Abstract
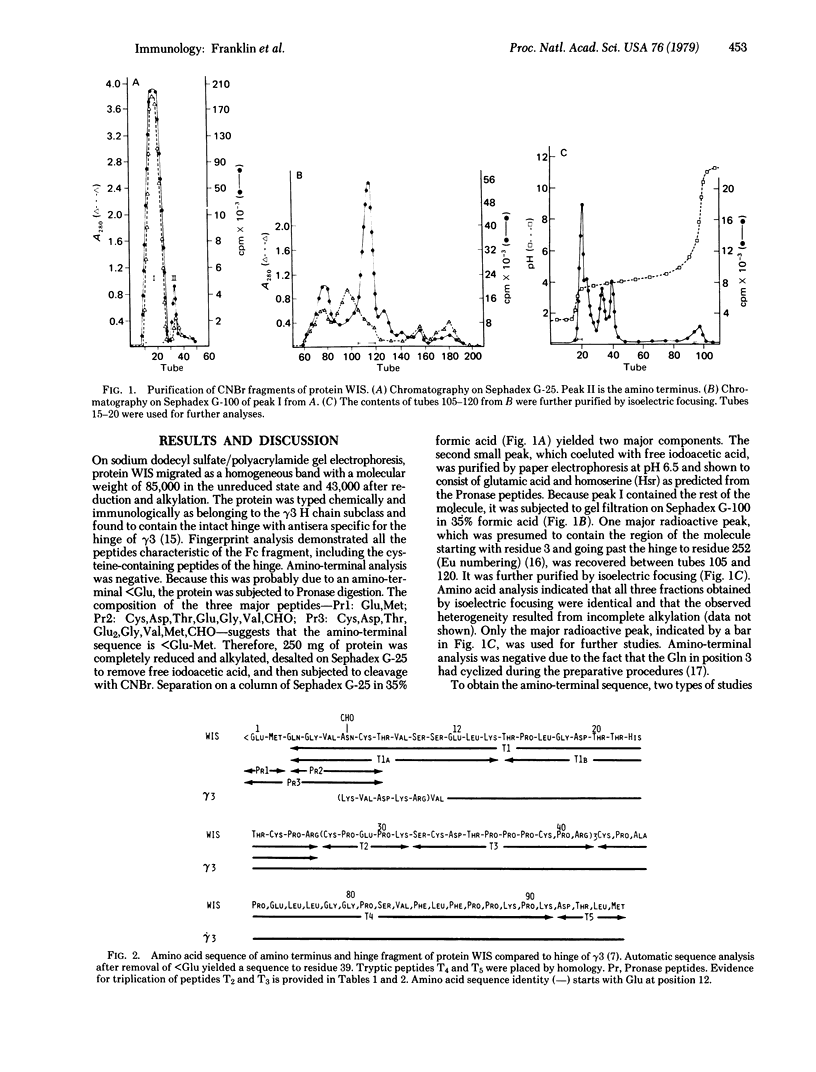
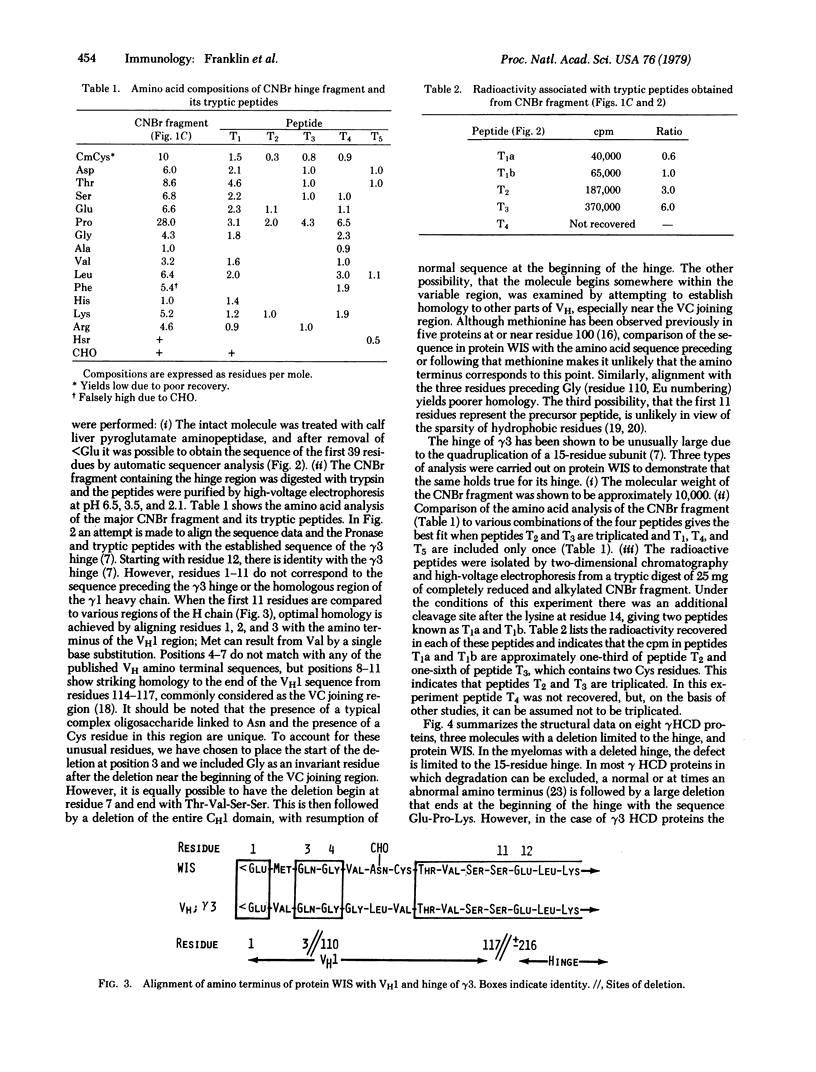
Protein WIS is a human gamma3 heavy (H) chain disease immunoglobulin variant whose amino acid sequence is most readily interpreted by postulating that three residues of the amino terminus are followed by a deletion of most of the variable (VH) domain, which ends at the variable-constant (VC) joining region. Then there is a stretch of eight residues, three of which are unusual, while the other five have striking homology to the VC junction sequence. This is followed by a second deletion, which ends at the beginning of the quadruplicated hinge region. These findings are consistent with mutations resulting in deletions of most of the gene coding for the V region and CH1 domain followed by splicing at the VC joining region and at the hinge. These structural features fit well the notion of genetic discontinuity between V and C genes and also suggest similar mechanisms of excision and splicing in the interdomain regions of the C gene of the heavy chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Amino acid sequence of the NH2-terminal extra piece segments of the precursors of mouse immunoglobulin lambda1-type and kappa-type light chains. Proc Natl Acad Sci U S A. 1977 Feb;74(2):716–720. doi: 10.1073/pnas.74.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett J. W., Deutsch H. F., Smithies O. Hinge-region deletion localized in the IgG-globulin Mcg. Immunochemistry. 1973 Feb;10(2):115–118. doi: 10.1016/0019-2791(73)90238-3. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Frangione B. A new immunoglobulin variant: gamma3 heavy chain disease protein CHI. Proc Natl Acad Sci U S A. 1976 May;73(5):1552–1555. doi: 10.1073/pnas.73.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C., Smithies O. Unusual genes at the aminoterminus of human immunoglobulin variants. Nature. 1978 Jun 1;273(5661):400–401. doi: 10.1038/273400a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Franklin E. C. Chemical typing of immunoglobulins. Nature. 1969 Jan 11;221(5176):149–151. doi: 10.1038/221149a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Partial deletion in the heavy chain disease protein ZUC. Nature. 1969 Nov 8;224(5219):597–599. doi: 10.1038/224597a0. [DOI] [PubMed] [Google Scholar]
- Franklin E. C., Frangione B., Adlersberg J. B. An antigenic determinant unique to the hinge of gamma3 heavy chains. J Immunol. 1975 Jul;115(1):314–315. [PubMed] [Google Scholar]
- Franklin E. C., Frangione B. The molecular defect in a protein (CRA) found in gamma-1 heavy chain disease, and its genetic implications. Proc Natl Acad Sci U S A. 1971 Jan;68(1):187–191. doi: 10.1073/pnas.68.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver F. A., Chang L., Mendicino J., Isobe T., Osserman E. F. Primary structure of a deleted human lambda type immunoglobulin light chain containing carbohydrate: protein Sm lambda. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4559–4563. doi: 10.1073/pnas.72.11.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka R. L., Pestka S. Amino acid sequence of the precursor region of MOPC-315 mouse immunoglobulin heavy chain. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5692–5696. doi: 10.1073/pnas.74.12.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen T. E., Frangione B., Franklin E. C. Primary structure of the "hinge" region of human IgG3. Probable quadruplication of a 15-amino acid residue basic unit. J Biol Chem. 1977 Feb 10;252(3):883–889. [PubMed] [Google Scholar]
- Podell D. N., Abraham G. N. A technique for the removal of pyroglutamic acid from the amino terminus of proteins using calf liver pyroglutamate amino peptidase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):176–185. doi: 10.1016/0006-291x(78)91646-7. [DOI] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat C., Schiff C., Rivat L., Ropartz C., Fougereau M. Deletion of hinge region of human myeloma IgG1 molecule (protein LEC) associated with nonexpression of G1m (3) and Km (1, 2) allotypes. A possible genetic explanation at the DNA level. Eur J Immunol. 1976 Aug;6(8):545–551. doi: 10.1002/eji.1830060804. [DOI] [PubMed] [Google Scholar]
- Secher D. S., Milstein C., Adetugbo K. Somatic mutants and antibody diversity. Immunol Rev. 1977;36:51–72. doi: 10.1111/j.1600-065x.1977.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]


