Abstract
Recent analysis of air samples from Chicago and Lake Michigan areas observed a ubiquitous airborne polychlorinated biphenyl (PCB) congener, 3,3′-dichlorobiphenyl (PCB11). Our analysis of serum samples also revealed the existence of hydroxylated metabolites of PCB11 in human blood. Because PCBs and PCB metabolites have been suggested to induce oxidative stress, this study sought to determine whether environmental exposure to PCB11 and its 4-hydroxyl metabolite could induce alterations in steady-state levels of reactive oxygen species (ROS) and cytotoxicity in immortalized human prostate epithelial cells (RWPE-1). This study also examines if antioxidants could protect the cells from PCB11-induced cytotoxicity. Exponentially growing RWPE-1 cells were exposed to PCB11 and its metabolite, 3,3′-dichlorobiphenyl-4-ol (4-OH-PCB11), as well as an airborne PCB mixture resembling the Chicago ambient air congener profile, every day for 5 days. Results showed that 4-OH-PCB11 could significantly induce cell growth suppression and decrease the viability and plating efficiency of RWPE-1 cells. 4-OH-PCB11 also significantly increased steady-state levels of intracellular superoxide, O2 •−, as well as hydroperoxides. Finally, treatment with the combination of polyethylene glycol–conjugated CuZn superoxide dismutase and catalase added 1h after 4-OH-PCB11 exposures, significantly protected RWPE-1 cells from PCB toxicity. The results strongly support the hypothesis that exposure to a hydroxylated metabolite of PCB11 can inhibit cell proliferation and cause cytotoxicity by increasing steady-state levels of ROS. Furthermore, antioxidant treatments following PCBs exposure could significantly mitigate the PCB-induced cytotoxicity in exponentially growing human prostate epithelial cells.
Key Words: 4-OH-PCB11, ROS, superoxide, cytotoxicity, antioxidant, oxidative stress.
Polychlorinated biphenyls (PCBs) are a group of 209 chemically related compounds, which consist of a biphenyl with a varying number of chlorines. Although the production of PCBs peaked in the 1970s and their manufacture curtailed due to production bans, a significant portion of PCBs is still present in the environment. Due to their environmental persistence, PCBs are routinely accumulated in the general environment as well as in human tissues such as breast milk and adipose tissue. They are also globally distributed throughout the atmosphere by upper level air currents (Demers et al., 2002; Flynn and Kleiman, 1997; Ritchie et al., 2003). PCBs so distributed are called airborne PCBs and represent the family of lower chlorinated PCBs that currently pose a potential health risk mostly through the inhalation route (Hu et al., 2013). Recent PCB inhalation studies demonstrated that airborne PCBs are quickly absorbed into the blood stream and distributed to the tissues (eg, brain) suggesting they may present different health effects than higher chlorinated PCBs (Dhakal et al., 2013; Hu et al., 2012).
Due to the strict regulation of PCB disposal, some reports suggest that the concentration of PCBs has decreased in the environment (Hu et al., 2008). However, recent research on air samples in the Chicago and Lake Michigan area discovered a wide distribution of 3,3′-dichlorobiphenyl (PCB11) (Basu et al., 2009; Hu et al., 2008). Although major airborne PCB congeners were almost certainly volatilized from the commercial mixture that contains about 50–100 PCB congeners (Cochran and Frame, 1999), the report also points out that PCB11 was not produced during Aroclor manufacturing or microbial dechlorination of Aroclor PCBs (Petrick et al., 1988). PCB11 might originate from the production of diarylide yellow and other pigments from 3,3′-dichlorbenzidine and is present in commercial paints, consumer goods including printed paper and cardboard packaging still in use (Hu and Hornbuckle, 2010; Rodenburg et al., 2010). These results imply that airborne PCBs continue to be an environmental problem affecting large populations. Therefore, an understanding of the biological effects of PCB11 and its hydroxylated metabolites could have important ramifications in clarifying the potential risks associated with airborne PCBs.
It has been known for more than 20 years that chronic PCB exposure contributes to human toxicity, including hepatotoxicity, neurotoxicity, immunotoxicity, carcinogenicity, and hormonal disruption (Demers et al., 2002; Moysich et al., 2002; Oakley et al., 1996; Ross, 2004). In epidemiological studies, increased mortality from cancers of the liver, gallbladder, biliary tract, gastrointestinal tract, malignant melanoma, as well as breast and prostate cancers were also observed in capacitor manufacturing workers exposed to a series of PCB mixtures (Bertazzi et al., 1987; Demers et al., 2002; Knerr and Schrenk, 2006; Moysich et al., 2002).
In addition to the well-known toxicity of Ah receptor agonists (dioxin-like PCBs), many new receptor-driven and metabolically driven PCB toxicities have been recently identified. Airborne PCBs, previously thought to be less of an environmental concern because they do not accumulate and have shorter half-lives, now are of increasing concern because of their demonstrated metabolism to stable metabolites and to more reactive electrophiles that may attack important cellular macromolecules. For example, OH-PCBs are substrates and inhibitors of sulfotransferases (Ekuase et al., 2011; Liu et al., 2011) and OH-PCBs as well as their sulfate esters bind, in some cases avidly, to serum proteins and thus interfere with the transport of vitamins and hormones (Grimm et al., 2013). Furthermore, the reactivities of PCB metabolites toward DNA (causing mutations, sister chromatid exchanges, aneuploidy, clastogenicity) have been elucidated (reviewed in Ludewig et al., 2008; Robertson and Ludewig, 2011). An expanded understanding of the variety of mechanisms/targets of PCBs has led International Agency for Research on Cancer (IARC) recently to upgrade PCBs, as a class, to Human Carcinogens (Group 1) and to declare sufficient evidence for human melanoma and limited evidence for non-Hodgkin lymphoma and breast cancer (Lauby-Secretan et al., 2013).
Previous research in Zhu et al. (2009) using PCB-exposed exponential growing human breast and prostate epithelial cells clearly showed PCBs and their metabolites could significantly affect cell growth and reproductive integrity by increasing the steady-state levels of reactive oxygen species (ROS).
However, there are relatively few reports available on recently discovered PCB11 in the Chicago ambient air, regarding PCB-induced ROS. The detection of PCB11 in human samples was first reported by Marek et al. (2013). In addition, unlike the heavily chlorinated PCBs, the family of lower chlorinated PCBs (like PCB11) is potentially much easier to metabolize to hydroquinones or quinones, which may contribute to cellular damage. Given the fact that those hydroquinones and quinones both had significant effects on oxidative metabolism leading to the increased level of ROS production, it is possible that the hydroxyl metabolites of airborne PCBs could also induce increased steady-state level of ROS and then play a significant role in cellular cytotoxicity.
In the current study, the hydroxylated metabolites of PCB congeners were determined in human serum samples, which confirmed the existence of PCB11 metabolites including 4-OH-PCB11 in humans for the first time in the literature. The 4-OH-PCB11 metabolite was shown to increase the steady-state levels of superoxide (O2 •−) and hydroperoxides in exponentially growing RWPE-1 human nonmalignant prostate epithelial cells. This increased level of ROS was accompanied by the inhibition of cell growth and clonogenic cell killing. Treatment of cells with antioxidants 1h following exposure to PCBs was able to significantly diminish the toxicity associated with PCBs in human prostate epithelial cells. These results support the hypothesis that airborne PCBs and/or their metabolites could increase the steady-state levels of ROS, which could further induce alterations in cell growth and proliferation. More importantly and consistent with earlier research, the data could provide information as to the efficacy of antioxidant manipulation following PCB exposure to protect human cells against PCB-induced intoxication.
MATERIALS AND METHODS
Cells and PCBs used in the experiments
The nonmalignant RWPE-1 human prostate epithelial cells were cultured in the Keratinocyte-SFM serum-free medium (Life Technologies, Grand Island, New York) with additives (epidermal growth factor 1–53 and bovine pituitary extract). Cells were maintained and experiments were accomplished in a humidified incubator at 37°C with 5% CO2. All experiments were done using exponentially growing cell cultures at 50% confluence. Cells were exposed to PCB11, 4-OH-PCB11, and a mixture of PCB congeners called the Chicago air mixture. Four methoxylated PCB11 derivatives were synthesized as described previously (Song et al., 2008) for use as standards. Additional details regarding their synthesis and characterization are provided as Supplementary material. A description of the preparation and characterization of the Chicago air mixture, a PCB mixture resembling the average profile in Chicago air, has been reported previously (Zhao et al., 2010). Diazomethane was prepared from N-methyl-N-nitroso-p-toluenesulfonamide (Diazald) using an Aldrich mini Diazald apparatus (Milwaukee, Wisconsin) following established procedures (Kania-Korwel et al., 2008). Dimethyl sulfoxide (DMSO) was used as the vehicle control in all experiments.
Human serum sample collection
Human serum samples were obtained from 3 volunteers (all males, ages between 26 and 55) for this study. Participants were asked to fast for 12h before blood extraction. The blood samples (100ml) were drawn by venipuncture, kept refrigerated, and centrifuged within 4h; the separated serum was first stored frozen at −20°C until analysis. All the protocols and procedures were approved by The University of Iowa Institutional Review Board committee.
Determinations of PCBs and OH-PCBs in human serum
The methods used to analyze the human serum for PCBs and OH-PCBs are described in Marek et al. (2013). Briefly, serum was spiked with PCB and OH-PCB surrogate standards, denatured, and extracted using liquid-liquid partitioning. OH-PCBs were derivatized to the methoxylated form using diazomethane, and both PCB and OH-PCB fractions were cleaned using concentrated sulfuric acid (Kania-Korwel et al., 2008; Marek et al., 2013). All solvents were pesticide grade quality and the water was optima quality (Fisher Scientific). PCB samples were spiked with internal standard PCB204 and d-PCB30 immediately prior to analysis on the instrument. A gas chromatography with tandem mass spectrometry (GC/MS/MS, Agilent 6890N Quattro Micro GC with Micromass MS Technologies) in multiple reaction monitoring mode was employed to analyze samples for all 209 PCBs as 159 individual or coeluting congener peaks. OH-PCB samples as MeO-PCBs were spiked with internal standard PCB209 immediately prior to analysis on the instrument. Gas chromatography with electron capture detection (GC-ECD, Agilent 6890N) was employed to analyze samples for 4 major OH-PCBs (4-OH-PCB107, 3-OH-PCB138, 4-OH-PCB146, and 4-OH-PCB187) and 4 hydroxylated metabolites of PCB11 (2-OH-PCB11, 4-OH-PCB11, 5-OH-PCB11, and 6-OH-PCB11). The detection limit for PCBs and OH-PCBs was 1pg/g fresh weight. Both the PCB and OH-PCB congener mass calculation was performed by applying a relative response factor obtained from the calibration standard for each congener. The surrogate standards were used to adjust the final concentrations to percent recovery on a per sample basis. Quality assurance and quality control were assessed for every sample using blanks and surrogate standards (see Supplementary material).
Growth curve and clonogenic survival
To determine whether the PCB exposure alters the cell proliferation in RWPE-1 cells, a growth curve was constructed. 100 000 cells per dish were plated in 60-mm tissue culture dishes. After 48h, the cells were treated with 3μM PCBs every day for 5 days. Media in all the dishes were changed daily, and fresh 3μM PCBs were added into media. The cell numbers were counted from day 1 through day 5 and used to construct the growth curve. At the same time, cells were replated using appropriate dilutions, and clonogenic survival evaluated after 14 days in regular growth medium in order to test the cytotoxicity of PCBs. Cells were stained with Coomassie blue, and colonies of more than 50 cells counted and utilized to calculate clonogenic survival as described (Spitz et al., 1990). We also tested the cytotoxic effects of 4-OH-PCB11 at different doses (0.03–30nM) using trypan blue exclusion as described (Aykin-Burns et al., 2009)
Estimation of intracellular superoxide levels using dihydroethidium oxidation
Steady-state levels of O2 •− were estimated using the oxidation of a fluorescent dye, dihydroethidium (DHE), purchased from Life Technologies. Cells were plated and treated for 5 days with PCBs as described earlier. On day 6, the cells were trypsinized and washed and then labeled in 5mM/l pyruvate containing PBS with DHE (10μM, in 0.1% DMSO, 40min) at 37°C. After labeling, cells were kept on ice. Samples were analyzed using a FACScan flowcytometer (Becton Dickinson Immunocytometry System, Inc, Mountain View, California) (excitation 488nm, emission 585/25nm band-pass filter). The mean fluorescence intensity (MFI) of 10 000 cells was analyzed in each sample and corrected for autofluorescence from unlabeled cells. The MFI data were normalized to control levels (Li et al., 2001; Slane et al., 2006).
Measurement of intracellular hydroperoxides
Steady-state levels of hydroperoxides were estimated using the oxidation-sensitive {CDCFH2 [5- (and 6-)carboxy-2′,7′-dichlorodihydrofluorescein diacetate]; 10 μg/ml} and oxidation-insensitive {CDCF [5- (and 6-)carboxy-2′,7′-dichlorofluorescein diacetate]; 10 μg/ml} fluorescent dyes (dissolved in 0.1% DMSO) that were obtained from Molecular Probes. Cells were plated and treated with PCBs for 5 days as described above. On day 6, the cells were trypsinized and washed with PBS once and then labeled with CDCFH2 or CDCF (10 μg/ml, in 0.1% DMSO, 15min) at 37°C. After labeling, cells were kept on ice. Samples were analyzed using a FACScan flowcytometer (Becton Dickinson Immunocytometry System, Inc) (excitation 488nm, emission 530/30nm band-pass filter). The MFI of 10 000 cells was analyzed in each sample and corrected for autofluorescence from unlabeled cells. The MFI data were normalized to control group (Slane et al., 2006).
Survival experiments using PEG-CAT/PEG-CuZnSOD and NAC treatments
In order to test for a possible causal relationship between the PCB-induced ROS and biological effects of PCBs and PCB metabolites, a clonogenic assay with PCBs and antioxidant treatments was performed. Cells were plated at a density of 50 000 cells per 60-mm dish, and first treated with 3μM PCBs after 48h. One hour after PCB treatment, polyethylene glycol–conjugated catalase (PEG-CAT) combined with polyethylene glycol–conjugated CuZn superoxide dismutase (PEG-CuZnSOD) (50U/ml each) or N-acetylcysteine (NAC) (5mM) was added into the cell culture media on target cells. This protocol was repeated with a fresh media change every 24h for 3 days. On day 4, cells were trypsinized, counted, and replated in complete control media using appropriate dilutions and clonogenic survival evaluated.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California). Data were expressed as mean ± SEM unless otherwise specified. One-way ANOVA analysis with Tukey’s postanalysis was used to study the differences among 3 or more means. Significance was determined at p < .05 and the 95% confidence interval.
RESULTS
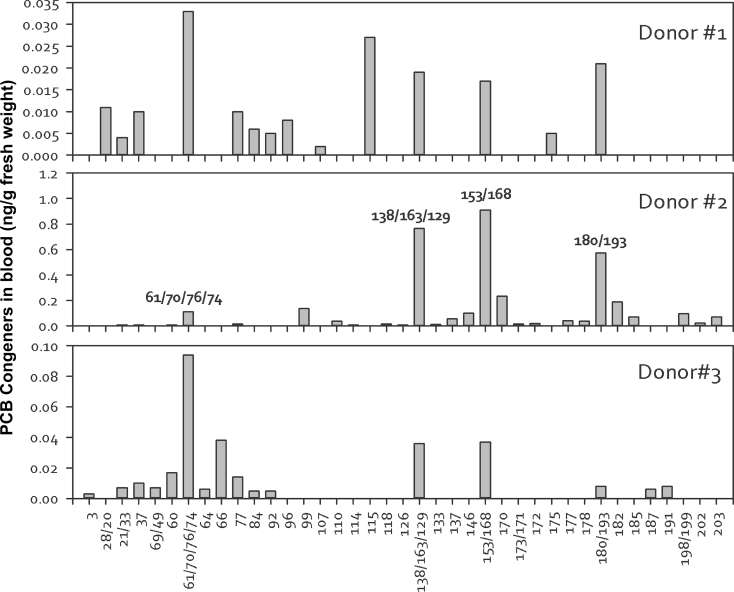
Fifty-one PCBs as individual or coeluting congeners were detected in the human blood obtained from 3 different donors (Fig. 1). The total PCB congener concentrations ranged from 0.179 to 3.55ng/g fresh weight (0.5–10nM). These results were compared with fresh weight concentrations in airborne exposure to semivolatile organic pollutants (AESOP) participants, where sum of 209 PCBs ranged from below detection to about 1.23ng/g fresh weight (0–3.5nM), with the highest concentration of 3.52ng/g fresh weight (10nM) being the only value outside this range (Marek et al., 2013). In contrast, PCB serum concentrations (calculated from the analysis of 13 PCBs) in Belgian and Romanian populations ranged 0.375–19.430ng/ml (1–60nM), with a median concentration of about 3ng/ml (9nM) (Dirtu et al., 2010). The National Health and Nutrition Examination Survey (NHANES) reports a median concentration calculated from analysis of 40 PCBs of 0.275ng/g fresh weight (0.8nM) for subjects 12–29 years, 0.470ng/g fresh weight (1nM) for subjects 20–39 years, and 1.206ng/g fresh weight (4nM) for subjects 40–59 years (Patterson et al., 2009). Figure 1 also clearly shows that the 3 donors had different distributions of PCB congeners. Although lower chlorinated PCBs were detected in all 3 donors, donor 2 had significantly higher levels of higher chlorinated PCBs with comparatively high PCB153 levels, which might be attributed to the lifestyle and dietary intake as well as physiological differences.
FIG. 1.
Congener distribution in blood serum of 3 different donors. Each column represents ng/g fresh weight. Abbreviations: ng/g, nanogram per gram; PCB, polychlorinated biphenyl.
Because lower chlorinated PCBs and their metabolites were detected in all 3 donors, we extended the analysis to examine the recently discovered PCB congener in Chicago air, PCB11 and its metabolites. Because PCB11 and other lower chlorinated PCBs can be rapidly metabolized, 4 specific hydroxylated PCB metabolites (OH-PCBs) were quantitatively examined in the human serum samples. Although PCB11 was not detected, Table 1 shows the existence of 3 different OH-PCB11 metabolites with detectable levels in human serum. Moreover, some of the major hydroxylated PCB metabolites for PCB107, PCB138, PCB146, and PCB187 were also detected (Table 1). Total concentrations of the major OH‐PCBs ranged from 0.022 to 0.313ng/g fresh weight (0.07–0.7nM). Donor 2, who had the highest PCB concentrations, also had the highest total OH‐PCB concentrations (Table 1). For comparison, total OH-PCB metabolites of 4 congeners detected in AESOP participants ranged from 0.011 to 1.18ng/g fresh weight (0.04–3.5nM), with a median concentration of 0.07ng/g fresh weight (0.2nM) (Marek et al., 2013). The Romanian and Belgian donors had a range of 0.050–0.795ng/ml fresh weight (0. 2–3nM) total OH-PCB concentration of 12 congeners (Dirtu et al., 2010). Based on a comparison of retention times between the calibration and sample, we also detected evidence of some PCB11 metabolites at very low concentrations (Table 1), which are at least an order of magnitude lower than the major human hydroxylated PCB metabolites of other congeners. The current study is the first to provide evidence of the 3 different OH-PCB11 metabolites existing in human samples.
TABLE 1.
Serum OH-PCB Congener Concentrations (ng/g Fresh Weight) by Donor
| OH-PCB Congener (ng/g Fresh Weight) | Donor 1 | Donor 2 | Donor 3 |
|---|---|---|---|
| 2-OH-PCB11 | 0.006 | ND | 0.001 |
| 4-OH-PCB11 | ND | 0.003 | ND |
| 5-OH-PCB11 | ND | ND | ND |
| 6-OH-PCB11 | ND | 0.002 | ND |
| Total PCB11 metabolites | 0.006 | 0.005 | 0.001 |
| 4-OH-PCB107 | 0.005 | 0.051 | 0.011 |
| 3′-OH-PCB138 | ND | 0.034 | ND |
| 4-OH-PCB146 | 0.006 | 0.103 | 0.007 |
| 4-OH-PCB187 | 0.011 | 0.123 | 0.010 |
| Total major metabolites | 0.022 | 0.311 | 0.028 |
Abbreviation: ND, not detected.
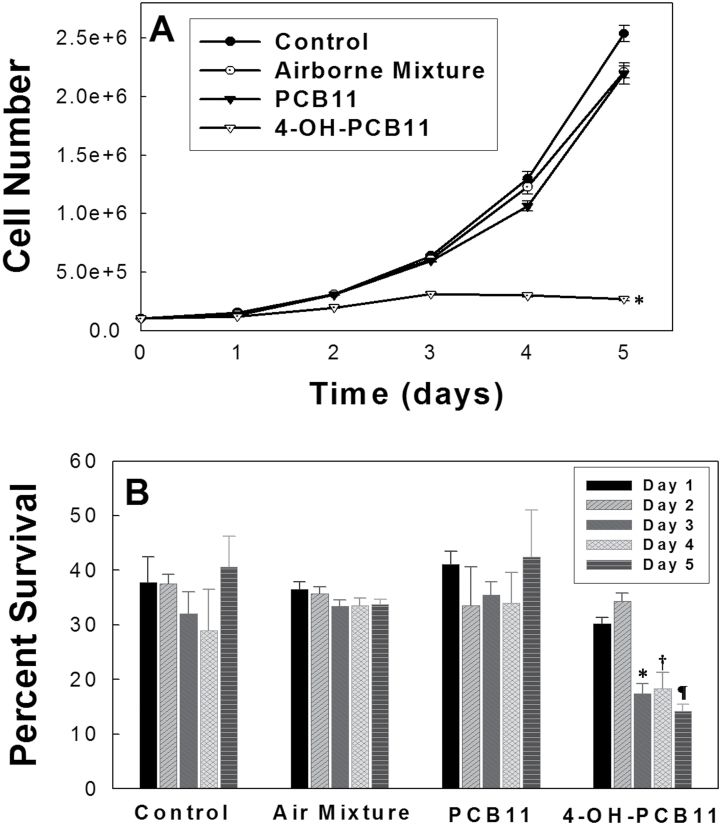
In order to test the hypothesis that PCB11 and its metabolites could cause disturbances in cell growth and clonogenic cell killing in human prostate epithelial cells (RWPE-1), experiments were conducted, the results of which are shown in Figure 2. RWPE-1 cells were chosen because they represent an immortalized but not fully malignantly transformed human prostate epithelial cell line. Exponentially growing RWPE-1 cells were exposed in complete Keratinocyte-SFM media to the indicated PCBs and PCB metabolites (3μM) for 5 days, and following trypsinization, cell number per dish was determined at each time point using a Coulter counter. Because the airborne PCBs contain not only PCB11 but include other major congeners, an airborne Aroclor mixture, resembling the Chicago ambient air congener profile formulated by The University of Iowa Superfund Research Program Synthesis Core, was also tested to understand the impact of these airborne PCBs and their metabolites on cell toxicity. To fully describe the toxicity of PCB11 in human cells, 4-OH-PCB11, a major metabolite of PCB11 was also included in the study. The dose of 3μM was chosen for the current study as representative of the upper limit of all PCB concentrations. This measurement occurs in the circulation of people living in the Anniston flood plain in Alabama, United States, which represents a heavily PCB-contaminated site (Silverstone, personal communication). Figure 2A shows that only the 4-OH-PCB11, a metabolite of PCB11, showed significant inhibitory effects on RWPE-1 cell growth, relatively to vehicle control. Cells treated with 4-OH-PCB11 grew slowly for the first 3 days; however, during the last 2 days, the treatment caused a complete growth arrest and cell number per dish decreased. This effect was not seen in other airborne PCBs treated RWPE-1 cells. The doubling time of 4-OH-PCB11-exposed cells before growth arrest (Fig. 2A) was calculated using the equation: T d = 0.693t/ln(N t/N 0), where N t and N 0 represent cell number at time t and time 0, respectively. Compared with the doubling time of cells exposed to PCB11 (26h) and cells exposed to Aroclor air mixture (27h), the RWPE-1 cells exposed to 4-OH-PCB11 demonstrated 70-h doubling time prior to complete growth arrest.
FIG. 2.
Airborne PCB-induced perturbations in RWPE-1 cell growth and decreased level of clonogenic survival. Asynchronously growing cultures of RWPE-1 cells were given fresh complete media daily containing 3μM PCBs for 5 days. Monolayer cultures were trypsinized, and cell numbers counted from day 1 to day 5 (Panel A). Each data point represents mean ± 1 SD of n = 6 treatment dishes from 2 separate experiments. *p < .001 compared with control. Panel B shows the data from the cells that were plated for clonogenic cell survival. Error bars represent mean ± 1 SD of n = 4 treatment dishes done in 2 separate experiments where each treatment dish was used to prepare 3 replicate cloning dishes for analysis. *p < .001 compared with control day 3, † p < .001 compared with control day 4, ¶ p < .001 compared with control day 5. Abbreviation: PCB, polychlorinated biphenyl.
To determine if the same PCBs could also induce the irreversible loss of reproductive integrity, a clonogenic survival assay was performed by replating the RWPE-1 cells in the absence of PCBs at various times during the exposure. Consistent with growth curve data, Figure 2B shows that 4-OH-PCB11-treated cells start exhibiting clonogenic cell killing starting from the third day of exposure by decreasing the percent survival from 30% to 15%, whereas the PCB11 and Aroclor air mixture did not show any significant inhibitory effect compared with vehicle control. Overall, the results in Figure 2 demonstrate that, compared with PCB11 or the Aroclor mixture, 4-OH-PCB11 had the most pronounced growth and proliferation inhibitory effects in human prostate epithelial cells.
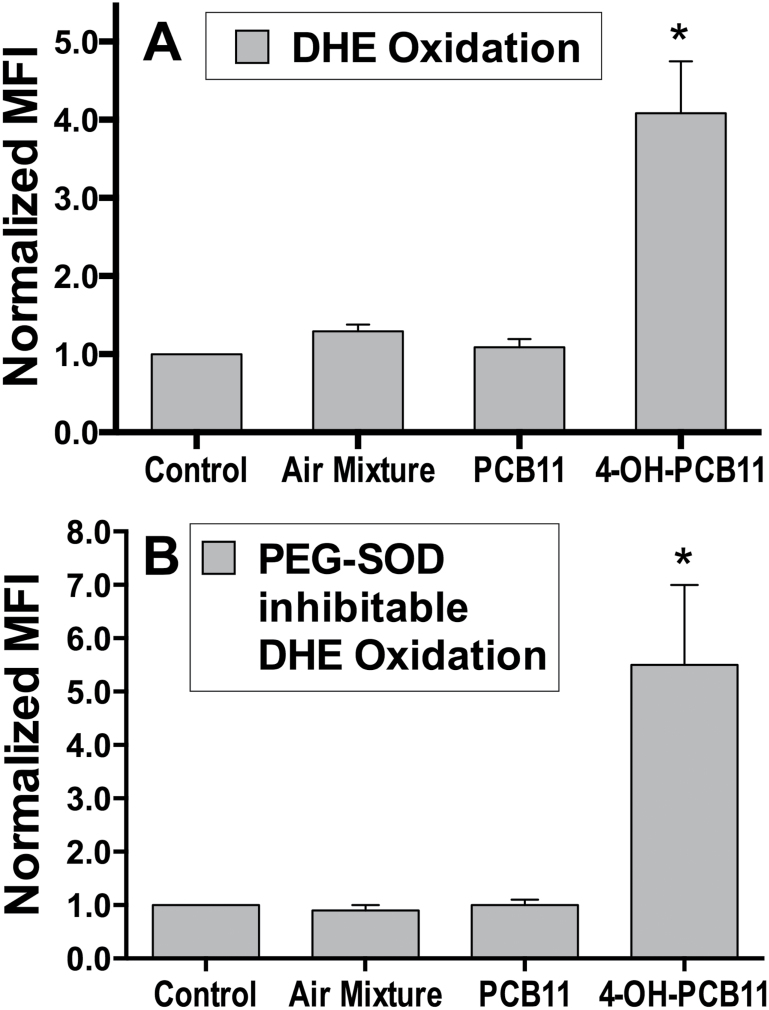
In order to determine if disrupted cell growth and decreased plating efficiency were also accompanied by the increased steady-state levels of O2 •−, RWPE-1 cells were treated with the same PCBs as in Figure 2, and DHE oxidation was measured as a surrogate marker of O2 •− levels. The results in Figure 3A shows that 4-OH-PCB11 induced a 4.5-fold increase in DHE oxidation compared with vehicle control. However, PCB11 and Chicago Aroclor air mixture only slightly increased the DHE oxidation. To further confirm whether this signal was truly representative of the O2 •− production in RWPE-1 cells, PEG-CuZnSOD-inhibitable DHE oxidation was determined. Similar increases in DHE oxidation after 4-OH-PCB11 treatment (5-fold) were also obtained when PEG-SOD-inhibitable DHE oxidation was determined, relative to PEG-alone vehicle controls (Fig. 3B). Because CuZnSOD is considered as a specific scavenger for O2 •−, results in Figure 3 strongly suggest that 4-OH-PCB11 could significantly increase intracellular steady-state levels of O2 •− in human prostate epithelial cells.
FIG. 3.
Steady-state levels of O2 •− in airborne PCB-exposed cells as determined by DHE oxidation. Asynchronously growing cultures of RWPE-1 were incubated with 3μM PCBs daily for 5 days. Monolayer cultures were harvested and trypsinized, washed once with PBS, and labeled 40min with 5μM DHE (in 0.1% DMSO) in PBS containing 5mM pyruvate at 37°C. Each sample was then analyzed for the MFI of 10 000 cells by flow cytometry (Panel A). Samples were assayed in triplicate; mean ± 1 SEM of 2 separate experiments containing 3 treatment dishes per group (n = 6), *p < .001 compared with control. Panel B shows the data from the third replicate of the experiments shown in panel A, in which the DHE labeling was done in the presence (100U/ml PEG-SOD) or absence (18μM PEG alone) of PEG-CuZnSOD given 2h before and during DHE labeling. PEG-SOD-inhibitable DHE oxidation was obtained by calculating the differences in DHE oxidation between cells pretreated with PEG-SOD and with PEG alone for each treatment group. The PEG-SOD-inhibitable DHE oxidation data were then normalized to the control (non-PCB-treated cells) and plotted in panel B; means ± 1 SD (n = 3), *p < .001 compared with control. Abbreviations: DHE, dihydroethidium; DMSO, dimethyl sulfoxide; MFI, mean fluorescence intensity; PCB, polychlorinated biphenyl; PEG-CuZnSOD, polyethylene glycol–conjugated CuZn superoxide dismutase.
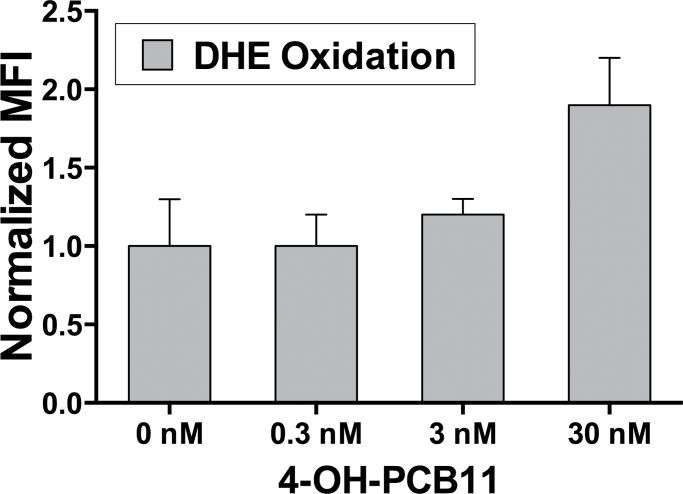
To analyze the dose-dependent impact of PCBs and 4-OH-PCB11 on cell viability and O2 •− levels, we performed trypan blue and DHE-labeling experiments with lower concentrations (0.03–30nM). Our results indicate that 30nM concentrations of 4-OH-PCB11 treatment caused an increase in the DHE oxidation (Fig. 4) and a decrease in cell viability (Table 2). However, these changes did not reach statistical significance (p = .055 for DHE oxidation and p = .062 for cell viability). At 3 and 0.3nM concentrations, we did not see any changes in either DHE oxidation or cell viability. At these low concentrations, longer than a 5-day exposure times may be required to see changes in PCB-induced ROS and/or cytotoxicity. Because exposure to 3μM of PCB11 and Chicago air mixture did not show any significant effects in ROS production or cytotoxicity in our model cell system, we were not surprised that treatment with these lower concentrations of PCB11 and air mixtures did not cause any changes in DHE oxidation and in cell viability (data not shown).
FIG. 4.
Dose-dependent impact of 4-OH-PCB11 on steady-state levels of O2 •− as determined by DHE oxidation. Asynchronously growing cultures of RWPE-1 were incubated with 0.3, 3, and 30nM 4-OH-PCB11 daily for 5 days. Monolayer cultures were harvested and trypsinized, washed once with PBS, and labeled with DHE as previously described. Each sample was then analyzed for the MFI of 10 000 cells by flow cytometry. Results were reported as mean ± 1 SD of 3 separate treatment dishes per group. Abbreviations: DHE, dihydroethidium; MFI, mean fluorescence intensity; PCB, polychlorinated biphenyl.
TABLE 2.
Dose-Dependent Impact of 4-OH-PCB11 on Cell Viability
| 4-OH-PCB11 (nM) | % Viability |
|---|---|
| 0 | 98 ± 4 |
| 0.3 | 99 ± 7 |
| 3 | 96 ± 2 |
| 30 | 84 ± 6 |
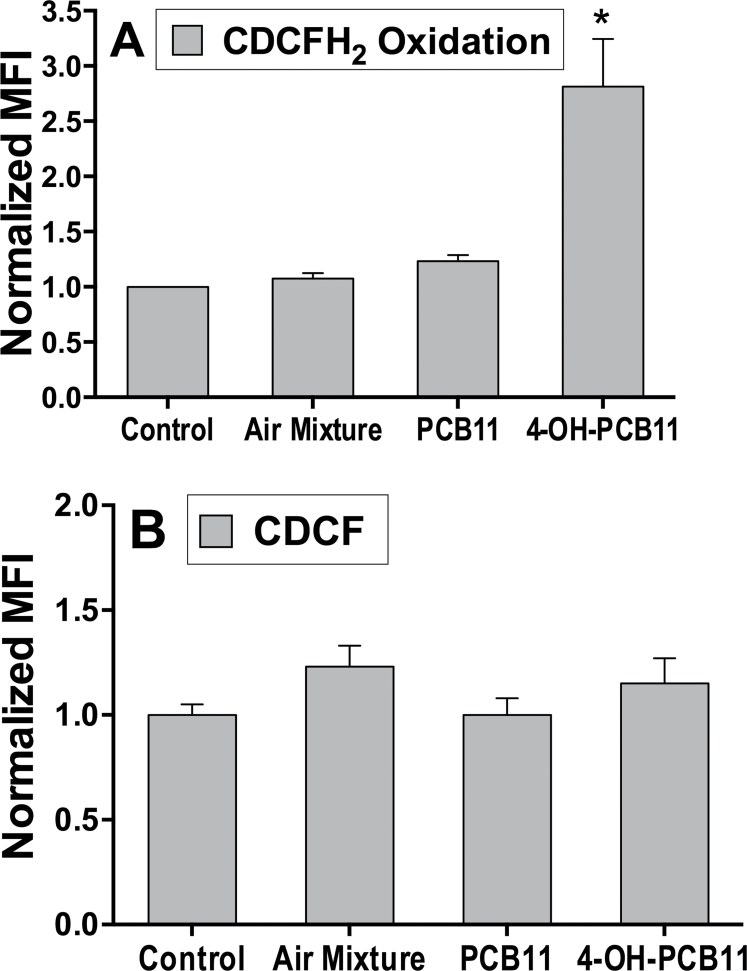
Superoxide generated intracellularly is rapidly converted to hydrogen peroxide (H2O2) by O2 •− dismutase enzymes at a reaction rate constant of 109 M−1 s−1, which is also capable of inducing the formation of organic hydroperoxides and aldehydes (reviewed in Spitz et al., 2004). Treatment with 4-OH-PCB11 was also able to significantly increase the steady-state levels of hydroperoxides in RWPE-1 cells as determined by CDCFH2 oxidation (Fig. 5A). Again, the PCB11-treated RWPE-1 cell group also suggested a slight increase in CDCFH2 oxidation, but this increase was not significant. The experiment was repeated using the oxidation-insensitive analog (CDCF), which measures changes in dye uptake, ester cleavage, and efflux (independent of oxidation). The results showed that there were no significant differences in CDCF labeling between any treatment groups compared with control (DMSO treated) cells (Fig. 5B). This result clearly demonstrates that the increase seen in CDCFH2 oxidation was truly represented the changes in probe oxidation presumably due to increased levels of ROS.
FIG. 5.
Steady-state levels of hydroperoxides as determined by CDCFH2 oxidation. Asynchronously growing cultures of RWPE-1 were incubated with 3μM PCBs daily for 5 days. Monolayer cultures were harvested and trypsinized, washed once with PBS, and then labeled in PBS with CDCFH2 (Panel A) or CDCF (Panel B) (10 μg/ml, in 0.1% DMSO 15min) at 37°C. MFI of 10 000 cells was analyzed by flow cytometry. Samples were assayed in triplicate; mean ± 1 SEM of 2 separate experiments containing 3 treatment dishes per group (n = 6), *p < .001 compared with control. Abbreviations: CDCFH2, 5-(and-6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate; DMSO, dimethyl sulfoxide; MFI, mean fluorescence intensity; PCB, polychlorinated biphenyl.
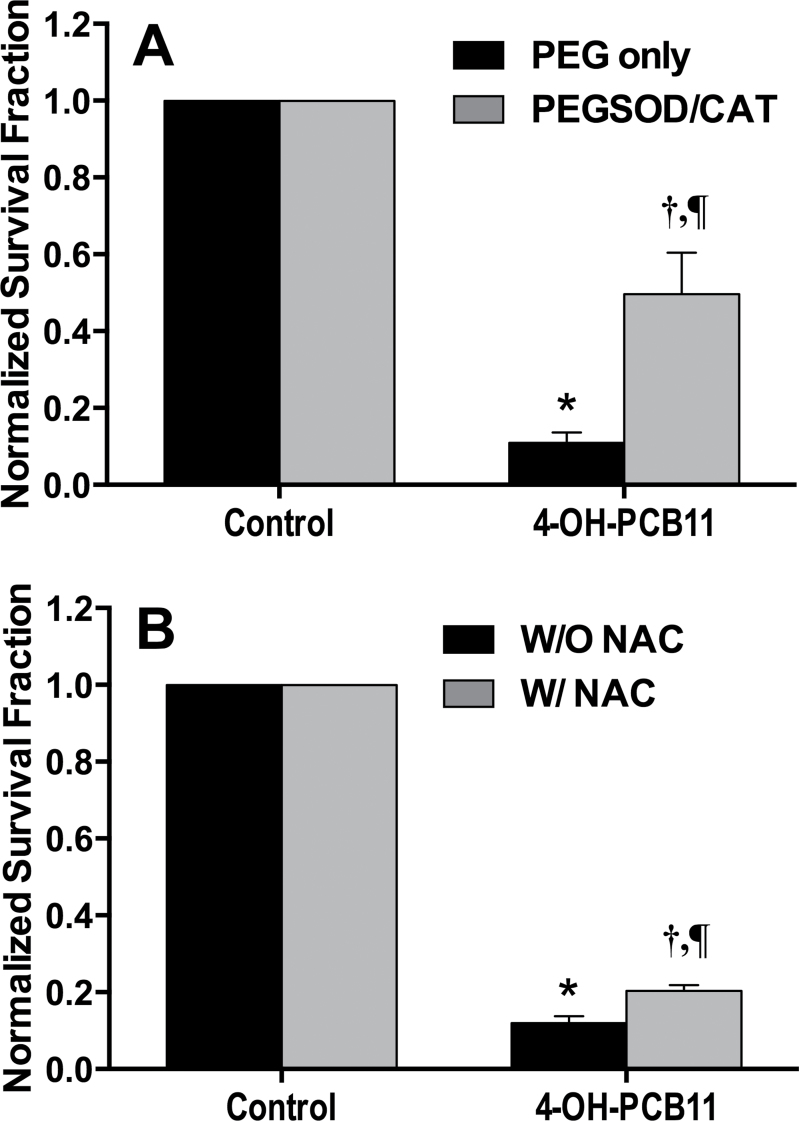
Based on the above results, it is noted that 4-OH-PCB11 could significantly inhibit the RWPE-1 cell growth and decrease the clonogenic cell survival as well as being capable of increasing the O2 •− and hydroperoxide levels in RWPE-1 cells. Our next step was to determine if treatment with antioxidants could protect human RWPE-1 cells from airborne PCB-induced cytotoxicity. In order to identify the causal relationship between the biological effects of airborne PCBs and PCB-induced increases in steady-state levels of ROS, RWPE-1 cells were treated with 4-OH-PCB11 for 1h followed by addition of antioxidant enzymes (ie, 50U/ml PEG-SOD and PEG-CAT) or a nonspecific thiol antioxidant (5mM NAC) for the next 23h. This treatment sequence was repeated for 3 consecutive days using 3μM 4-OH-PCB11, and then the cells were assayed for clonogenic cell survival. In Figure 6A, the results clearly show that treatment with the combination of PEG-CAT and PEG-SOD could significantly protect the RWPE-1 cells from 4-OH-PCB11-induced toxicity, which was not seen in the PEG-only treatment group. These data demonstrate the important role that ROS play in the 4-OH-PCB11-induced cytotoxicity. When the cells were treated with 5mM NAC in Figure 6B, it is interesting to note that NAC only showed a modest effect in protecting the RWPE-1 cells from 4-OH-PCB11-induced cytotoxicity. Because NAC is a nonspecific thiol antioxidant and our previous data suggested NAC could fully rescue the RWPE-1 cell from some PCBs (PCB153, Aroclor1254, 4ClBQ) (Zhu et al., 2009), the results in Figure 6B suggest that the spectrum of toxins produced by 4-OH-PCB11-induced cytotoxicity compared with other PCB congeners or their metabolites may differ significantly. The toxic effects of PCBs have been attributed to induction of polyploidy, telomere shortening, and micronuclei formation for genotoxicity as well as hepatotoxicity, neurotoxicity, immunotoxicity, carcinogenicity, and hormonal disruption (Demers et al., 2002; Flor and Ludewig, 2010; Jacobus et al., 2008; Moysich et al., 2002; Oakley et al., 1996; Ross, 2004). In addition, the above results also strongly support the hypothesis that the ROS are at least in part significant mediators of cytotoxicity PCB-induced cell growth alterations in our model system.
FIG. 6.
RWPE-1 cells treated with PEG-CAT + PEG-SOD or 5mM NAC 1h following PCB exposure are protected from PCB-induced toxicity. Asynchronously growing cultures of RWPE-1 were incubated with a daily dose of 3μM PCBs for 3 days. One hour after PCB treatment each day, PEG-CAT/SOD (50U/ml each) (Panel A) or NAC (5mM) (Panel B) was added to the dishes. Three days later, cells were replated for clonogenic survival assay. Errors represent ± 1 SEM of 3 separate experiments, each experiment had 1 treatment dish and 3 replicate cloning dishes (n = 3); in panel A, *p < .001 compared with control PEG alone, † p < .01 compared with control + PEG-SOD/CAT, ¶ p < .01 compared with 4-OH-PCB11 group + PEG alone; in panel B, *p < .001 compared with control without NAC, † p < .01 compared with control + NAC, ¶ p < .01 compared with 4-OH-PCB11 without NAC. Abbreviations: NAC, N-acetylcysteine; PCB, polychlorinated biphenyl; PEG-CAT, polyethylene glycol–conjugated catalase; PEG-SOD, polyethylene glycol–conjugated CuZn superoxide dismutase.
DISCUSSION
Human exposure to PCBs is mainly from food, PCB-contaminated sites, and ambient air (Demers et al., 2002; Flynn and Kleiman, 1997). Recent research on the air quality sample in the Chicago area discovered a unique PCB congener, PCB11, which is inadvertently formed during the manufacturing of pigments that are now commonly used in commercial paints, printed paper, cardboard packaging, inks, textiles, etc. and underscores that the general population is still being exposed daily to airborne PCBs indoors (Hu and Hornbuckle, 2010; Hu et al., 2008; Rodenburg et al., 2010). Although the strict regulation of PCB disposal laws has reduced the possibility of human exposure to PCBs from food and hazardous sites, there is still no effective regulation or removal of ambient air PCB pollution. Thus, it is important to understand the biological effects and mechanisms of PCB11 toxicity to develop protective strategies and predict risk factors especially for the populations exposed to airborne PCBs.
Many in vivo and in vitro studies clearly demonstrated that PCBs could cause a number of serious health effects, including effects to the immune, reproductive, nervous, and endocrine systems. Occupational research also suggests that PCBs might also relate to increased incidences of prostate and breast cancers in chronically exposed populations (Glauert et al., 2001; Laden et al., 2002; Ritchie et al., 2003). Recent research in our group and others also indicated that exposure to PCBs could cause alterations in cell growth and proliferation through a mechanism involving oxidative stress (Hennig et al., 2002; Hassoun et al., 2002; Twaroski et al., 2001; Venkatesha et al., 2008). It is also noted that the majority of airborne PCBs are lower chlorinated PCBs. Instead of inducing the receptor-driven toxicity, lower chlorinated PCBs are more likely to affect metabolic oxidative metabolism and induce oxidative stress. In addition, these metabolites can also react with DNA that may play an important role in carcinogenesis. For example, 4ClBQ, a metabolite of lower chlorinated PCB3, could significantly increase the steady-state levels of ROS and induce cell growth perturbation and cytotoxicity in human breast and prostate epithelial cells (Zhu et al., 2009). However, despite the interest in PCB-induced oxidative stress, there are few new data available about airborne PCBs, particularly PCB11- and OH-PCB11-induced oxidative stress and their role in cytotoxicity and other deleterious health effects. Furthermore, obtaining this information could benefit the design of pharmaceutical antioxidant intervention to help people who are environmentally exposed to PCBs.
In this study, we have successfully detected OH-PCB11 metabolites as well as other PCB congers in human serum. The various lower chlorinated PCBs were detected in 3 volunteers shown in Figure 1, which supports the hypothesis that inhalation of PCBs might constitute an important exposure route to humans. The high variability seen in different PCB congener profiles and concentrations in the serum samples obtained from 3 donors could be explained by the different dietary intake or inhalation as well as the physiological conditions of the different subjects. Moreover, those concentrations are still typical relative to the ranges of PCBs found in humans in previously published reports (Marek et al., 2013). PCB11 was not detected in serum of our donors. This could be due to our limited samples size (n = 3). It is also possible that PCB11 could be metabolized rapidly, thus allowing its detection if there has been a recent exposure. More importantly, the current study is the first to provide evidence for existence of OH-PCB11 metabolites in human blood. 2-OH-PCB11, 4-OH-PCB11, and 6-OH-PCB11 were all detected in the serum samples obtained from the 3 donors (Table 1). To understand if PCB11 and OH-PCBs are capable of altering cell proliferation, in the current study, RWPE-1 cells received 3μM PCBs for 5 consecutive days. Airborne PCBs used in the experiments includes PCB11, 4-OH-PCB11, and Aroclor air mixture. As mentioned, the metabolite of lower chlorinated PCBs could have an important role in inducing ROS. The results in Figure 2 indicate that only 4-OH-PCB11 could significantly inhibit RWPE-1 cell growth and induce clonogenic cell killing, whereas PCB11 did not show significant effects. This result is consistent with a previous study that postulates that metabolites of lower chlorinated PCB are the major mediator of PCB-induced cytotoxicity (Zhu et al., 2009). It is interesting that the Aroclor air mixture did not exhibit any effects on the RWPE-1 cell growth. This result might be due to the lower level of heavily chlorinated PCBs and an absence of PCB metabolites from lower chlorinated PCB in airborne Aroclor mixtures.
In addition to the cell growth disturbances and disrupted cell proliferation, the results seen in Figure 3 suggest that 4-OH-PCB11 could significantly increase the steady-state level of O2 •− as determined by the SOD-inhibitable DHE oxidation. 4-OH-PCB11 was also capable of increasing hydroperoxide production as determined by the CDCFH2 oxidation (Fig. 5). It is important to know if administration of antioxidants following the PCB exposure could protect the cells from PCB-induced deleterious effects, which is the situation most often faced in human environmental exposure. RWPE-1 cells were exposed to PEG-SOD/CAT, which can be endocytosed, or a nonspecific thiol antioxidant NAC 1h following PCB exposure. A clonogenic survival assay was performed after 3 days of exposure. Results in Figure 6A suggest that combination of PEG-SOD and PEG-CAT treatment could significantly increase the clonogenic survival following PCB exposure. Because PEG-SOD and PEG-CAT are specific scavengers for O2 •− and H2O2, this figure clearly demonstrates that 4-OH-PCB could induce growth inhibition and irreversible loss of reproductive integrity through increased steady-state levels of ROS. Furthermore, the results in Figure 6B suggest that NAC could slightly but significantly protect the cell from PCB cytotoxicity. However, this modest protection was also accompanied by a slight decrease in survival in DMSO-treated vehicle control. Because it was previously reported (Menon et al., 2003; Menon et al., 2005) that NAC not only was able to act as an antioxidant molecule but also could induce cell cycle arrest and further decrease plating efficiency in some cell lines, this modest rescue effect might be due to NAC-induced alteration in the cell cycle. Overall, results in Figure 6 strongly support the hypothesis that 4-OH-PCB11-induced cytotoxicity can be mitigated by manipulation of specific antioxidant enzymes targeting O2 •− and H2O2 following the exposure.
To the best of our knowledge, this is the first study revealing the existence of hydroxylated metabolites of PCB11 in human blood. The current study is also the first to demonstrate that 4-OH-PCB11 was able to induce cell growth inhibition as well as decreased clonogenic survival through increased steady-state levels of O2 •− and H2O2. Furthermore, our results also provide important information that administration of antioxidants targeting specific ROS (ie, O2 •− and H2O2) could rescue human prostate cells from airborne PCB intoxication following exposure. Future experiments will be focusing on the specific sites of ROS production following exposure to airborne PCB congeners and their effects on intracellular antioxidant profiles. In order to further provide proof-of-principle information for designing the pharmaceutical interventions to protect people from PCB-induced adverse health effects, animal experiments will be also included in our future studies.
Overall, results presented in this study and Marek et al. (2013) as well as recent in vivo inhalation studies conducted with lower chlorinated PCBs, strongly suggest that the potential risks of exposure to airborne PCBs (including inadvertent by-products of paint industry such as PCB11) are far more significant than originally thought. In addition, the current regulatory practices do not take into account of the possible risk factors linked with long exposures to low levels of airborne PCBs, which can be easily metabolized in mammalian tissues. Therefore, comprehensive future studies are required to understand the risks and mechanisms of toxicity associated with airborne PCBs to reexamine our current regulatory standards for these sources of PCBs.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES 013661, ES 05605, CA 086862). Y.Z. and R.F.M. gratefully acknowledge support from the Iowa Superfund Research Program (P42 ES013661) Training Core.
Supplementary Material
ACKNOWLEDGMENTS
Authors thank George Rasmussen, Heath Vignes, and Justin Fishbaugh for technical assistance with flow cytometry. The authors also thank Jane Hershberger for her help during blood tests and Gareth Smith for editorial assistance. The opinions expressed are solely those of the authors and do not reflect an official policy of the NIH. The authors declare they have no actual or potential competing financial interests.
REFERENCES
- Aykin-Burns N., Ahmad I. M., Zhu Y., Oberley L. W., Spitz D. R. (2009). Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 18, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu I., Arnold K. A., Venier M., Hites R. A. (2009). Partial pressure of PCB-11 in air from several Great Lakes sites. Environ. Sci. Technol. 43, 6488–6492 [DOI] [PubMed] [Google Scholar]
- Bertazzi P. A., Riboldi L., Pesatori A., Radice L., Zocchetti C. (1987). Cancer mortality of capacitor manufacturing workers. Am. J. Ind. Med. 11, 165–176 [DOI] [PubMed] [Google Scholar]
- Cochran J. W., Frame G. M. (1999). Recent developments in the high-resolution gas chromatography of polychlorinated biphenyls. J. Chromatogr. A 843, 323–368 [DOI] [PubMed] [Google Scholar]
- Demers A., Ayotte P., Brisson J., Dodin S., Robert J., Dewailly E. (2002). Plasma concentrations of polychlorinated biphenyls and the risk of breast cancer: A congener-specific analysis. Am. J. Epidemiol. 155, 629–635 [DOI] [PubMed] [Google Scholar]
- Dhakal K., Adamcakova-Dodd A., Lehmler H. J., Thorne P. S., Robertson L. W. (2013). Sulfate conjugates are urinary markers of inhalation exposure to 4-chlorobiphenyl (PCB3). Chem. Res. Toxicol. 26, 853–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirtu A. C., Jaspers V. L., Cernat R., Neels H., Covaci A. (2010). Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries. Environ. Sci. Technol. 44, 2876–2883 [DOI] [PubMed] [Google Scholar]
- Ekuase E., Liu Y., Lehmler H. J., Robertson L. W., Duffel M. W. (2011). Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 24, 1720–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor S., Ludewig G. (2010). Polyploidy-induction by dihydroxylated monochlorobiphenyls: Structure-activity-relationships. Environ. Int. 36, 962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn L. T., Kleiman C. F. (1997). Position paper of the American Council on Science and Health: Public health concnerns about environmental polychorinated biphenyls (PCBs). Ecotoxicol. Environ. Saf. 38, 71–84 [DOI] [PubMed] [Google Scholar]
- Glauert H. P., Robertson L. W., Silberhorn E. M. (2001). PCBs Recent Advances in Enviormental Toxicology and Health Effects. The University Press of Kentucky, Lexington, Kentucky [Google Scholar]
- Grimm F. A., Lehmler H. J., He X., Robertson L. W., Duffel M. W. (2013). Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect. 121, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun E. A., Wang H., Abushaban A., Stohs S. J. (2002). Induction of oxidative stress in the tissues of rats after chronic exposure to TCDD, 2,3,4,7,8-pentachlorodibenzofuran, and 3,3’,4,4’,5-pentachlorobiphenyl. J. Toxicol. Environ. Health A 65, 825–842 [DOI] [PubMed] [Google Scholar]
- Hennig B., Hammock B. D., Slim R., Toborek M., Saraswathi V., Robertson L. W. (2002). PCB-induced oxidative stress in endothelial cells: Modulation by nutrients. Int. J. Hyg. Environ. Health 205, 95–102 [DOI] [PubMed] [Google Scholar]
- Hu D., Hornbuckle K. C. (2010). Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 44, 2822–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Martinez A., Hornbuckle K. C. (2008). Discovery of non-aroclor PCB (3,3’-dichlorobiphenyl) in Chicago air. Environ. Sci. Technol. 42, 7873–7877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Adamcakova-Dodd A., Lehmler H. J., Hu D., Hornbuckle K. C., Thorne P. S. (2012). Subchronic inhalation exposure study of an airborne polychlorinated biphenyl mixture resembling the Chicago ambient air congener profile. Environ. Sci. Technol. 46, 9653–9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Lehmler H. J., Adamcakova-Dodd A., Thorne P. S. (2013). Elimination of Inhaled 3,3′-Dichlorobiphenyl and the Formation of the 4-Hydroxylated Metabolite. Environ. Sci. Technol. 47, 4743–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J. A., Flor S., Klingelhutz A., Robertson L. W., Ludewig G. (2008). 2-(4’-Chlorophenyl)-1,4-benzoquinone increases the frequency of micronuclei and shortens telomeres. Environ. Toxicol. Pharmacol. 25, 276–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Zhao H., Norstrom K., Li X., Hornbuckle K. C., Lehmler H. J. (2008). Simultaneous extraction and clean-up of PCBs and their metabolites from small tissue samples using pressurized liquid extraction. J. Chromatogr. A 1214, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr S., Schrenk D. (2006). Carcinogenicity of “non-dioxinlike” polychlorinated biphenyls. Crit. Rev. Toxicol. 36, 663–694 [DOI] [PubMed] [Google Scholar]
- Laden F., Ishibe N., Hankinson S. E., Wolff M. S., Gertig D. M., Hunter D. J., Kelsey K. T. (2002). Polychlorinated biphenyls, cytochrome P450 1A1, and breast cancer risk in the Nurses’ Health Study. Cancer Epidemiol. Biomarkers Prev. 11, 1560–1565 [PubMed] [Google Scholar]
- Lauby-Secretan B., Loomis D., Grosse Y., Ghissassi F. E., Bouvard V., Benbrahim-Tallaa L., Guha N., Baan R., Mattock H., Straif K. (2013). Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 14, 287 – –288 [DOI] [PubMed] [Google Scholar]
- Li W. G., Miller F. J., Jr, Zhang H. J., Spitz D. R., Oberley L. W., Weintraub N. L. (2001). H2O2-induced O2 production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J. Biol. Chem. 276, 29251–29256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lehmler H. J., Robertson L. W., Duffel M. W. (2011). Physicochemical properties of hydroxylated polychlorinated biphenyls aid in predicting their interactions with rat sulfotransferase 1A1 (rSULT1A1). Chem. Biol. Interact. 189, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig G., Lehmann L., Esch H., Robertson L. W. (2008). Metabolic activation of PCBs to carcinogens in vivo - A review. Environ. Toxicol. Pharmacol. 25, 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R. F., Thorne P. S., Wang K., DeWall J., Hornbuckle K. C. (2013). PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol. 47, 3353–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S. G., Coleman M. C., Walsh S. A., Spitz D. R., Goswami P. C. (2005). Differential susceptibility of nonmalignant human breast epithelial cells and breast cancer cells to thiol antioxidant-induced G(1)-delay. Antioxid. Redox Signal. 7, 711–718 [DOI] [PubMed] [Google Scholar]
- Menon S. G., Sarsour E. H., Spitz D. R., Higashikubo R., Strum M., Zhang H., Goswami P. C. (2003). Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 63, 2109–2117 [PubMed] [Google Scholar]
- Moysich K. B., Menezes R. J., Baker J. A., Falkner K. L. (2002). Environmental exposure to polychlorinated biphenyls and breast cancer risk. Rev. Environ. Health 17, 263–277 [DOI] [PubMed] [Google Scholar]
- Oakley G. G., Devanaboyina U., Robertson L. W., Gupta R. C. (1996). Oxidative DNA damage induced by activation of polychlorinated biphenyls (PCBs): Implications for PCB-induced oxidative stress in breast cancer. Chem. Res. Toxicol. 9, 1285–1292 [DOI] [PubMed] [Google Scholar]
- Patterson D. G., Wong L. Y., Turner W. E., Caudill S. P., Dipietro E. S., McClure P. C., Cash T. P., Osterloh J. D., Pirkle J. L., Sampson E. J, et al. (2009). Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ. Sci. Technol. 43, 1211–1218 [DOI] [PubMed] [Google Scholar]
- Petrick G., Schulz D. E., Duinker J. C. (1988). Clean-up of environmental samples by high-performance liquid chromatography for analysis of organochlorine compounds by gas chromatography with electron-capture detection. J. Chromatogr. 435, 241–248 [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Vial S. L., Fuortes L. J., Guo H., Reedy V. E., Smith E. M. (2003). Organochlorines and risk of prostate cancer. J. Occup. Environ. Med. 45, 692–702 [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Ludewig G. (2011). Polychlorinated Biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst. Reinhalt. Luft 71, 25–32 [PMC free article] [PubMed] [Google Scholar]
- Rodenburg L. A., Guo J., Du S., Cavallo G. J. (2010). Evidence for unique and ubiquitous environmental sources of 3,3′-dichlorobiphenyl (PCB 11). Environ. Sci. Technol. 44, 2816–2821 [DOI] [PubMed] [Google Scholar]
- Ross G. (2004). The public health implications of polychlorinated biphenyls (PCBs) in the enviorment. Ecotoxicol. Environ. Saf. 59, 275–291 [DOI] [PubMed] [Google Scholar]
- Slane B. G., Aykin-Burns N., Smith B. J., Kalen A. L., Goswami P. C., Domann F. E., Spitz D. R. (2006). Mutation of succinate dehydrogenase subunit C results in increased O2.-, oxidative stress, and genomic instability. Cancer. Res. 66, 7615–7620 [DOI] [PubMed] [Google Scholar]
- Song Y., Buettner G. R., Parkin S., Wagner B. A., Robertson L. W., Lehmler H. J. (2008). Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones. J. Org. Chem. 73, 8296–8304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz D. R., Azzam E. I., Li J. J., Gius D. (2004). Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 23, 311–322 [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Malcolm R. R., Roberts R. J. (1990). Cytotoxicity and metabolism of 4-hydroxy-2-nonenal and 2-nonenal in H2O2-resistant cell lines. Do aldehydic by-products of lipid peroxidation contribute to oxidative stress? Biochem. J. 267, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroski T. P., O’Brien M. L., Robertson L. W. (2001). Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: Implications for oxidative stress. Biochem. Pharmacol. 62, 273–281 [DOI] [PubMed] [Google Scholar]
- Venkatesha V. A., Venkataraman S., Sarsour E. H., Kalen A. L., Buettner G. R., Robertson L. W., Lehmler H. J., Goswami P. C. (2008). Catalase ameliorates polychlorinated biphenyl-induced cytotoxicity in nonmalignant human breast epithelial cells. Free Radic. Biol. Med. 45, 1094–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Adamcakova-Dodd A., Hu D., Hornbuckle K. C., Just C. L., Robertson L. W., Thorne P. S., Lehmler H. J. (2010). Development of a synthetic PCB mixture resembling the average polychlorinated biphenyl profile in Chicago air. Environ. Int. 36, 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Kalen A. L., Li L., Lehmler H. J., Robertson L. W., Goswami P. C., Spitz D. R., Aykin-Burns N. (2009). Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free Radic. Biol. Med. 47, 1762–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.