Abstract
STUDY QUESTION
What is the time course of production of vascular endothelial growth factor-A (VEGF-A), angiopoietin (ANGPT)-1 and ANGPT-2 by primate follicles during encapsulated three-dimensional culture, and what conditions affect their production?
SUMMARY ANSWER
Primate follicles produce VEGF-A and ANGPT-2 in vitro, particularly after developing to the antral stage, with VEGF production influenced by FSH concentration and O2 tension.
WHAT IS KNOWN ALREADY
Folliculogenesis, i.e. the development of primordial follicles into mature, antral follicles, requires the creation of a vascular network in the follicle wall via a process called angiogenesis. Angiogenic factors including VEGFs and ANGPTs have documented roles in angiogenesis. However, direct studies on the production and regulation of angiogenic factors by individual, growing follicles are limited.
STUDY DESIGN, SIZE, DURATION
Ovaries (n = 9 pairs) were obtained from rhesus macaques during the early follicular phase of the menstrual cycle (cycle days 1–4). Secondary (125–225 µm) follicles were isolated mechanically, encapsulated into alginate (0.25% w/v) and cultured for 40 days.
MATERIALS, SETTING, METHODS
Individual follicles were cultured in a 5 or 20% O2 environment in alpha minimum essential medium supplemented with recombinant human (h) FSH. Half of the follicles had recombinant hLH added to the media from Days 30 to 40. Follicle diameters were measured weekly. Follicles were categorized at Week 5 as no-grow (NG; <250 μm in diameter), slow-grow (SG; 251–499 μm) and fast-grow (FG; >500 μm). VEGF-A, ANGPT-1 and -2 concentrations in media were measured by ELISA.
MAIN RESULTS AND THE ROLE OF CHANCE
VEGF concentrations were low throughout the culture for NG follicles. SG and FG follicles had detectable VEGF concentrations at Week 2, which continued to rise throughout culture. VEGF concentrations were distinct (P < 0.05) among all three follicle categories during Weeks 4 and 5. VEGF concentrations were higher (P < 0.05) in SG follicles in the presence of high/mid-dose FSH at 5% O2. In contrast, there were no dose-dependent differences in VEGF production for FG follicles based on FSH concentrations or O2 tension. At Week 5, follicles that produced metaphase II oocytes, following exposure to an ovulatory hCG dose, secreted higher concentrations of VEGF than those containing germinal vesicle-intact oocytes. Media concentrations of ANGPT-1 were low throughout culture for all three follicle categories. ANGPT-2 concentrations were low throughout culture for NG follicles. In contrast, ANGPT-2 concentrations of SG and FG follicles continued to rise from Weeks 1 to 4. During Weeks 2–4, ANGPT-2 concentrations in FG follicles were significantly higher than those of SG and NG follicles (P < 0.05).
LIMITATION, REASONS FOR CAUTION
This study reports VEGF-A, ANGPT-1 and -2 production by in vitro-developed individual primate (macaque) follicles, that is limited to the interval from the secondary to small antral stage. After VEGF and ANGPT-1 assays, the limited remaining samples did not allow assessment of the independent effects of gonadotrophin and O2 on the ANGPT-2 production by cultured follicles. Findings await translation to human follicles.
WIDER IMPLICATION OF THE FINDINGS
The above findings provide novel information on the process of primate follicle maturation. We hypothesize that a symbiotic relationship between elevated concentrations of ANGPT-2 and VEGF allows FG antral follicles to excel in follicle maturation, e.g. by promoting its vascularization. Elevated ANGPT-2 may also offer possible insight into future oocyte quality as early as Week 2, compared with Week 4 for VEGF and follicle size.
STUDY FUNDING/COMPETING INTEREST(S)
The study was funded by the following grants: NIH U54 RR024347/HD058294/PL1-EB008542 (Oncofertility Consortium), NIH U54-HD018185 (SCCPIR), NIH ORWH/NICHD 2K12HD043488 (BIRCWH), NIH FIC TW/HD-00668, ONPRC 8P51OD011092. There are no conflicts of interest to declare.
Keywords: VEGF, angiopoietin, follicle culture, angiogenesis, follicle development
Introduction
The ovarian vasculature is distinguished from that of many other organs by the creation and degeneration of vessels in response to physiologic conditions, notably the development of new blood vessels from pre-existing vessels to supply growing follicles. Non-growing primordial and growing early pre-antral follicles do not possess their own vascular supply, but rely on vessels within the surrounding stroma. As follicle development continues, the growing follicles form their own individualized vascular supply (Hazzard and Stouffer, 2000; Fraser, 2006; Robinson et al., 2009). This process of angiogenesis appears critical for follicular function, including steroidogenesis and oocyte maturation, and locally regulated by angiogenic factors including vascular endothelial growth factor (notably VEGF-A) and angiopoietins (ANGPTs) (Hazzard and Stouffer, 2000; Robinson et al., 2009).
VEGF-A is a potent mitogenic factor that stimulates the proliferation and migration of vascular endothelial cells for the creation and maintenance of vascular structures (Araújo et al., 2013). In contrast, ANGPTs are not mitogenic, but their actions are still fundamental in the construction of vessels (Maisonpierre et al., 1997). As an agonist to the Tie2 receptor, ANGPT-1, helps to mature and maintain blood vessels by recruiting peri-endothelial cells; whereas, the endogenous antagonist ANGPT-2 assists in loosening the support cell framework to allow for further vascular expansion (Hazzard and Stouffer, 2000). The angiolytic (degenerative) effects of ANGPT-2 may become pronounced in the relative absence of VEGF (Maisonpierre et al., 1997).
These angiogenic factors have been the focus of studies evaluating their presence and specific role(s) in ovarian angiogenesis and folliculogenesis. VEGF mRNA and protein were localized to ovarian follicles/cells in rodents (Celik-Ozenci et al., 2003; Danforth et al., 2003), cattle (Greenaway et al., 2004) and primates (Christenson and Stouffer, 1997; Zimmermann et al., 2001, 2002; Wulff et al., 2002; Martinez-Chequer et al., 2003; Fraser et al., 2005), primarily through in situ hybridization and immunohistochemistry. Also, a number of in vivo studies, performed primarily by administering agents that block VEGF action (Roberts et al., 2007), generated data supporting a causal role of VEGF in promoting the growth and maturation of ovarian follicles, including primate follicles (Zimmerman et al., 2001, 2002; Wulff et al., 2001, 2002; Hazzard et al., 2002). There have been fewer studies examining the presence or role(s) of ANGPTs in the follicle (Parborell et al., 2008), especially in primate follicles (Xu and Stouffer, 2005).
Moreover, there are few reports of direct analyses of the production, regulation or action of angiogenic factors on follicles at specific stages of their development. Recently, techniques were developed for growing individual follicles of rhesus monkeys in three-dimensional (3-D) culture (Xu et al., 2010); small pre-antral follicles grew to the antral stage, developed steroidogenic function and, in some cases, yielded meiotically mature oocytes after exposure to an ovulatory hCG bolus (Xu et al., 2010, 2011). Preliminary evidence also suggested that these follicles could produce VEGF-A in vitro (Xu et al., 2010). Therefore, further studies were designed to examine VEGF-A, plus ANGPT-1 and ANGPT-2, production by primate follicles in vitro and its regulation by gonadotrophins and O2 tension.
Materials and Methods
Animal use and ovary collection
The general care and housing of rhesus macaques was performed by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC), Oregon Health & Science University. The environment was temperature controlled at 22°C in a light-regulated 12L:12D room. Animals were caged in social pairs. Diet consisted of Purina monkey chow (Ralston-Purina, Richmond, IN, USA) provided twice a day, supplemented with fresh fruit or vegetables once a day and water ad libitum. Animal treatment was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol for ovary collection was previously approved by the ONPRC Institutional Animal Care and Use Committee (Xu et al., 2010, 2011).
Adult, female rhesus macaques (n = 9) with a history of regular menstrual cycles were observed daily for menstruation. Ovariectomies were performed under anesthesia between days 1 and 4 of the cycle as described previously (Duffy and Stouffer, 2002). Ovaries were placed in HEPES-buffered holding media (Cooper Surgical, Inc., Trumbull, CT, USA) supplemented with 0.2% (v/v) human serum protein supplement (SPS; Cooper Surgical, Inc.) and 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C and immediately transferred to the laboratory (Xu et al., 2010, 2011).
Follicle isolation, encapsulation and culture
The process of follicle isolation, encapsulation and culture has been described previously (Xu et al., 2009). In short, the ovarian cortex was separated from the medulla, cut into 2 × 2 × 1 mm cortical strips and incubated in 6 ml holding media containing 275 U/ml collagenase type I and 585 U/ml deoxyribonuclease I (Worthington Biochemical Corp., Lakewood, NJ, USA) at 37°C for 20 min. Follicles were mechanically isolated using 31-gauge needles. Secondary follicles (diameter 125–225 μm) met the criteria for encapsulation if they exhibited an intact basement membrane, two to three layers of granulosa cells and a healthy, centrally located oocyte.
Follicles were individually transferred into 5 µl 0.25% (w/v) sterile sodium alginate (FMC BioPolymers, Philadelphia, PA, USA)-PBS (137 mM NaCl, 10 M phosphate, 2.7 mM KCI, Invitrogen, Carlsbad, CA, USA) and the droplets were cross linked in 50 mM CaCl2, 140 mM NaCl, 10 mM HEPES solution for 1 min. Each encapsulated follicle was placed in individual wells containing 300 µl alpha minimum essential medium (Invitrogen), 0.3% (v/v) SPS, 1 mg/ml bovine fetuin, 5 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml sodium selenite (Sigma-Aldrich) (Xu et al., 2011).
Follicles (an average of 64 follicles/monkey from 9 monkeys) were randomly assigned to culture groups with 0.3, 3 or 15 ng/ml recombinant human (h) FSH (low-, mid-, high-dose; NV Organon, Oss, Netherlands), and cultured at 37°C in a 5 or 20% O2 environment (in 6% CO2/89% N2 or 5% CO2 in air atmosphere, respectively) for 40 days. The small difference in CO2 concentration had no apparent effect on the pH. Half of the follicles from each culture condition had 0.4 ng/ml recombinant hLH (EMB Serono, Inc., Randolph, MA, USA) added to the culture media from Days 30 to 40. Each culture group contained follicles from a minimum of three monkeys. Follicles that developed an antrum were treated with 100 ng/ml recombinant human chorionic gonadotrophin (hCG; Merck Serono, Geneva, Switzerland) for 34 h before removal of the enclosed oocyte for evaluation of meiotic maturation. Every other day, half (150 µl) of the media was removed and replaced (Xu et al., 2011). Media removed from individual follicles were stored at −20°C until analysis of angiogenic factors (VEGF, ANGPT-1 and ANGPT-2). Follicles and media samples were used previously to evaluate follicle survival, growth and steroid hormone (estradiol, progesterone) production (Xu et al., 2011).
Follicle survival and growth
Follicle survival and maturation were assessed weekly using an Olympus CK-40 inverted microscope and an Olympus DP11 digital camera (Olympus Imaging America, Inc., Center Valley, PA, USA) as described previously (Xu et al., 2009, 2010, 2011). Follicle diameter was determined by measuring the distance from the outer layer of cells at the widest diameter and then the diameter perpendicular to the first measurement. The mean of the two values was considered the follicle's overall diameter. The measurements were performed using Image J 1.42 software (National Institutes of Health, Bethesda, MD, USA). Follicles were considered atretic if the oocyte developed a dark appearance or was no longer surrounded by a layer of granulosa cells, if the granulosa cells appeared dark or fragmented, or if the follicle diameter decreased.
Macaque follicles developed in 3-D culture can be divided into three distinct cohorts based on their growth rates by Week 5 (Xu et al., 2010, 2011). Follicles whose diameter increased a minimum of 3-fold (>500 μm) were considered in the fast-grow (FG) group. Those whose diameter increased a minimum of 2-fold (250–499 μm) were categorized as slow-grow (SG) follicles. And follicles whose diameter did not significantly increase ( < 250 μm) were assigned to the no-grow (NG) group. As development continued, FG and SG follicles developed an antrum between 3 and 4 weeks of culture. Media from NG, SG and FG follicles were analysed for VEGF and ANGPT production.
Granulosa cell retrieval and culture
Granulosa cells were collected from large antral follicles of rhesus monkeys (n = 5) following controlled ovarian stimulation, as described previously (Martinez-Chequer et al., 2003). Granulosa cells were obtained by follicle aspiration either before or 27 h after administration of the bolus of recombinant hCG (Merck Serono). The cells were cultured in fibronectin-coated 96-well plates, each well contained 200 μl DMEM/F12 media, 2 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml sodium selenite, 25 µm/ml aprotinin and 25 µg/ml low density lipoprotein (Sigma-Aldrich) at 37°C in a 20% O2/5% CO2/75% N2 environment. After 24 h, the media was exchanged and the cells were cultured with the presence or absence of 100 ng/ml of hLH in 20% O2/5% CO2/75% N2 or 0% O2/5% CO2/95% N2. After 48 h of culture, the media was retrieved for analyses.
VEGF, ANGPT and progesterone assays
VEGF-A concentrations were determined in media collected at Weeks 2–5 of the follicle culture. Assays were performed using a Human VEGF Quantikine ELISA kit (R&D Systems, Minneapolis, MN), which was previously validated for macaque VEGF in culture media (Christenson and Stouffer, 1997).
ANGPT-1 and ANGPT-2 concentrations were determined using media from Weeks 1–4 of the follicle culture, as well as from granulosa cells after plating and 24 h of culture. Assays were performed using Human ANGPT-1 or ANGPT-2 Quantikine ELISA kits (R&D Systems), previously validated for measurement of macaque ANGPTs (Hurliman et al., 2010).
Progesterone concentrations in media from granulosa cell cultures were assayed by the Endocrine Technology Support Core at the ONPRC using an Immulite 2000, a chemiluminescence-based automatic clinical platform (Siemens Healthcare Diagnostics, Deerfield, IL, USA), validated for macaque follicle culture media as reported previously (Xu et al., 2009).
Statistical analysis
Statistical differences over time and between groups in the follicle culture experiments were analysed using mixed modeling of repeated measures using SAS Enterprise (9.2) with support from the ONPRC Biostatistics Unit. VEGF and ANGPT production was analysed using linear mixed models of the media concentrations over time that incorporated a random intercept for the follicle and treated measurements over time as repeated measures for the follicle. In order to meet the assumption of a normal distribution of the outcome variable and the model residuals, the analysis modeled natural log-transformed concentrations by week, group and the interaction between week and group. The reported differences between groups at each week were derived from contrasts of the interaction effect and were adjusted for multiple comparisons using Bonferroni's method. The residuals from the model did not deviate significantly from those expected from normal distribution, indicating that the use of a parametric model was justified. Because the follicles were nested within individual monkeys, an additional random effect specification for follicle source was not necessary in the models, and the model residuals did not differ significantly by the source monkey.
Statistical differences between groups in the granulosa cell experiments were analysed using one-way ANOVA using a randomized block design, followed by Duncan's multiple range test to determine differences between groups. Assay values that were below the detection limit were assigned the lowest detection value for statistical analyses. A P value < 0.05 was considered a significant difference between groups or time points.
Results
VEGF-A concentrations
Since preliminary studies did not detect VEGF-A in media during Week 1 of culture (Xu et al, 2010), VEGF concentrations were measured from Weeks 2 to 5. Values from follicles exposed to high- and mid-doses of FSH at 5% O2 were combined (Fig. 1) since there was no significant difference in VEGF concentrations between these treatment groups (data not shown). Media VEGF concentrations for NG follicles remained low and unchanged throughout culture. In contrast, SG and FG follicles produced detectable VEGF concentrations at Week 2, which increased (P < 0.05) continuously during the subsequent culture. VEGF concentrations were distinct (P < 0.05) among all three follicle categories at Weeks 4 and 5 (Fig. 1).
Figure 1.
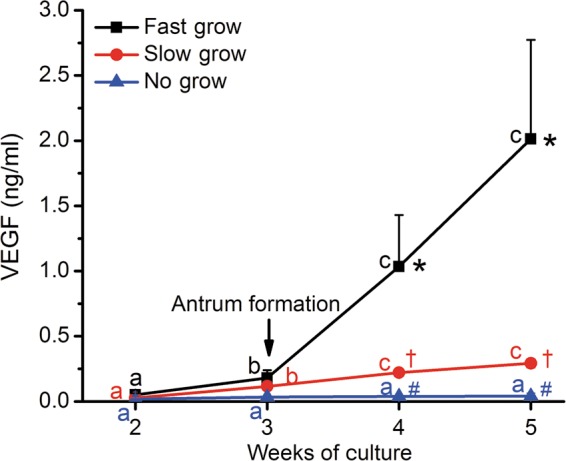
VEGF-A concentrations in the media of no-grow (NG) (n = 27), slow-grow (SG) (n = 49) and fast-grow (FG) (n = 15) follicles (from 6 monkeys) as a function of weeks in culture in the presence of 5% O2 and high/mid FSH levels. Data are presented as non-transformed—mean ± SEM. a,b,cSignificant differences between Weeks 2, 3, 4 and 5 for NG (blue), SG (red) and FG (black) follicles, respectively (P < 0.05). *,†,#Significant differences between NG, SG and FG follicles at Weeks 4 and 5 (P < 0.05). Growing follicles reached the antral stage (arrow) at 3–4 weeks of culture (Xu et al., 2011).
At Week 5 of culture, the high/mid-dose of FSH did not promote an increase in VEGF concentrations by NG follicles, compared with low-dose FSH, at 5% O2 (Fig. 2A). In contrast, the high/mid-dose of FSH increased (P < 0.05) concentrations of VEGF produced by SG follicles. For FG follicles, both high/mid- and low-dose of FSH induced a higher (P < 0.05) level of VEGF production compared with SG and NG groups, but there was no dose-dependent difference despite the ∼2-fold greater VEGF concentrations with high/mid-FSH treatment (Fig. 2A).
Figure 2.
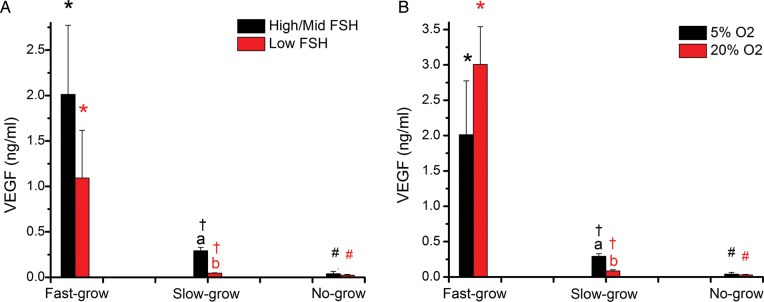
VEGF-A concentrations in media from no-grow (NG), slow-grow (SG) and fast-grow (FG) follicles following 5 weeks of culture in (A) low or high/mid FSH (NG, n = 11 and 27; SG, n = 13 and 49; FG, n = 6 and 15 from 9 monkeys) at 5% O2 and (B) 5 or 20% O2 (NG, n = 27 and 20; SG, n = 49 and 8; FG, n = 15 and 2 from 6 monkeys) in the presence of high/mid FSH. Data are presented as mean ± SEM. Panel A: “a”s on FG data points mean VEGF concentrations in high/mid FSH culture is not different from low FSH culture for fast-grow follicles; a and b on SG data points mean VEGF concentrations in high/mid FSH culture is different from low FSH culture for slow-grow follicles; “a”s on NG data points mean VEGF concentrations in high/mid FSH culture is not different from low FSH culture for no-grow follicles. *,† and # on high/mid FSH data points mean VEGF concentrations in FG is different from SG and NG, and SG is different from NG at high/mid dose of FSH; *,† and # on low FSH data points mean VEGF concentrations in FG is different from SG and NG, and SG is different from NG at low dose of FSH. a,bSignificant differences between low (red) and high/mid (black) FSH doses for SG follicles (P < 0.05). *,†,#Significant differences between NG, SG and FG follicles in the presence of low and high/mid FSH (P < 0.05). Panel B: “a”s on FG data points mean VEGF concentrations in 5% O2 culture is not different from 20% O2 culture for fast-grow follicles; a and b on SG data points mean VEGF concentrations in 5% O2 culture is different from 20% O2 culture for slow-grow follicles; “a”s on NG data points mean VEGF concentrations in 5% O2 culture is not different from 20% O2 culture for no-grow follicles. a,bSignificant differences between 20% (red) and 5% (black) O2 concentrations for SG follicles (P<0.05). *,†,#Significant differences between NG, SG and FG follicles in the presence of 20% and 5% O2 (P<0.05). *,† and # on 5% O2 data points mean VEGF concentrations in FG is different from SG and NG, and SG is different from NG at 5% O2; *,† and # on 20% O2 data points mean VEGF concentrations in FG is different from SG and NG, and SG is different from NG at 20% O2.
In the presence of high/mid-dose of FSH, there was no difference in the VEGF concentrations for NG follicles at Week 5 with the different O2 tensions (5 versus 20%) (Fig. 2B). In contrast, higher (P < 0.05) VEGF concentrations were produced by SG follicles in the 5% O2, compared with 20% O2, milieu. For FG follicles, VEGF concentrations were higher (P < 0.05) than those of SG and NG follicles, but there was no observed change between different O2 tensions (5 versus 20%) (Fig. 2B).
There was no significant effect of adding LH after Day 30 on VEGF concentrations in any treatment group (data not shown).
Oocyte quality related to VEGF-A production
Following 5 weeks of culture, FG and SG follicles were exposed to hCG with oocytes retrieved subsequently. Oocytes retrieved from follicles cultured in different conditions (FSH doses and O2 concentrations) were combined for the following analysis. The majority of follicles contained oocytes that degenerated (n = 45), and those with healthy oocytes typically remained at germinal vesicle (GV) stage (n = 19). However, three follicles produced oocytes that progressed to metaphase II (MII) stage. Follicles containing healthy immature (GV intact) oocytes produced higher (P < 0.05) concentrations of VEGF than those with degenerated oocytes, which were lower (P < 0.05) than those producing meiotically matured (MII) oocytes (Fig. 3).
Figure 3.
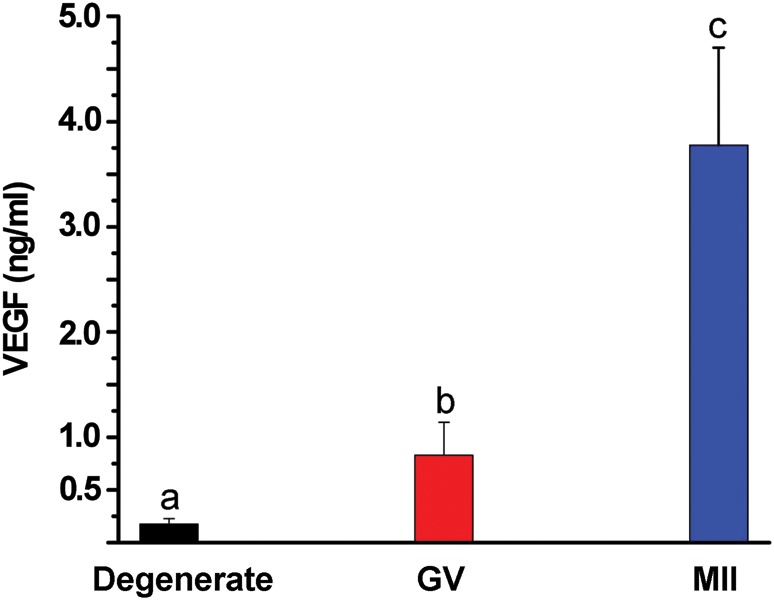
VEGF-A concentrations at Week 5 from cultures of growing (slow- or fast-grow) follicles as a function of oocyte quality/maturation following exposure to 100 ng/ml hCG. Values are the mean ± SEM, n = 45, 19 and 3 for follicles from 9 monkeys providing degenerate (black), germinal-vesicle (GV, red) or metaphase II (MII, blue) oocytes, respectively, at retrieval. Significant differences between groups are denoted by different letters.
ANGPT-1 and -2 concentrations
In the presence of high/mid-FSH and 5% O2, media from all three follicle categories contained ANGPT-1 concentrations near the limit of detection (64 pg/ml) throughout culture (Fig. 4A). No change in ANGPT-1 concentrations was noted for follicles cultured in different FSH concentrations (high/mid- versus low-dose) or O2 tensions (5 versus 20%) (data not shown).
Figure 4.
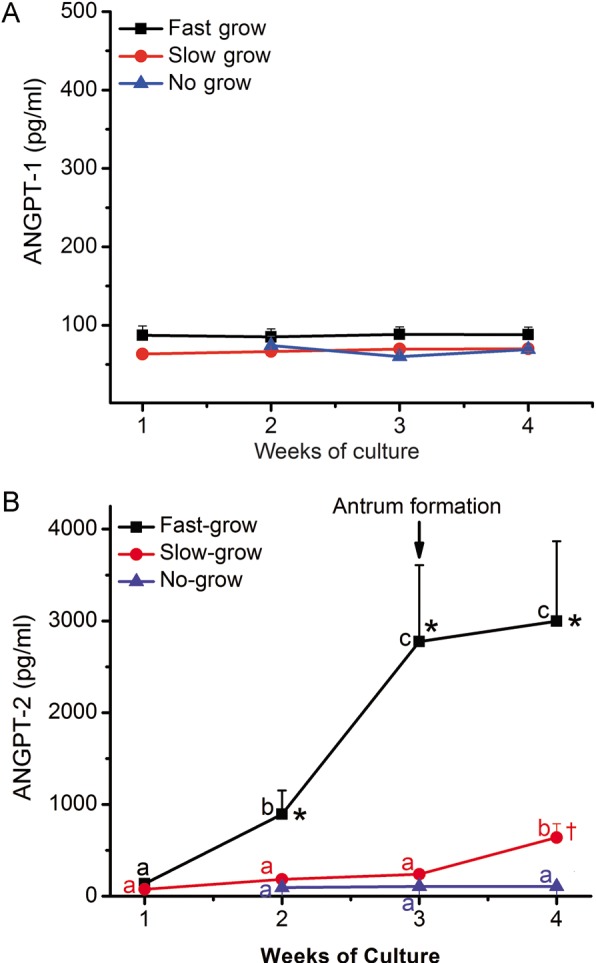
Angiopoietin (ANGPT)-1 (A) and ANGPT-2 (B) concentrations in the media of no-grow (NG), slow-grow (SG) and fast-grow (FG) follicles (n = 9, 25 and 16, respectively, from 9 monkeys) as a function of weeks in culture. Data from different culture conditions (FSH concentrations and O2 concentrations) were combined for analysis for ANGPT-2 due to the limited sample sizes. Data are presented as non-transformed mean ± SEM. a,b,cSignificant differences between Weeks 1, 2, 3 and 4 for NG (blue), SG (red) and FG (black) follicles (P < 0.05). *Significant differences between FG and SG/NG follicles at Weeks 2, 3 and 4 (P < 0.05). * and † on week 2 data points mean ANGPT-2 concentrations in FG is different from SG and NG, but SG is not different from NG at week 2; * and † on week 3 data points mean ANGPT-2 concentrations in FG is different from SG and NG, but SG is not different from NG at week 3; *,† and # on week 4 data points mean ANGPT-2 concentrations in FG is different from SG and NG, and SG is different from NG at week 4. †Significant differences between SG and NG follicles at Week 4 (P<0.05). Growing follicles reached the antral stage (arrow) at 3–4 weeks of culture (Xu et al., 2011).
Because of limited sample size and/or media volume after measuring VEGF and ANGPT-1 (plus steroids and anti-Müllerian hormone in other studies, Xu et al., 2011), data from different culture conditions (FSH doses and O2 concentrations) were combined for analysis of ANGPT-2 (Fig. 4B). ANGPT-2 concentrations remained low in NG follicles throughout culture. For SG follicles, there were detectable ANGPT-2 concentrations at Week 1, which increased (P < 0.05) by Week 4. ANGPT-2 concentrations produced by FG follicles increased (P < 0.05) at Weeks 2–4, and were greater (P < 0.05) than those produced by SG and NG follicles. Limited sample sizes also did not allow the assessment of LH effects on ANGPT production.
To initially consider the cell source(s) of ANGPTs in the macaque antral follicle, media from short-term cultures of granulosa cells collected from large antral follicles of controlled ovarian stimulation protocols were analysed (Table I). Non-luteinized and luteinizing granulosa cells collected before and after the hCG bolus, respectively, produced >10-fold more (P < 0.05) ANGPT-2 than ANGPT-1 at 20% O2. Granulosa cells responded to hCG in vivo (luteinized versus non-luteinized controls) and LH in vitro (non-luteinized controls versus LH) with enhanced progesterone concentrations. However, there were no significant differences in ANGPT concentrations produced by luteinized or non-luteinized granulosa cells when cultured in different O2 tensions (20 versus 0% O2; data not shown) or exposed to LH in vitro, or following hCG in vivo (Table I).
Table I.
Angiopoietin (ANGPT) and progesterone levels in media from acute (24 h) culture at 20% O2 of macaque granulosa cells collected before (non-luteinized) or after (luteinized) administration of an hCG bolus in controlled ovarian stimulation cycles.
| Granulosa cells | ANGPT-1 (pg/ml) | ANGPT-2 (pg/ml) | Progesterone (ng/ml) |
|---|---|---|---|
| Luteinized | |||
| Control | 39 ± 2 | 944 ± 438 | 923 ± 385b,d |
| LH | 39 ± 2 | 1480 ± 1675 | 1380 ± 397b,d |
| Non-luteinized | |||
| Control | 36 ± 2a | 906 ± 780 | 68 ± 50b,e |
| LH | 39 ± 2 | 527 ± 991 | 482 ± 276c,e |
aLimit of assay detection.
b,cDifferent letters indicate significant differences(P < 0.05) in progesterone levels between treatments with the same cell type.
d,eDifferent letters indicate significant differences(P < 0.05) in progesterone levels between cell types with the same treatment.
Discussion
Using 3-D culture technology, this study directly evaluated the production of angiogenic factors, notably VEGF-A, ANGPT-1 and ANGPT-2, by individual primate follicles at discrete (secondary to small antral) stages of development in vitro, as well as its regulation by gonadotrophins and O2 tension. The data suggest that growing (SG and FG) follicles produce significant quantities of VEGF-A and ANGPT-2, but not ANGPT-1, especially after antrum formation. VEGF production by SG follicles was influenced by FSH concentration and O2 tension.
VEGF-A accumulation in media was a function of follicle growth rate in vitro. Prior to antrum formation, and if follicles failed to form an antrum (NG follicles), VEGF concentrations did not increase. However, following antrum formation of growing (SG and FG) follicles, VEGF increased and reached appreciable concentrations (ng/ml) in FG follicles. Our findings are consistent with previous reports of absent VEGF mRNA expression, as judged from in situ hybridization on ovarian sections, in primordial, primary and early secondary follicles in non-human primates (Wulff et al., 2002; Taylor et al., 2004), and VEGF mRNA expression increased after antrum formation in rodent and monkey follicles (Taylor et al., 2004; Abramovich et al., 2009). Whereas the onset of VEGF production appears to be related to the stage of follicle growth (antrum formation), the magnitude of VEGF production may be influenced by follicle size and activity. VEGF secretion correlates with its purported angiogenic actions as the antrum forms to create an extensive surrounding vasculature for increasing transport of nutrients and hormones to/from the growing follicle (Taylor et al., 2004; Abramovich et al., 2009). As follicles grow larger and mature, their need for vasculature increases. In the current study, FG follicles produced higher concentrations of VEGF, which supports previous data that larger or faster growing follicles from various species produced more VEGF (Danforth et al., 2003; Greenaway et al., 2004; Chowdhury et al., 2010).
VEGF-A production by in vitro-developed follicles can be dependent on FSH and O2 milieu. Increasing FSH concentrations promoted VEGF production by SG follicles at 5% O2. The dose-dependent, stimulatory effect of FSH in small antral follicles extends the previous evidence that gonadotrophins promote VEGF production by granulosa cells in the periovulatory follicle. Since survival and growth of macaque follicles in 3-D culture requires FSH (Xu et al., 2011), FSH may have, at least in part, a ‘permissive’ action that allows other factors to regulate VEGF production. Notably, decreasing O2 concentration, from the typical tissue culture milieu (20%) to concentrations commonly found in vascularized tissues (5%), increased VEGF production by SG follicles in the presence of high/mid-dose of FSH. Hypoxia is a primary stimulus for VEGF production in many tissues (Lee et al., 1997; Neeman et al., 1997; Tesone et al., 2005). The current data extend the evidence that hypoxic-to-normoxic (0–5%) O2 concentrations promote VEGF production by luteinizing granulosa cells collected from women during controlled ovarian stimulation cycles (Friedman et al., 1997) or dispersed luteal cells from monkeys or women (Tesone et al., 2005). VEGF production by FG follicles was not influenced by FSH or O2 concentrations, which is consistent with the conclusions that cells in the tertiary or large pre-ovulatory follicle produce VEGF independent from gonadotrophins (Taylor et al., 2004) and O2 (Martinez-Chequer et al., 2003). Due to limited sample sizes, the interaction between FSH dose and O2 concentration on VEGF production was not evaluated.
Notably, follicles that contained an oocyte capable of reinitiating meiosis in response to hCG produced higher concentrations of VEGF at Week 5 in vitro. In vivo studies support an angiogenic or angiotropic action of VEGF that promotes follicular development and steroidogenic function (Zimmerman et al., 2001, 2002; Wulff et al., 2002; Celik-Ozenci et al., 2003; Roberts et al., 2007). There is also increasing evidence that VEGF has extra-vascular effects in the ovary (Luo et al., 2002; Araújo et al., 2013; Greenaway et al., 2004). Further studies are warranted to discern vascular versus extra-vascular actions of VEGF in the growing follicle, and whether VEGF is a possible marker for high-quality follicles destined to provide a mature oocyte capable of fertilization.
In the present study, ANGPT-1 production was low for in vitro-developed follicles regardless of culture conditions and growth rates. Previous evidence in rodent (Abramovich et al., 2009), bovine (Hayashi et al., 2004) and monkey (Wulff et al., 2001) follicles suggests that ANGPT-1 mRNA and protein expression increases in antral follicles. The limited ANGPT-1 production in SG and FG follicles after antrum formation may be due to the immaturity of growing follicles. In contrast to ANGPT-1, in vitro-developed follicles produced ANGPT-2 with increasing concentrations after antrum formation, especially in FG follicles which display considerable estrogenic activity (Xu et al., 2011) and produce mature oocytes. The high ANGPT-2:ANGPT-1 ratio in developing follicles in vitro is consistent with the current granulosa cell study, where non-luteinized cells produced more ANGPT-2 than ANGPT-1 during short-term culture. There is also evidence of similar characteristics in the follicular fluid of ‘lead’ follicles from controlled ovarian stimulation protocols in women (Hurliman et al., 2010; Nishigaki et al., 2011). These data suggest that a high ANGPT-2:ANGPT-1 ratio is associated with final maturation of the periovulatory follicle in primates. Due to limited sample sizes, gonadotrophin and O2 effects on ANGPT-2 production were not assessed for cultured follicles. But, effects of gonadotrophins and O2 on ANGPT production by cultured granulosa cells were also not detected in the current study. Further studies are needed to examine the regulation of ANGPT-2 expression in the developing follicle and clarify its vascular and extravascular actions (Hayashi et al., 2004; Parborell et al., 2008). We hypothesize that a symbiotic relationship between elevated concentrations of ANGPT-2 and VEGF allows FG follicles to excel in follicle maturation. Also, elevated ANGPT-2 production may offer possible insight into future oocyte quality as early as Week 2, compared with Week 4 for VEGF and follicular size.
The current data add to our understanding of the dynamic activity of primate follicles as they develop from the early secondary stage to the small antral (∼1 mm in diameter) stage during 3-D culture. The production of angiogenic factors, VEGF-A and ANGPT-2, as well as other local factors (e.g. anti-Müllerian hormone) and steroid hormones (progesterone, androstenedione and estradiol) (Xu et al., 2010, 2011, 2013), is a function of the follicle growth rate, with FG follicles producing higher concentrations than SG and NG follicles. Likewise, their production is a function of the stage of follicular development, e.g. the production of VEGF and ANGPT-2 correlates with steroid hormone production (Xu et al., 2011); concentrations are low prior to onset of antrum formation and continue to rise after antrum formation. The 3-D culture system provides a unique opportunity to explore possible local actions of follicular factors that promote the follicular development, both from a steroidogenic and gametogenic perspective, including VEGF and ANGPTs independent of angiogenesis.
Authors' roles
As lead author, T.E.F. performed VEGF and ANGPT assays of media from follicle cultures, analysed the data, led the discussion of data and its relevance to the literature, and provided the first draft of the manuscript. T.A.M. and A.V. performed the granulosa cell experiments, including ANGPT assays, analysed the data and participated in preparation of the manuscript. M.B.Z. assisted with follicle isolation. M.B.Z. and R.L.S., as collaborators and leaders of their respective laboratories, provided oversight of the research trainees (T.E.F. and A.V.) during experimental design and performance, data analyses, literature evaluation and manuscript preparation. J.X. performed the follicle culture, analyzed follicle growth and oocyte parameters, as well as contributed to data interpretation, critical manuscript revising for important intellectual content and preparation of the final manuscript be submitted for publication. All authors approved the final version to be submitted for publication.
Funding
This work was supported by the National Institute of Health (NIH) U54 RR024347, RL1HD058294, PL1EB008542 (the Oncofertility Consortium), NIH-NICHD through cooperative agreement as part of the Specialized Cooperative Center Program in Reproduction and Infertility Research U54HD018185, NIH ORWH/NICHD 2K12HD043488 (Building Interdisciplinary Research Careers in Women's Health), NIH Fogarty International Center TW/HD-00668 (to P. Michael Conn) and Oregon National Primate Research Center 8P51OD011092.
Conflict of interest
The authors have no conflict of interests to disclose.
Acknowledgements
We appreciate the assistance provided by members of the Division of Comparative Medicine and the Endocrine Technology Support Core at ONPRC. We also acknowledge expertise and valuable assistance of Drs Min Xu, Teresa Woodruff and Lonnie Shea at the Northwestern University. The assistance of Dr Richard Yeoman and Maralee Lawson, ONPRC, with follicle culture is appreciated. Statistical analysis of the follicle culture data was performed by Jean O'Malley with support from the ONPRC Biostatistics Unit. The Principal Investigator of the NIH Fogarty International Center is P. Michael Conn, ONPRC.
References
- Abramovich D, Rodriguez Celin A, Hernandez F, Tesone M, Parborell F. Spatiotemporal analysis of the protein expression of angiogenic factors and their related receptors during folliculogenesis in rats with and without hormonal treatment. Reproduction. 2009;137:309–320. doi: 10.1530/REP-08-0130. [DOI] [PubMed] [Google Scholar]
- Araújo VR, Duarte AB, Bruno JB, Pinho Lopes CA, de Figueiredo JR. Importance of vascular endothelial growth factor (VEGF) in ovarian physiology of mammals. Zygote. 2013;21:295–304. doi: 10.1017/S0967199411000578. [DOI] [PubMed] [Google Scholar]
- Celik-Ozenci C, Akkoyunlu G, Kayisli UA, Arici A, Demir R. Localization of vascular endothelial growth factor in the zona pellucida of developing ovarian follicles in the rat: a possible role in destiny of follicles. Histochem Cell Biol. 2003;120:383–390. doi: 10.1007/s00418-003-0586-4. [DOI] [PubMed] [Google Scholar]
- Chowdhury MW, Scaramuzzi RJ, Wheeler-Jones CPD, Khalid M. The expression of angiogenic growth factors and their receptors in ovarian follicles throughout the estrous cycle in the ewe. Theriogenology. 2010;73:856–872. doi: 10.1016/j.theriogenology.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Christenson L, Stouffer RL. Follicle-stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J Clin Endocrinol Metab. 1997;82:2135–2142. doi: 10.1210/jcem.82.7.4169. [DOI] [PubMed] [Google Scholar]
- Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod. 2003;68:1736–1741. doi: 10.1095/biolreprod.101.000679. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte reals without alternation of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Wilson H, Rudge JS, Wiegand SJ. Single injections of vascular endothelial growth factor trap block ovulation in the macaque and produce a prolonged, dose-related suppression of ovarian function. J Clin Endocrinol Metab. 2005;90:1114–1122. doi: 10.1210/jc.2004-1572. [DOI] [PubMed] [Google Scholar]
- Friedman CI, Danforth DR, Herbosa-Encarnacion C, Arbogast L, Alak BM, Seifer DB. Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril. 1997;68:607–612. doi: 10.1016/s0015-0282(97)00278-1. [DOI] [PubMed] [Google Scholar]
- Greenaway J, Connor K, Pedersen HG, Coomber BL, Lamarre J, Petrik J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145:2896–2905. doi: 10.1210/en.2003-1620. [DOI] [PubMed] [Google Scholar]
- Hayashi KG, Berisha B, Matsui M, Schams D, Miyamoto A. Expression of mRNA for the angiopoietin-tie system in granulosa cells during follicular development in cows. J Reprod Dev. 2004;50:477–480. doi: 10.1262/jrd.50.477. [DOI] [PubMed] [Google Scholar]
- Hazzard TM, Stouffer RL. Angiogenesis in ovarian follicular and luteal development. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:883–900. doi: 10.1053/beog.2000.0133. [DOI] [PubMed] [Google Scholar]
- Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod. 2002;67:1305–1312. doi: 10.1095/biolreprod67.4.1305. [DOI] [PubMed] [Google Scholar]
- Hurliman AK, Speroff L, Stouffer RL, Patton PE, Lee A, Molskness TA. Changes in circulating levels and ratios of angiopoietins during pregnancy but not during the menstrual cycle and controlled ovarian stimulation. Fertil Steril. 2010;93:1493–1499. doi: 10.1016/j.fertnstert.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Christenson LK, Patton PE, Burry KA, Stouffer RL. Vascular endothelial growth factor production by human luteinized granulosa cells in vitro. Hum Reprod. 1997;12:2756–2761. doi: 10.1093/humrep/12.12.2756. [DOI] [PubMed] [Google Scholar]
- Luo H, Kimura K, Aoki M, Hirako M. Effect of vascular endothelial growth factor on maturation, fertilization and developmental competence of bovine oocytes. J Vet Med Sci. 2002;64:803–806. doi: 10.1292/jvms.64.803. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Martinez-Chequer JC, Stouffer RL, Hazzard TM, Patton PE, Molskness TA. Insulin-like growth factor-1 and -2, but not hypoxia, synergize with gonadotropin hormone to promote vascular endothelial growth factor—a secretion by monkey granulosa cells from preovulatory follicles. Biol Reprod. 2003;68:1112–1118. doi: 10.1095/biolreprod.102.011155. [DOI] [PubMed] [Google Scholar]
- Nishigaki A, Okada H, Tsuzuki T, Cho H, Yasuda K, Kanzaki H. Angiopoietin 1 and angiopoietin 2 in follicular fluid of women undergoing a long protocol. Fertil and Steril. 2011;96:1378–1383. doi: 10.1016/j.fertnstert.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Neeman M, Abramovitch R, Schiffenbauer YS, Tempel C. Regulation of angiogenesis by hypoxic stress: from solid tumours to the ovarian follicle. Int J Exp Pathol. 1997;78:57–70. doi: 10.1046/j.1365-2613.1997.d01-247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parborell F, Abramovich D, Tesone M. Intrabursal administration of the antiangiopoietin 1 antibody produces a delay in rat follicular development associated with an increase in ovarian apoptosis mediated by changes in the expression of BCL2 related genes. Biol Reprod. 2008;78:506–513. doi: 10.1095/biolreprod.107.063610. [DOI] [PubMed] [Google Scholar]
- Roberts AE, Arbogast LK, Friedman CI, Cohn DE, Kaumaya PT, Danforth DR. Neutralization of endogenous vascular endothelial growth factor depletes primordial follicles in the mouse ovary. Biol Reprod. 2007;76:218–223. doi: 10.1095/biolreprod.106.050880. [DOI] [PubMed] [Google Scholar]
- Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138:869–881. doi: 10.1530/REP-09-0283. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Hillier SG, Fraser HM. Effects of GnRH antagonist treatment on follicular development and angiogenesis in the primate ovary. J Endocrinol. 2004;183:1–17. doi: 10.1677/joe.1.05685. [DOI] [PubMed] [Google Scholar]
- Tesone M, Stouffer RL, Borman SM, Hennebold JD, Molskness TA. Vascular endothelial growth factor (VEGF) production by the monkey corpus luteum during the menstrual cycle: isoform-selective mRNA expression in vivo and hypoxia regulated protein secretion in vitro. Biol Reprod. 2005;73:927–934. doi: 10.1095/biolreprod.105.039875. [DOI] [PubMed] [Google Scholar]
- Wulff C, Wiegand SJ, Saunders PTK, Scobie GA, Fraser HM. Angiogenesis during follicular development in the primate and its inhibition by treatment with truncated Flt-1-Fc (vascular endothelial growth factor trap) Endocrinology. 2001;142:3244–3254. doi: 10.1210/endo.142.7.8258. [DOI] [PubMed] [Google Scholar]
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor trap R1R2. Endocrinology. 2002;143:2797–2807. doi: 10.1210/endo.143.7.8886. [DOI] [PubMed] [Google Scholar]
- Xu F, Stouffer RL. Local delivery of angiopoietin-2 into the preovulatory follicle terminates the menstrual cycle in rhesus monkeys. Biol Reprod. 2005;72:1352–1358. doi: 10.1095/biolreprod.104.037143. [DOI] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–2200. doi: 10.1093/humrep/det093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab. 2001;86:768–772. doi: 10.1210/jcem.86.2.7181. [DOI] [PubMed] [Google Scholar]
- Zimmermann RC, Xiao E, Bohlen P, Ferin M. Administration of antivascular endothelial growth factor receptor 2 antibody in the early follicular phase delays follicular selection and development in the rhesus monkey. Endocrinology. 2002;143:2496–2502. doi: 10.1210/endo.143.7.8896. [DOI] [PubMed] [Google Scholar]


