pretreatment with vehicle or 30 μg/kg TCDD. They were euthanized 24h later. At all intervals evaluated, TCDD/Con A-treated mice had increased ALT activity in plasma compared with vehicle/Con A-treated mice (Supplementary Figure 1), and the increase in ALT was greatest with the 7-day interval.
Inflammatory Chemokines in TCDD/Con A-Induced Liver Injury
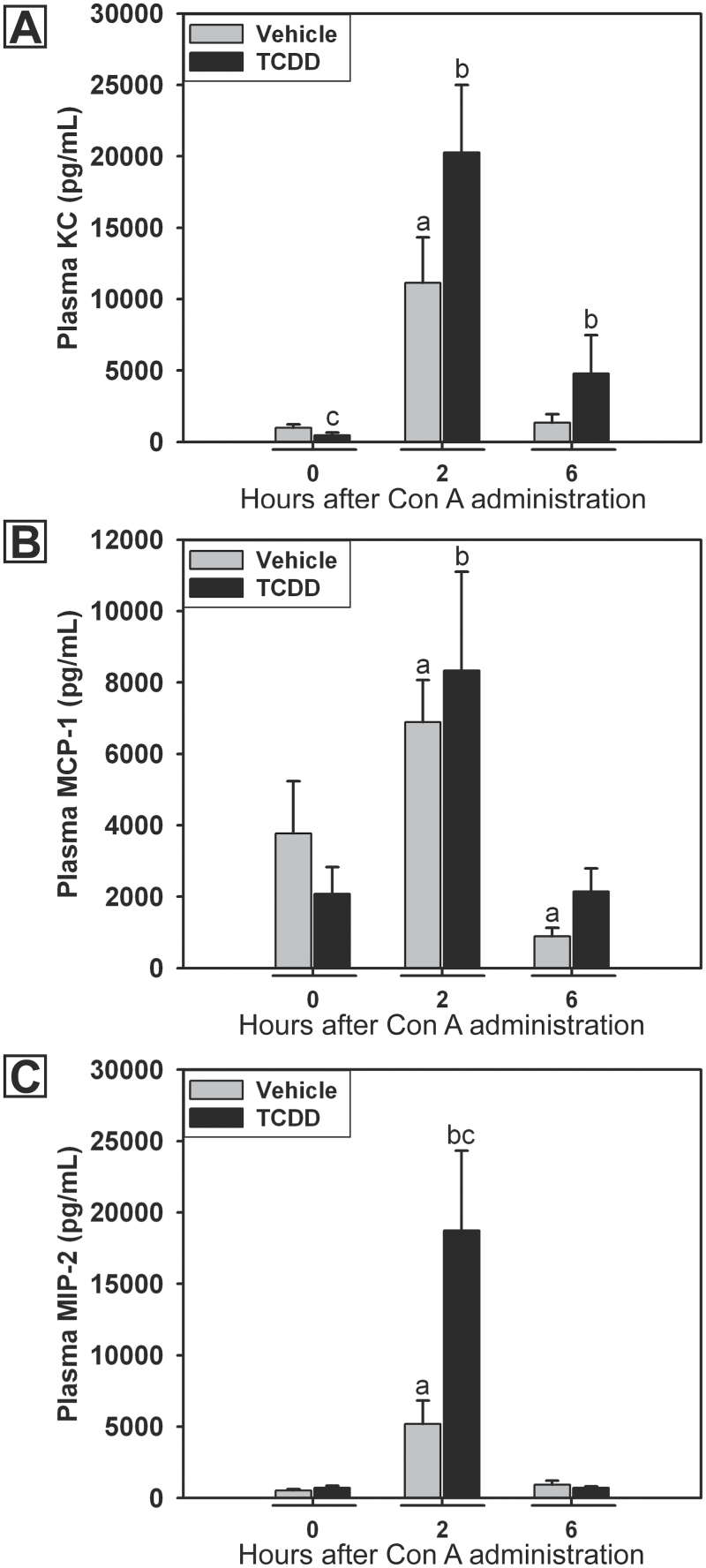
Inflammatory liver injury often involves the recruitment of numerous cell types to the liver; such recruitment is mediated by various chemokines. Mice treated with TCDD/Con A developed liver injury that was first evident 4h after the administration of Con A and peaked at 8h (Fullerton et al., 2013). In this study, the production of chemokines was measured in the plasma at times prior to the peak of TCDD/Con A-induced liver injury. TCDD pretreatment alone decreased plasma concentration of KC compared with vehicle pretreatment in the absence of Con A (Fig. 2A). Concentrations of KC, MCP-1, and MIP-2 were significantly increased 2h after Con A administration and returned to baseline by 6h (Figs. 2A–C). TCDD pretreatment did not alter the induction of KC, or MCP-1 by Con A, at any time but led to a significant increase in the plasma concentration of MIP-2 two hours after Con A administration. In TCDD/Con A-treated mice, the concentration of KC in plasma remained elevated at 6h compared with 0h.
Fig. 2.
Concentrations of KC (A), MCP-1 (B), and MIP-2 (C) in plasma after Con A administration. Mice were treated on day 0 with vehicle (gray bars) or 30 μg/kg TCDD (black bars) and on day 4 with 6mg/kg Con A. Plasma samples were collected at various times after the administration of Con A. a, p < 0.05 versus vehicle pretreatment at 0h. b, p < 0.05 versus TCDD pretreatment at 0h. c, p < 0.05 TCDD pretreatment versus vehicle pretreatment at the same time point. Data represent the mean ± SE of 5–10 independent replicates per treatment group. Data were combined from 2 separate experiments.
Hepatic Neutrophil Accumulation in TCDD/Con A-Induced Liver Injury
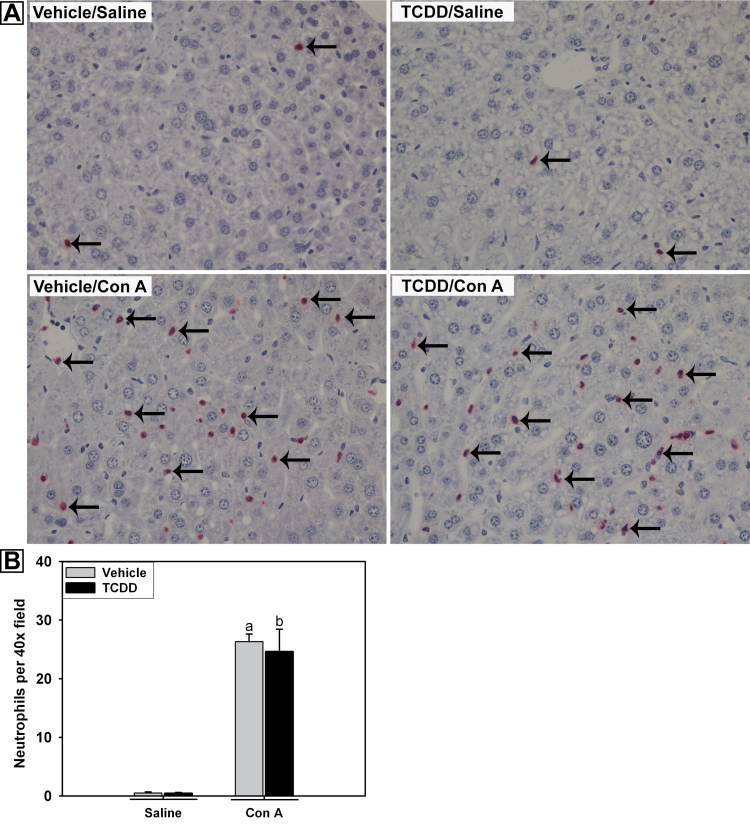
The increase in plasma KC and MIP-2 concentrations in TCDD/Con A-treated mice raised the possibility of a greater influx of neutrophils into livers of those animals. Accumulation of neutrophils in the liver was determined by immunohistochemical staining of liver samples collected 4h after the administration of saline or Con A. TCDD pretreatment alone did not result in increased numbers of neutrophils in the liver (Figs. 3A and 3B). Con A administration significantly increased the number of neutrophils compared with vehicle/saline treatment, and TCDD pretreatment did not alter the accumulation or distribution of neutrophils in the liver.
Fig. 3.
Hepatic neutrophil accumulation after Con A administration. Mice were treated as described in the legend to Figure 2 with vehicle/saline, TCDD/saline, vehicle/Con A, or TCDD/Con A. A, Mice were euthanized 4h after saline or Con A administration. Paraffin-embedded liver sections were stained for neutrophils. Representative liver sections were photographed at ×40 magnification. Examples of positive neutrophil staining are indicated by arrows. B, Immunohistochemical staining of neutrophils in the livers at 4h was quantified as described in Materials and Methods. a, p < 0.05 vehicle/Con A versus vehicle/saline. b, p < 0.05 TCDD/Con A versus TCDD/saline. Data represent the mean ± SEM of 3–4 independent replicates per treatment group.
Flow cytometry was also used to evaluate the percentage of neutrophils (Gr-1+, CD11b+ cells) in the isolated hepatic leukocyte population at 0, 2, 4, and 24h after Con A administration. TCDD pretreatment did not alter the percentage of neutrophils in the liver at any time after Con A administration (data not shown).
Role of Hepatic Macrophages in TCDD/Con A-Induced Liver Injury
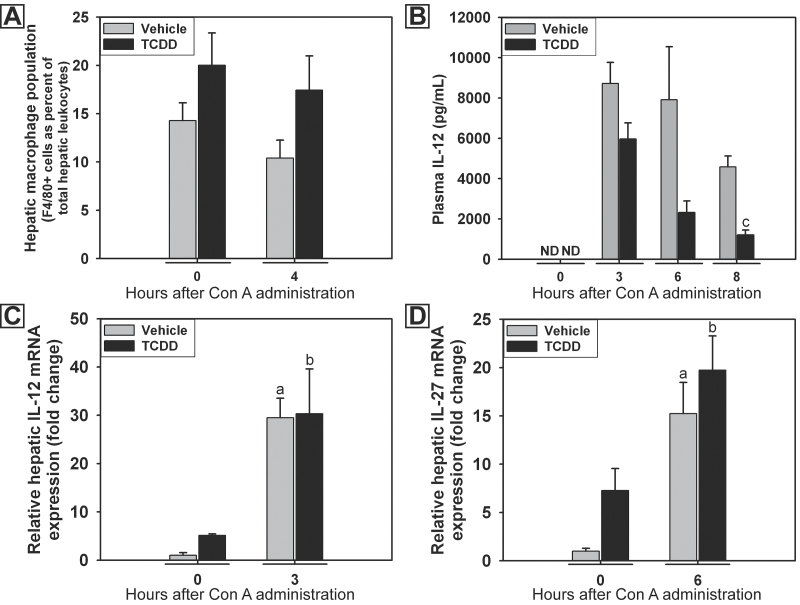
Macrophages play an accessory role in the development of Con A hepatitis (Hatano et al., 2008; Nakamura et al., 2001; Schümann et al., 2000). MIP-2 is produced by activated macrophages, and its concentration was increased in plasma of TCDD-pretreated mice (Fig. 2C). Accordingly, the role of hepatic macrophages in the development of TCDD/Con A-induced liver injury was evaluated. The percentage of monocytes/macrophages (F4-80+ cells) in the population of intrahepatic leukocytes isolated after Con A administration was evaluated by flow cytometry at times before the development of liver injury. Compared with vehicle, TCDD pretreatment 4 days earlier did not alter the percentage of macrophages measured in the liver at 0h (Fig. 4A). The percentage of macrophages observed was not affected by Con A administration in either vehicle- or TCDD-pretreated mice.
Fig. 4.
Hepatic macrophages and macrophage-associated cytokines after Con A administration. Mice were treated as described in the legend to Figure 2 with vehicle/Con A (gray bars) or TCDD/Con A (black bars). A, percentage of F4/80-positive hepatic leukocytes. B, concentration of IL-12 in plasma. C, hepatic expression of IL-12 mRNA after Con A administration. D, hepatic expression of IL-27 mRNA after Con A administration. a, p < 0.05 versus vehicle at 0h. b, p < 0.05 versus TCDD at 0h. c, p < 0.05 TCDD pretreatment versus vehicle pretreatment at the same time. Abbreviation: ND, not detected (value below the limit of detection; 3.2 pg/ml). Data represent the mean ± SEM of 4–6 independent replicates per treatment group. Data were combined from at least 2 separate experiments.
In addition to the quantification of macrophages by flow cytometry, the effect of TCDD pretreatment on the production of macrophage-derived cytokines in response to Con A was evaluated. The plasma concentration of IL-12 was measured at times before the development and at the peak of liver injury. Con A administration resulted in an increased concentration of IL-12 in plasma at 3, 6, and 8h after treatment (Fig. 4B). TCDD pretreatment decreased the concentration of IL-12 in plasma measured 8h after Con A administration. The hepatic expression of IL-12 and IL-27 mRNA was assessed by real-time PCR. Expression of both was increased in Con A-treated mice at 3 and 6h after administration, respectively, but expression was not affected by pretreatment with TCDD (Figs. 4C and 4D).
NKT and T Cells in TCDD-Induced Sensitization of Mice to Con A Hepatotoxicity
Both NKT and conventional CD4+ T cells have been implicated as major effector cells in the development of Con A-induced liver injury, so the requirement for these cell types in the sensitization to Con A hepatotoxicity induced by TCDD pretreatment was evaluated. NKT cell-deficient B6.129S6-Cd1d1/Cd1d2tm1Spb/J (CD1d KO) mice were used for this purpose. In addition, B6.129S7-Rag1 tm1Mom/J (RAG1 KO) mice lacking mature T cells but with intact NK cells were used to determine if CD4+ T cells are required for injury and if the presence of NK cells alone is sufficient to cause injury following TCDD/Con A treatment.
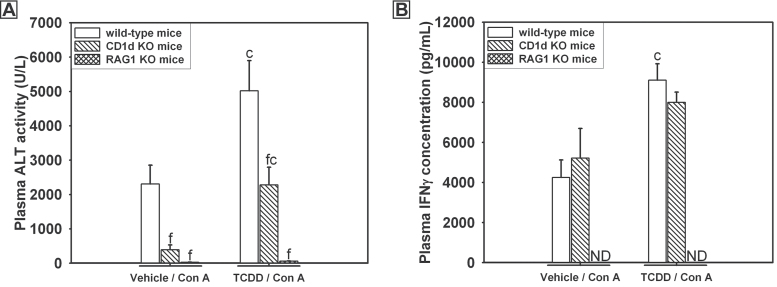
TCDD treatment alone did not cause liver injury in CD1d KO, Rag1 KO, or C57Bl/6J (wild type) mice (data not shown). TCDD-pretreated wild-type mice given Con A had increased plasma ALT activity compared with vehicle-pretreated, wild-type mice (Fig. 5A). Plasma ALT activity was reduced in CD1d KO mice regardless of TCDD treatment; however, ALT activity in CD1d KO mice treated with TCDD/Con A was reduced only to the level in wild-type mice treated with Con A. RAG1 KO mice were completely protected from liver injury induced by either vehicle/Con A or TCDD/Con A treatments.
Fig. 5.
Liver injury (A) and plasma IFNγ concentration (B) after TCDD/Con A treatment in CD1d KO and RAG1 KO mice. Wild-type (open bars), B6.129S6-Cd1d1/Cd1d2tm1Spb/J (CD1d KO) (striped bars), and B6.129S7-Rag1 tm1Mom/J (RAG1 KO) (cross-hatched bars) mice were treated as described in the legend to Figure 2 with vehicle/Con A or TCDD/Con A. Samples were collected 8h after Con A administration. c, p < 0.05 TCDD/Con A versus vehicle/Con A in the same mouse genotype. f, p < 0.05 versus the same treatment in wild-type mice. Abbreviation: ND, not detected (value below the limit of detection). Data represent the mean ± SEM of 4–10 independent replicates per treatment group. Data were combined from at least 2 separate experiments.
As previously stated, enhanced IFNγ production is required for liver injury after TCDD/Con A treatment. In vehicle/Con A-treated mice, the plasma concentration of IFNγ was similar in wild-type and CD1d KO mice but undetectable in RAG1 KO mice (Fig. 5B). In mice treated with Con A, TCDD-pretreated, wild-type mice had increased concentrations of IFNγ compared with wild-type mice given Con A alone. The plasma concentration of IFNγ in TCDD/Con A-treated CD1d KO mice was not different from wild-type mice given the same treatment or from vehicle/Con A-treated CD1d KO mice. In RAG1 KO mice treated with TCDD/Con A, IFNγ was not detectable in plasma.
Effect of TCDD Pretreatment on In Vivo and Ex Vivo Activation of Hepatic Lymphocytes by Con A
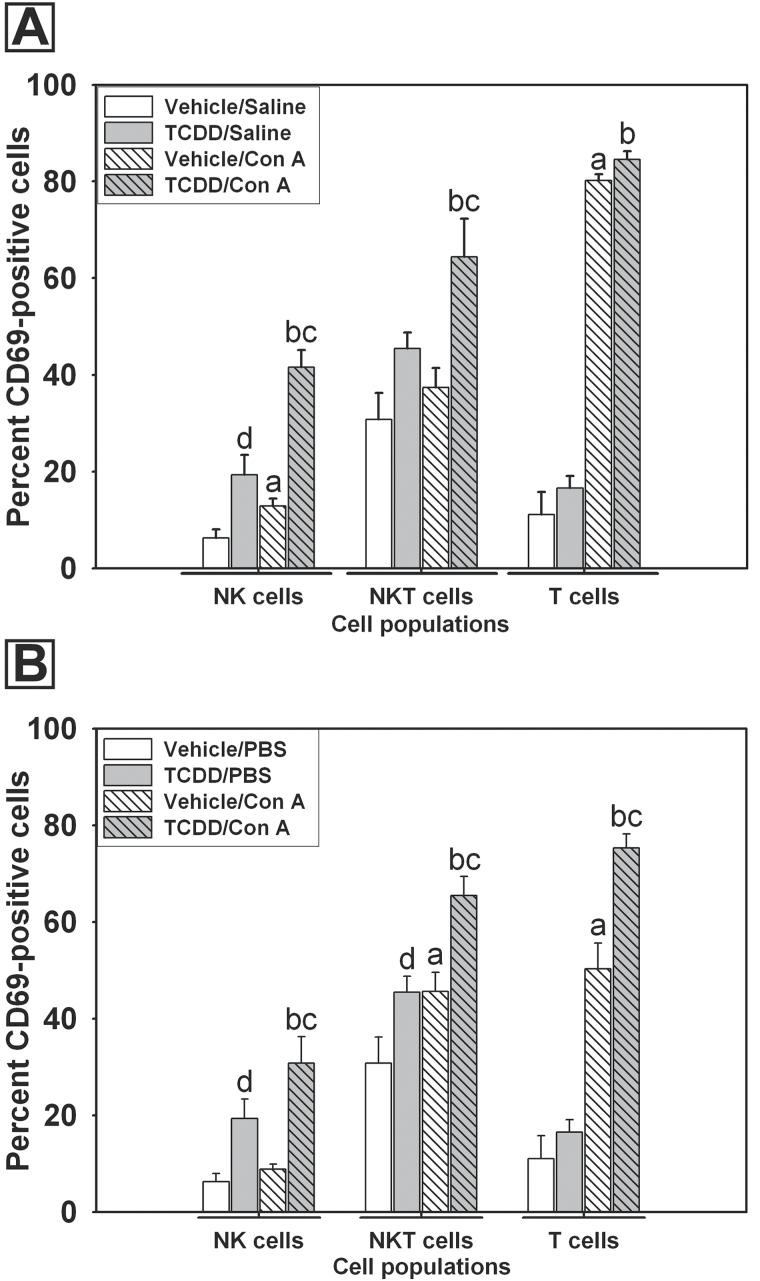
Lymphocytes were isolated from livers of mice pretreated with vehicle or TCDD 2h after administration of either saline or Con A. Expression of the early lymphocyte activation marker CD69 was evaluated by flow cytometry. TCDD pretreatment alone increased the percentage of activated CD69-positive NK (NK1.1+, CD3ε−) cells compared with vehicle pretreatment (Fig. 6A). Con A administration also increased the percentage of CD69-positive NK cells, and this response was further increased by TCDD pretreatment. An increase in the percentage of CD69-positive NKT (NK1.1+, CD3ε+) cells was only observed in the TCDD/Con A group. Con A administration increased the percentage of CD69-positive T (NK1.1−, CD3ε+) cells, but this response was not altered by TCDD pretreatment.
Fig. 6.
Effect of pretreatment with TCDD on in vivo and ex vivo activation of hepatic lymphocytes by Con A. A, Mice were pretreated with vehicle (white bars) or TCDD (gray bars) as described in the legend to Figure 2, and intrahepatic leukocytes were isolated 2h after saline (open bars) or Con A (striped bars) administration. Cells were stained for expression of activation marker CD69 and analyzed by flow cytometry. NK cells were identified as (NK1.1+, CD3ε−), NKT cells as (NK1.1+, CD3ε+), and T cells as (NK1.1−, CD3ε+). B, Intrahepatic leukocytes were isolated from mice treated 4 days earlier with vehicle (white bars) or 30 μg/kg TCDD (gray bars) and were treated ex vivo with either PBS (open bars) or 7.5 μg/ml Con A (striped bars) in culture for 5h. NK cells, NKT cells, and T cells were stained for the lymphocyte activation marker CD69 and analyzed by flow cytometry. a, p < 0.05 vehicle/Con A versus vehicle/saline in the same cell type. b, p < 0.05 TCDD/Con A versus TCDD/saline in the same cell type. c, p < 0.05 TCDD/Con A versus vehicle/Con A in the same cell type. d, p < 0.05 TCDD/saline versus vehicle/saline in the same cell type. Data represent the mean ± SEM of 6–7 independent replicates per treatment group. Data were combined from 3 separate experiments.
Increased activation of NK and NKT cells after treatment with TCDD/Con A could arise from exposure of these cells in vivo to danger signals released from damaged parenchymal cells. To investigate the effect of TCDD on the response of hepatic leukocytes to Con A in the absence of hepatocellular damage (Fig. 1), hepatic leukocytes were isolated from mice treated 4 days earlier with TCDD or saline, and ex vivo activation of lymphocytes by Con A was evaluated. Cells were exposed in culture to PBS (vehicle) or to Con A (7.5 μg/ml) for 5h and analyzed by flow cytometry. A greater percentage of NK cells isolated from TCDD-pretreated mice were CD69-positive compared with those isolated from vehicle-pretreated mice (Fig. 6B). Con A stimulation of NK cells isolated from TCDD-pretreated mice, but not vehicle-pretreated mice, resulted in significantly increased percentage of CD69-positive cells. Con A activation increased the percentage of CD69-positive NKT cells, and pretreatment with TCDD increased the percentage of CD69-positive NKT cells in the absence and presence of Con A stimulation. Exposure of T cells isolated from vehicle-pretreated mice to Con A led to an increased percentage of CD69-positive T cells, and this response was further increased in T cells isolated from TCDD-pretreated mice.
Activation of NK Cells in TCDD/Con A-induced Liver Injury
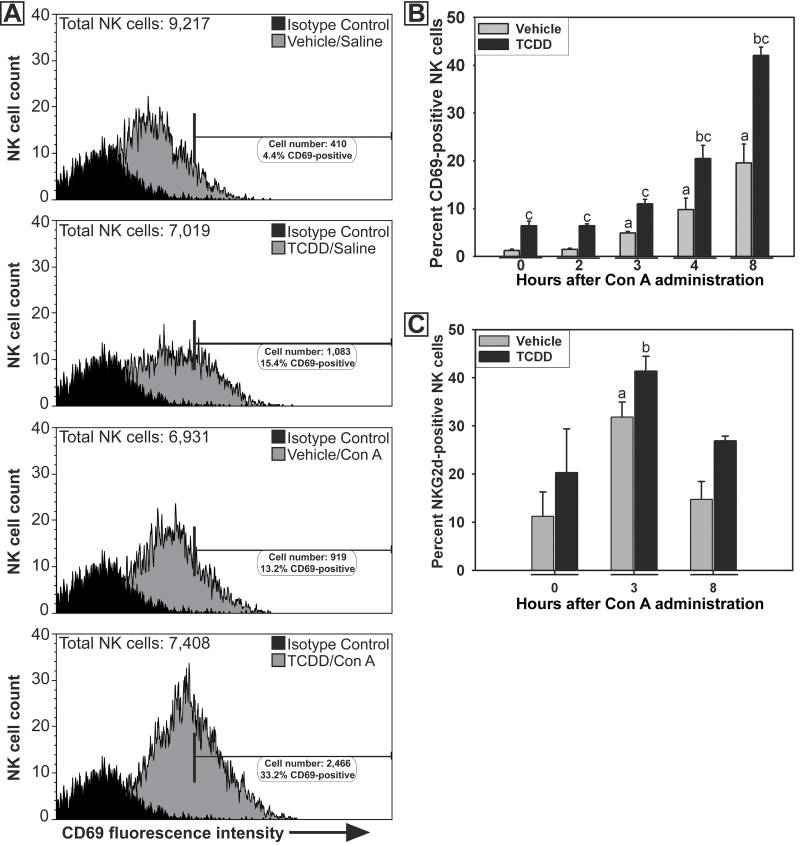
Flow cytometry was used to develop a more extensive time course of the activation of NK cells after TCDD/Con A treatment. Hepatic lymphocytes were isolated from mice at 0, 2, 3, 4, or 8h after Con A administration. In vehicle/Con A-treated mice, increased percentages of CD69-positive NK cells were detected at 3, 4, and 8h (Figs. 7A and 7B). TCDD pretreatment increased the percentage of CD69-positive NK cells compared with vehicle pretreatment at all times evaluated. The percentage of NK cells expressing the activating receptor NKG2d was increased 3h after Con A administration (Fig. 7C). This response to Con A was not altered by pretreatment with TCDD.
Fig. 7.
Activation of NK cells after TCDD/Con A treatment. A, Representative histograms showing fluorescence intensity of CD69 staining on NK cells 4h after Con A or saline administration. Treatments are indicated in the panels. The horizontal bar represents the area of positive staining based on the isotype control. In each histogram, total NK cells refer to the number of NK cells measured in the treatment group sample. B, The percentage of NK cells staining positive for CD69 at 0, 2, 3, 4, and 8h after Con A administration. a, p < 0.05 versus vehicle pretreatment at 0h. b, p < 0.05 versus TCDD pretreatment at 0h. c, p < 0.05 TCDD/Con A versus vehicle/Con A at the same time point. Data represent the mean ± SEM of 4–7 independent replicates per treatment group. Data were collected from at least 2 separate experiments. C, The percentage of NK cells staining positive for NKG2d at 0, 3, and 8h after Con A or saline administration. a, p < 0.05 versus vehicle pretreatment at 0h. b, p < 0.05 versus TCDD pretreatment at 0h. Data represent the mean ± SEM of 3–5 independent replicates per treatment group. Data were combined from 2 separate experiments.
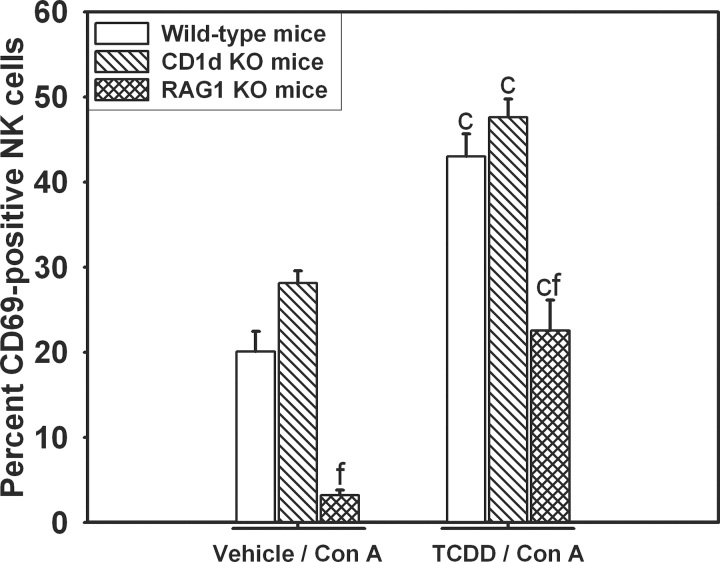
The percentage of CD69-positive NK cells was decreased in RAG1 KO mice compared with wild-type or CD1d KO mice in both vehicle/Con A and TCDD/Con A treatments at 8h (Fig. 8). In each genotype, TCDD/Con A treatment significantly increased the percentage of CD69-positive NK cells compared with vehicle/Con A treatment.
Fig. 8.
Activation of NK cells in CD1d KO and RAG1 KO mice after TCDD/Con A treatment. Wild-type (open bars), B6.129S6-Cd1d1/Cd1d2tm1Spb/J (CD1d KO; striped bars), and B6.129S7-Rag1 tm1Mom/J (RAG1 KO; cross-hatched bars) mice were treated as described in the legend to Figure 2 with vehicle/Con A or TCDD/Con A. Intrahepatic leukocytes were isolated from mice 8h after Con A administration, and NK cells were stained for CD69 expression. c, p < 0.05 TCDD/Con A versus vehicle/Con A in the same mouse genotype. f, p < 0.05 versus the same treatment in wild-type. Data represent the mean ± SEM of 3–5 independent replicates per treatment group.
Role of NK Cells in Exacerbation of Con A Hepatotoxicity by TCDD Pretreatment
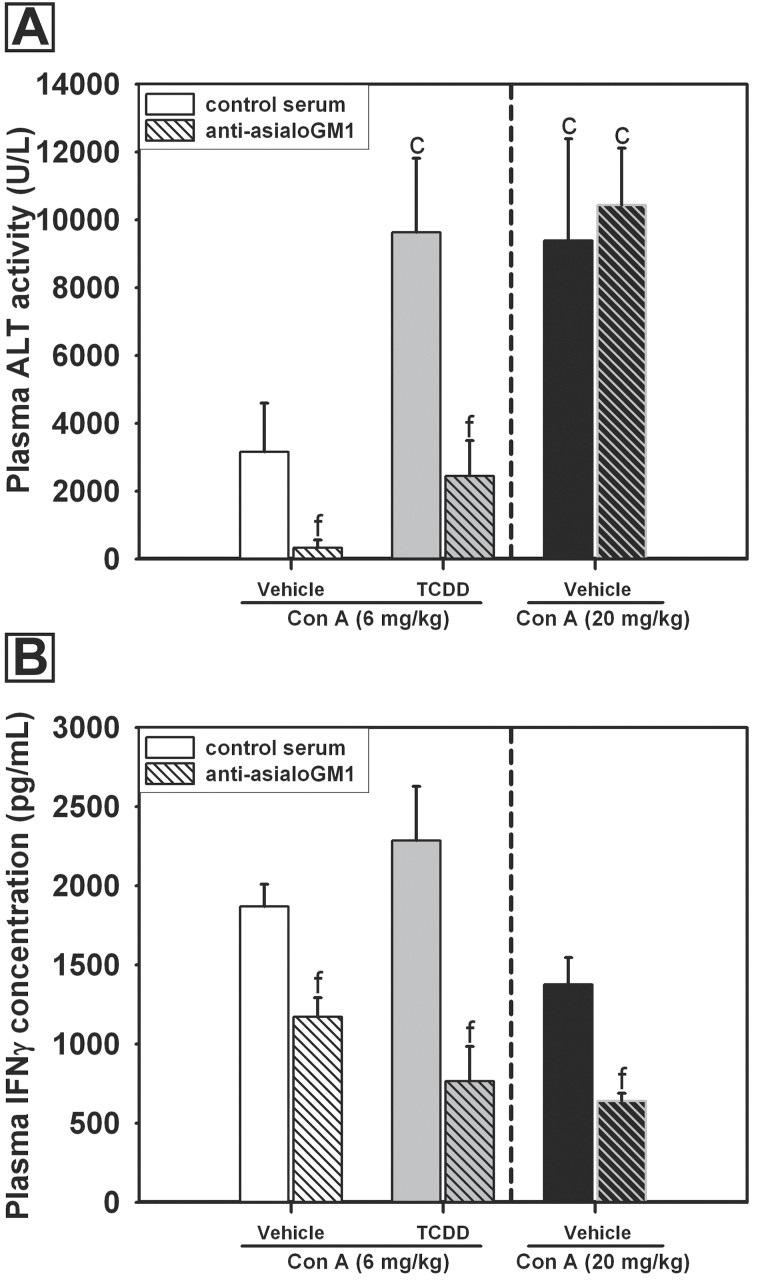
NK cells are not considered to be important effectors of liver injury caused by large doses (15–25mg/kg) of Con A (Takeda et al., 2000; Toyabe et al., 1997); however, the TCDD-mediated activation of NK cells prompted the investigation of the importance of these cells in the development of liver injury after TCDD/Con A treatment. Pretreatment with TCDD led to increased plasma ALT activity in Con A (6mg/kg)-treated mice given control serum (Fig. 9A). Anti-asialoGM1 depletion of NK cells significantly reduced injury in vehicle- and TCDD-pretreated mice administered 6mg/kg Con A. In these experiments, a separate group of mice was pretreated with vehicle and then given a larger dose (20mg/kg) of Con A to compare the effects of anti-asialoGM1 treatment in our studies with results previously reported in the literature (Toyabe et al., 1997). The plasma ALT activity in control serum-treated mice given this larger dose of Con A was comparable with ALT activity in TCDD/Con A-treated mice given control serum. Anti-asialoGM1 treatment did not protect against injury in mice given 20mg/kg Con A.
Fig. 9.
The effect of NK cell depletion on TCDD/Con A-mediated liver injury (A) and IFNγ production (B). Mice were treated as described in the legend to Figure 2 with vehicle (white bars) or TCDD (gray bars) on day 0, then treated with either normal rabbit serum (open bars) or rabbit anti-mouse/rat asialoGM1 polyclonal antibody (striped bars), as described in Materials and Methods section, 18h prior to the administration of 6mg/kg (white and gray bars) or 20mg/kg (black bars) Con A. c, p < 0.05 versus vehicle/6mg/kg Con A in the same control serum or anti-asialo treatment. f, p < 0.05 versus the same treatment with control serum. Data represent the mean ± SEM of 7–11 independent replicates for all groups treated with 6mg/kg Con A and 3–4 independent replicates for all groups treated with 20mg/kg Con A. Data were combined from 2 separate experiments.
In addition, compared with mice treated with control serum, anti-asialoGM1 treatment significantly reduced the concentration of IFNγ in plasma 8h after Con A administration in all treatment groups (Fig. 9B).
DISCUSSION
TCDD is disproportionately distributed to hepatic tissue after exposure, resulting in relatively large hepatic concentrations, and the liver is a major target organ for TCDD toxicity in many species (Abraham et al., 1988; Birnbaum and Tuomisto, 2000; Diliberto et al., 1995; Poland and Knutson, 1982; Thoma et al., 1990). In the liver, TCDD induces prolonged activation of the AhR, resulting in extensive changes in gene expression, and altered expression of many AhR-regulated genes associated with immune cell activity has been identified (Dere et al., 2011; Kerkvliet, 2009; Stevens et al., 2009). However, despite the importance of the liver in AhR-mediated gene expression after TCDD treatment and the increasing experimental evidence that TCDD treatment alters the development of various immune-mediated diseases, the effect of TCDD exposure on the development of autoimmune liver disease has not been addressed thoroughly.
We previously determined that TCDD increased the sensitivity of mice to liver injury in a model of autoimmune hepatitis induced by the administration of Con A (Fullerton et al., 2013). Here, we present data demonstrating that a 10-fold smaller dose of TCDD produced an equivalent degree of liver injury in Con A-treated mice (Fig. 1A). Decreasing the TCDD dose to 0.3 μg/kg resulted in loss of the TCDD-induced sensitization. These results suggest that the threshold dose for the exacerbation of Con A-induced liver injury is between 0.3 and 3 μg/kg TCDD. Toxicokinetic studies revealed that accumulation of TCDD in the liver peaks 4 days following treatment, and the elimination half-life of TCDD is approximately 8 days in mice (Birnbaum, 1986). TCDD exacerbated the hepatotoxic response to Con A when given 4, 7, or 10 days earlier (Supplementary Figure 1), and the greatest response was seen with Con A administration 7 days after TCDD pretreatment. Because liver concentration decreases from 4 to 7 days after a single TCDD administration (Birnbaum, 1986; Kopec et al., 2008), our results suggest that the degree of injury in TCDD/Con A-treated mice is not simply related to the hepatic concentration of TCDD at the time of Con A administration but rather is likely the result of other changes that follow TCDD treatment. It has been reported that peak induction of chemokines such as KC and MCP-1 occurs at 1 and 7 days, respectively, after a single administration of TCDD, and various batteries of genes are altered in expression differently during this same time period (Boverhof et al., 2005; Nault et al., 2013). Additionally, the effect of TCDD on the development of inflammatory liver injury varies greatly with the time between TCDD exposure and administration of the inflammagen lipopolysaccharide (Patterson et al., 2003). Although the mechanism is unknown, the TCDD-induced sensitization to Con A is a persistent effect.
Several chemokines are responsible for the recruitment of inflammatory cells into the liver during the development of injury. KC and MIP-2 both belong to the CXC-type chemokine family and act on CXC receptor 2. These chemokines are synthesized by activated tissue macrophages. KC and MIP-2 perform similar functions to increase neutrophil egress from the bone marrow and mediate transmigration of these cells into the peripheral tissues (De Filippo et al., 2008; Lee et al., 1995; Sadik et al., 2011). MCP-1 is a CCL-type chemokine responsible for the recruitment of monocytes to sites of inflammation (Zimmermann et al., 2012). TCDD treatment enhanced the expression of MCP-1 and KC and increased the recruitment of neutrophils and monocytes to sites of tissue injury (Vogel et al., 2007). Con A treatment induced the production of MIP-2 in a TNF-α-dependent manner, and neutralization of MIP-2 decreased the accumulation of neutrophils in the liver and reduced hepatocellular injury resulting from Con A administration (Nakamura et al., 2001). In the studies presented here, administration of Con A induced KC, MIP-2, and MCP-1 production. Although TCDD treatment alone did not affect the plasma concentration of these chemokines, in Con A-treated mice, TCDD increased the concentration of MIP-2 in plasma (Figs. 2A–C).
Neutrophils contribute to the development of injury and to the production of IFNγ by lymphocytes in Con A-treated mice (Hatada et al., 2005), and neutrophil depletion reduced the severity of liver injury (Bonder et al., 2004). Despite the increased concentration of MIP-2 in plasma in TCDD/Con A-treated mice, TCDD pretreatment did not alter the accumulation of neutrophils in the liver in response to Con A (Fig. 3). Based on these results, it is unlikely that neutrophils play an important role in the exacerbation of Con A-induced liver injury by TCDD.
Macrophages are another hepatic immune cell known to play an accessory role in hepatitis and liver damage induced by large doses of Con A. Hepatic macrophages contribute to injury via the production of inflammatory mediators such as TNF-α, which can induce hepatic parenchymal cell death (Gantner et al., 1996; Schümann et al., 2000). In addition, stimulated macrophages produce IL-12 and IL-27, which activate hepatic lymphocytes and enhance cytolytic activity of NK and NKT cells while promoting Th1 polarization of T cells. IL-12 and IL-27 also increase the production of IFNγ from NK and CD4+ T cells (Pflanz et al., 2002; Vignali and Kuchroo, 2012). IFNγ and TNF-α can act synergistically to kill hepatic parenchymal cells (Adamson and Billings, 1993). In studies presented here, TCDD pretreatment of mice given Con A did not change the percentage of macrophages (F4/80-positive cells) recovered from the liver (Fig. 4A). In addition, there was no difference in plasma concentration of IL-12 in TCDD-pretreated and vehicle-pretreated mice at 3h (Fig. 4B), a time before the development of liver injury (Fullerton et al., 2013). Furthermore, TCDD pretreatment decreased IL-12 production induced by Con A. TCDD pretreatment also did not alter hepatic mRNA expression of IL-12 or IL-27 (Figs. 4C and 4D). Collectively, these results suggest that while macrophages are likely involved in the development of injury, they do not play an important role in the increased sensitivity to Con A observed in TCDD-pretreated mice.
The importance of NKT cells and conventional CD4+ T cells has been well documented in the development of Con A-induced liver injury. In particular, NKT cells are required for the development of injury and directly contribute to the killing of hepatic parenchymal cells via expression of cytolytic effectors such as Fas ligand (FasL; Seino et al., 1997; Tagawa et al., 1998). A number of studies have demonstrated protection from Con A-induced liver injury in mice deficient in NKT cells and in Rag1 KO mice lacking mature T cells (Kaneko et al., 2000; Takeda et al., 2000). Using a smaller dose of Con A (6mg/kg), we saw results similar to those previously reported with larger Con A doses (Fig. 5A). TCDD/Con A-induced liver injury was abolished in RAG1 KO mice, confirming the essential role of T cells in the pathogenesis. Pretreatment with TCDD increases the activation of NKT cells and promotes expression of FasL in these cells after Con A administration (Fullerton et al., 2013). However, CD1d KO mice were only partially protected from TCDD/Con A-induced liver injury. In fact, ALT activity in the plasma of TCDD/Con A-treated CD1d KO mice was comparable with the ALT activity measured in the plasma of vehicle/Con A-treated wild-type mice. These results suggested that a cell type in addition to NKT cells contributes to TCDD-induced sensitization to Con A hepatotoxicity.
To identify this cell type, the activation of hepatic lymphocytes was assessed. TCDD/Con A treatment in vivo resulted in a greater percentage of activated NK and NKT cells than either treatment alone (Fig. 6A). Interestingly, this response did not require exposure to Con A in vivo; a greater percentage of NK and NKT cells isolated from TCDD-treated mice than from vehicle-treated mice became activated upon exposure to Con A ex vivo (Fig. 6B).
Lymphocyte activation occurs 2h after Con A administration (Fig. 6A) prior to any increase in plasma ALT activity (Fullerton et al., 2013). This suggests that lymphocyte activation occurs before initial hepatocyte injury. However, endogenous alarmins such as high-mobility group box 1 released from damaged hepatocytes and sinusoidal endothelial cells can activate lymphocytes and exacerbate hepatic injury (Gong et al., 2010). Furthermore, Con A given at larger doses induces hepatic sinusoidal endothelial cell damage within 15min. Such damage can induce lymphocyte activation (Knolle et al., 1996). The observation that a greater percentage of lymphocytes isolated from TCDD-treated mice became activated upon ex vivo stimulation with Con A compared with cells from vehicle-treated mice (Fig. 6) suggests that TCDD alters lymphocyte activation by Con A independent of products released by dying cells.
One interesting result was that TCDD alone increased activation of NK cells (Fig. 6). Upon further investigation, it was observed that TCDD pretreatment increased the activation of NK cells at all times examined (0–8h) after Con A administration (Figs. 7A and 7B). These results were unexpected because the role of NK cells as effectors in Con A-induced liver injury has been discounted (Dong et al., 2007; Kaneko et al., 2000; Takeda et al., 2000). Despite not being associated with Con A-induced liver injury, NK cells are known to play important roles in human autoimmune disease and inflammatory liver injury (Schleinitz et al., 2010). For example, NK-cell activation is increased in the livers of patients with primary biliary cirrhosis (Chuang et al., 2006; Shimoda et al., 2011). In addition, a clear role for NK cells has been demonstrated in other animal models of immune-mediated liver injury. The administration of alpha-galactoceramide causes liver injury that is mediated by both NK and NKT cells (Trobonjaca et al., 2002). In alpha-galactoceramide-induced liver injury, NKT cells are responsible for activating NK cells by producing IFNγ (Carnaud et al., 1999; Eberl and MacDonald, 2000). In studies presented here, there was no difference in the percentage of CD69-positive NK cells detected after TCDD/Con A treatment of wild-type and CD1d KO mice. This result indicates that increased NK-cell activation is independent of the presence of NKT cells in this model. Interestingly, after either vehicle/Con A or TCDD/Con A treatment, the percentage of CD69-positive NK cells in RAG1 KO mice was decreased compared with wild-type and CD1d KO mice indicating a role for conventional T cells in the activation of NK cells following TCDD/Con A administration.
To determine if increased NK-cell activity could be a contributor to injury in TCDD/Con A-treated mice, NK cells were depleted with anti-asialoGM1 prior to the administration of Con A. As previously mentioned, NK-cell activity is reported to be inconsequential in the development of hepatitis from large doses of Con A (Toyabe et al., 1997). Our results are consistent with this finding (Fig. 9A): anti-asialoGM1 did not diminish injury in mice treated with 20mg/kg Con A. This treatment resulted in injury comparable with TCDD-pretreated mice given only 6mg/kg Con A. However, in vehicle- or TCDD-pretreated mice administered 6mg/kg Con A, NK-cell depletion by anti-asialoGM1 significantly protected against the development of injury. The reduction in hepatotoxicity was accompanied by a decrease in the concentration of IFNγ (Fig. 9B), which is critical to the development of liver injury.
These results clearly demonstrate a role for increased NK-cell activation by TCDD pretreatment in the development of hepatotoxicity from TCDD/Con A administration. Although the mechanisms underlying this response are not yet determined, a number of possibilities exist. TCDD treatment of mice increases the activity of NK cells in the spleen and blood (Funseth and Ilback, 1992). In studies of human cohorts exposed occupationally to TCDD, an increase in the number of NK cells was observed in peripheral blood. In addition, TCDD treatment alters the expression of numerous immune-related genes through the activation of AhR signaling pathways that include coregulatory NK-cell receptors (Kerkvliet, 2009; Sun et al., 2004). TCDD pretreatment did not alter the expression of the stimulatory receptor NKG2d in NK cells after Con A treatment (Fig. 7C), but there are many other coregulatory receptors that were not investigated in this model. In addition, TCDD pretreatment did not alter the production of IL-12 after Con A administration; however, TCDD can increase the expression of IL-12 receptor β2 and might increase the sensitivity of NK cells to the stimulatory effects of IL-12 via that mechanism (Kerkvliet, 2009). These studies did not directly investigate the role of AhR signaling in the effect of TCDD on NK cells in this model; this represents an important topic for future research given the vast array of functions that NK cells play in many diseases.
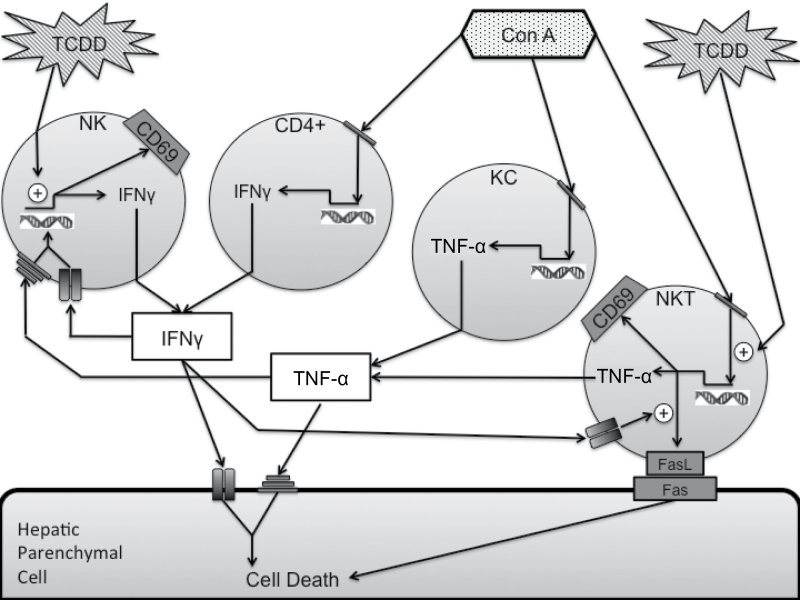
In summary, the results presented here demonstrate that pretreatment with TCDD exacerbates liver injury in a model of autoimmune hepatitis induced by Con A administration. Furthermore, TCDD pretreatment increased the activation of NK cells by Con A, and NK cells play an important role in the development of TCDD/Con A-induced liver injury (Fig. 10). This mechanism is distinctly different from the development of comparable injury obtained by administration of a larger dose of Con A. As such, the enhanced immune response induced by TCDD treatment warrants further investigation into mechanisms of NK cell activation and the larger role that exposure to TCDD and other environmental xenobiotics that influence AhR signaling might play in the development of autoimmune liver disease.
Fig. 10.
Proposed pathway by which TCDD pretreatment exacerbates Con A-induced liver injury. Con A activates NKT, CD4+ T-cells, and Kupffer cells, increasing production of cytokines such as IFNγ and TNF-α, as well as expression of cytolytic molecules such as Fas ligand. These mediators directly kill hepatic parenchymal cells. TCDD pretreatment increased NKT cell activation and FasL expression while also activating NK cells and increasing IFNγ production resulting in increased HPC death. Also depicted are positive feedback loops for IFNγ and TNF-α. Abbreviations: Con A, Concanavalin A; CD4+, conventional CD4+ T cells; CD69, early lymphocyte activation marker; IFNγ, interferon gamma; KC, Kupffer cells; NK, natural killer cells; NKT, natural killer T cells; TCDD, 2,3,7,8 tetrachlorodibenzo-para-dioxin; TNF-α, tumor necrosis factor alpha.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health grant (ES004911); National Institute of Environmental Health Sciences training grant (T32 ES007255 to A.M.F.).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Nicole Crisp for experimental support regarding flow cytometry and Dr Christine Dugan for assistance in developing protocols for intrahepatic immune cell isolation. The authors also thank Ryan Albee for technical assistance.
REFERENCES
- Abraham K., Krowke R., Neubert D. (1988). Pharmacokinetics and biological activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. 1. Dose-dependent tissue distribution and induction of hepatic ethoxyresorufin O-deethylase in rats following a single injection. Arch. Toxicol. 62, 359–368 [DOI] [PubMed] [Google Scholar]
- Adamson G. M., Billings R. E. (1993). Cytokine toxicity and induction of NO synthase activity in cultured mouse hepatocytes. Toxicol. Appl. Pharmacol. 119, 100–107 [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S. (1986). Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab. Dispos. 14, 34–40 [PubMed] [Google Scholar]
- Birnbaum L. S., Tuomisto J. (2000). Non-carcinogenic effects of TCDD in animals. Food Addit. Contam. 17, 275–288 [DOI] [PubMed] [Google Scholar]
- Bonder C. S., Ajuebor M. N., Zbytnuik L. D., Kubes P., Swain M. G. (2004). Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 172, 45–53 [DOI] [PubMed] [Google Scholar]
- Boverhof D. R., Burgoon L. D., Tashiro C., Chittim B., Harkema J. R., Jump D. B., Zacharewski T. R. (2005). Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicol. Sci. 85, 1048–1063 [DOI] [PubMed] [Google Scholar]
- Carnaud C., Lee D., Donnars O., Park S. H., Beavis A., Koezuka Y., Bendelac A. (1999). Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163, 4647–4650 [PubMed] [Google Scholar]
- Chuang Y. H., Lian Z. X., Tsuneyama K., Chiang B. L., Ansari A. A., Coppel R. L., Gershwin M. E. (2006). Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J. Autoimmun. 26, 232–240 [DOI] [PubMed] [Google Scholar]
- Czaja A. J., Manns M. P. (2010). Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology 139, 58–72.e4 [DOI] [PubMed] [Google Scholar]
- De Filippo K., Henderson R. B., Laschinger M., Hogg N. (2008). Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 180, 4308–4315 [DOI] [PubMed] [Google Scholar]
- Dere E., Lo R., Celius T., Matthews J., Zacharewski T. R. (2011). Integration of genome-wide computation DRE search, AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver. BMC Genomics 12, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diliberto J. J., Akubue P. I., Luebke R. W., Birnbaum L. S. (1995). Dose-response relationships of tissue distribution and induction of CYP1A1 and CYP1A2 enzymatic activities following acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Toxicol. Appl. Pharmacol. 130, 197–208 [DOI] [PubMed] [Google Scholar]
- Dong Z., Wei H., Sun R., Tian Z. (2007). The roles of innate immune cells in liver injury and regeneration. Cell. Mol. Immunol. 4, 241–252 [PubMed] [Google Scholar]
- Dugan C. M., Fullerton A. M., Roth R. A., Ganey P. E. (2011). Natural killer cells mediate severe liver injury in a murine model of halothane hepatitis. Toxicol. Sci. 120, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G., MacDonald H. R. (2000). Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30, 985–992 [DOI] [PubMed] [Google Scholar]
- Esser C., Rannug A., Stockinger B. (2009). The aryl hydrocarbon receptor in immunity. Trends Immunol. 30, 447–454 [DOI] [PubMed] [Google Scholar]
- Faggioni R., Jones-Carson J., Reed D. A., Dinarello C. A., Feingold K. R., Grunfeld C., Fantuzzi G. (2000). Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: Role of tumor necrosis factor alpha and IL-18. Proc. Natl. Acad. Sci. U.S.A. 97, 2367–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld J. J., Heathcote E. J. (2003). Epidemiology of autoimmune liver disease. J. Gastroenterol. Hepatol. 18, 1118–1128 [DOI] [PubMed] [Google Scholar]
- Fullerton A. M., Roth R. A., Ganey P. E. (2013). 2,3,7,8-TCDD enhances the sensitivity of mice to concanavalin A immune-mediated liver injury. Toxicol. Appl. Pharmacol. 266, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funseth E., Ilback N. G. (1992). Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) increases blood and spleen natural killer cell activity in the mouse. Chemosphere. 25, 7–10 [DOI] [PubMed] [Google Scholar]
- Gantner F., Leist M., Küsters S., Vogt K., Volk H. D., Tiegs G. (1996). T cell stimulus-induced crosstalk between lymphocytes and liver macrophages results in augmented cytokine release. Exp. Cell Res. 229, 137–146 [DOI] [PubMed] [Google Scholar]
- Gilbert K. M. (2010). Xenobiotic exposure and autoimmune hepatitis. Hepat. Res. Treat. 2010, 248157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., Zhang H., Li J. H., Duan L. H., Zhong S., Kong X. L., Zheng F., Tan Z., Xiong P., Chen G., et al. (2010). High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J. Mol. Med. (Berl). 88, 1289–1298 [DOI] [PubMed] [Google Scholar]
- Hatada S., Ohta T., Shiratsuchi Y., Hatano M., Kobayashi Y. (2005). A novel accessory role of neutrophils in concanavalin A-induced hepatitis. Cell. Immunol. 233, 23–29 [DOI] [PubMed] [Google Scholar]
- Hatano M., Sasaki S., Ohata S., Shiratsuchi Y., Yamazaki T., Nagata K., Kobayashi Y. (2008). Effects of Kupffer cell-depletion on Concanavalin A-induced hepatitis. Cell. Immunol. 251, 25–30 [DOI] [PubMed] [Google Scholar]
- Holladay S. D., Mustafa A., Gogal R. M., Jr (2011). Prenatal TCDD in mice increases adult autoimmunity. Reprod. Toxicol. 31, 312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Harada M., Kawano T., Yamashita M., Shibata Y., Gejyo F., Nakayama T., Taniguchi M. (2000). Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J. Exp. Med. 191, 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I. (1995). Immunological effects of chlorinated dibenzo-p-dioxins. Environ. Health Perspect. 103(Suppl. 9), 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I. (2009). AHR-mediated immunomodulation: The role of altered gene transcription. Biochem. Pharmacol. 77, 746–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I., Shepherd D. M., Baecher-Steppan L. (2002). T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol. Appl. Pharmacol. 185, 146–152 [DOI] [PubMed] [Google Scholar]
- Kerkvliet N. I., Steppan L. B., Vorachek W., Oda S., Farrer D., Wong C. P., Pham D., Mourich D. V. (2009). Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy 1, 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle P. A., Gerken G., Loser E., Dienes H. P., Gantner F., Tiegs G., Meyer zum Buschenfelde K. H., Lohse A. W. (1996). Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology 24, 824–829 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Okamoto H., Iwamoto T., Toyama Y., Tomatsu T., Yamanaka H., Momohara S. (2008). A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology (Oxford). 47, 1317–1322 [DOI] [PubMed] [Google Scholar]
- Kopec A. K., Boverhof D. R., Burgoon L. D., Ibrahim-Aibo D., Harkema J. R., Tashiro C., Chittim B., Zacharewski T. R. (2008). Comparative toxicogenomic examination of the hepatic effects of PCB126 and TCDD in immature, ovariectomized C57BL/6 mice. Toxicol. Sci. 102, 61–75 [DOI] [PubMed] [Google Scholar]
- Lee J., Cacalano G., Camerato T., Toy K., Moore M. W., Wood W. I. (1995). Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155, 2158–2164 [PubMed] [Google Scholar]
- Longhi M. S., Ma Y., Mieli-Vergani G., Vergani D. (2010). Aetiopathogenesis of autoimmune hepatitis. J. Autoimmun. 34, 7–14 [DOI] [PubMed] [Google Scholar]
- Moos A. B., Baecher-Steppan L., Kerkvliet N. I. (1994). Acute inflammatory response to sheep red blood cells in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: The role of proinflammatory cytokines, IL-1 and TNF. Toxicol. Appl. Pharmacol. 127, 331–335 [DOI] [PubMed] [Google Scholar]
- Moos A. B., Oughton J. A., Kerkvliet N. I. (1997). The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on tumor necrosis factor (TNF) production by peritoneal cells. Toxicol. Lett. 90, 145–153 [DOI] [PubMed] [Google Scholar]
- Mustafa A., Holladay S. D., Goff M., Witonsky S., Kerr R., Weinstein D. A., Karpuzoglu-Belgin E., Gogal R. M., Jr (2009). Developmental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters postnatal T cell phenotypes and T cell function and exacerbates autoimmune lupus in 24-week-old SNF1 mice. Birth Defects Res. A. Clin. Mol. Teratol. 85, 828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Okada M., Yoneda M., Takamoto S., Nakade Y., Tamori K., Aso K., Makino I. (2001). Macrophage inflammatory protein-2 induced by TNF-alpha plays a pivotal role in concanavalin A-induced liver injury in mice. J. Hepatol. 35, 217–224 [DOI] [PubMed] [Google Scholar]
- Nault R., Kim S., Zacharewski T. R. (2013). Comparison of TCDD-elicited genome-wide hepatic gene expression in Sprague-Dawley rats and C57BL/6 mice. Toxicol. Appl. Pharmacol. 267, 184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Di Marco R., Zaccone P., Salvaggio A., Magro G., Bendtzen K., Meroni P. (2000). Murine concanavalin A-induced hepatitis is prevented by interleukin 12 (IL-12) antibody and exacerbated by exogenous IL-12 through an interferon-gamma-dependent mechanism. Hepatology 32(Pt 1), 728–733 [DOI] [PubMed] [Google Scholar]
- Patterson R. M., Stachlewitz R., Germolec D. (2003). Induction of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin following endotoxin exposure. Toxicol. Appl. Pharmacol. 190, 120–134 [DOI] [PubMed] [Google Scholar]
- Peters M. G. (2002). Animal models of autoimmune liver disease. Immunol. Cell Biol. 80, 113–116 [DOI] [PubMed] [Google Scholar]
- Pflanz S., Timans J. C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., et al. (2002). IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 16, 779–790 [DOI] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. (1982). 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: Examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 22, 517–554 [DOI] [PubMed] [Google Scholar]
- Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., Weiner H. L. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 [DOI] [PubMed] [Google Scholar]
- Sadik C. D., Kim N. D., Luster A. D. (2011). Neutrophils cascading their way to inflammation. Trends Immunol. 32, 452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleinitz N., Vély F., Harlé J. R., Vivier E. (2010). Natural killer cells in human autoimmune diseases. Immunology 131, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann J., Wolf D., Pahl A., Brune K., Papadopoulos T., van Rooijen N., Tiegs G. (2000). Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am. J. Pathol. 157, 1671–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino K., Kayagaki N., Takeda K., Fukao K., Okumura K., Yagita H. (1997). Contribution of Fas ligand to T cell-mediated hepatic injury in mice. Gastroenterology 113, 1315–1322 [DOI] [PubMed] [Google Scholar]
- Shimoda S., Harada K., Niiro H., Shirabe K., Taketomi A., Maehara Y., Tsuneyama K., Nakanuma Y., Leung P., Ansari A. A., et al. (2011). Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology 53, 1270–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E. A., Mezrich J. D., Bradfield C. A. (2009). The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology 127, 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulentic C. E., Kaminski N. E. (2011). The long winding road toward understanding the molecular mechanisms for B-cell suppression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 120(Suppl. 1), S171–S191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. V., Boverhof D. R., Burgoon L. D., Fielden M. R., Zacharewski T. R. (2004). Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 32, 4512–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y., Kakuta S., Iwakura Y. (1998). Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. Eur. J. Immunol. 28, 4105–4113 [DOI] [PubMed] [Google Scholar]
- Takeda K., Hayakawa Y., Van Kaer L., Matsuda H., Yagita H., Okumura K. (2000). Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. U.S.A. 97, 5498–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske S., Bohn A. A., Regal J. F., Neumiller J. J., Lawrence B. P. (2005). Activation of the aryl hydrocarbon receptor increases pulmonary neutrophilia and diminishes host resistance to influenza A virus. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L111–L124 [DOI] [PubMed] [Google Scholar]
- Thoma H., Mücke W., Kauert G. (1990). Comparison of the polychlorinated dibenzo-p-dioxin and dibenzofuran in human tissue and human liver. Chemosphere 20, 433–442 [Google Scholar]
- Tiegs G., Gantner F. (1996). Immunotoxicology of T cell-dependent experimental liver injury. Exp. Toxicol. Pathol. 48, 471–476 [DOI] [PubMed] [Google Scholar]
- Tiegs G., Hentschel J., Wendel A. (1992). A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Invest. 90, 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyabe S., Seki S., Iiai T., Takeda K., Shirai K., Watanabe H., Hiraide H., Uchiyama M., Abo T. (1997). Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J. Immunol. 159, 1537–1542 [PubMed] [Google Scholar]
- Trobonjaca Z., Kröger A., Stober D., Leithäuser F., Möller P., Hauser H., Schirmbeck R., Reimann J. (2002). Activating immunity in the liver. II. IFN-beta attenuates NK cell-dependent liver injury triggered by liver NKT cell activation. J. Immunol. 168, 3763–3770 [DOI] [PubMed] [Google Scholar]
- Vignali D. A., Kuchroo V. K. (2012). IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 13, 722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C. F., Nishimura N., Sciullo E., Wong P., Li W., Matsumura F. (2007). Modulation of the chemokines KC and MCP-1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Arch. Biochem. Biophys. 461, 169–175 [DOI] [PubMed] [Google Scholar]
- Wang H. X., Liu M., Weng S. Y., Li J. J., Xie C., He H. L., Guan W., Yuan Y. S., Gao J. (2012). Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J. Gastroenterol. 18, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Nishimura N., Kuo V., Fiehn O., Shahbaz S., Van Winkle L., Matsumura F., Vogel C. F. (2011). Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler. Thromb. Vasc. Biol. 31, 1260–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. B., Hanumegowda U. M., Hotchkiss J. A., Ganey P. E., Roth R. A. (2003). Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol. Sci. 72, 43–56 [DOI] [PubMed] [Google Scholar]
- Zimmermann H. W., Trautwein C., Tacke F. (2012). Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front. Physiol. 3, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.