Abstract
Background
Little information is available regarding the effect of conventional radiotherapy on glioma-related seizures.
Methods
In this retrospective study, we analyzed the seizure response and outcome following conventional radiotherapy in a cohort of 43 patients with glioma (33 grade II, 10 grade III) and medically intractable epilepsy.
Results
At 3 months after radiotherapy, seizure reduction was significant (≥50% reduction of frequency compared with baseline) in 31/43 patients (72%) of the whole series and in 25/33 patients (76%) with grade II gliomas, whereas at 12 months seizure reduction was significant in 26/34 (76%) and in 19/25 (76%) patients, respectively. Seizure reduction was observed more often among patients displaying an objective tumor response on MRI, but patients with no change on MRI also had a significant seizure reduction. Seizure freedom (Engel class I) was achieved at 12 months in 32% of all patients and in 38% of patients with grade II tumors. Timing of radiotherapy and duration of seizures prior to radiotherapy were significantly associated with seizure reduction.
Conclusions
This study showed that a high proportion of patients with medically intractable epilepsy from diffuse gliomas derive a significant and durable benefit from radiotherapy in terms of epilepsy control and that this positive effect is not strictly associated with tumor shrinkage as shown on MRI. Radiotherapy at tumor progression seems as effective as early radiotherapy after surgery. Prospective studies must confirm and better characterize the response to radiotherapy.
Keywords: diffuse gliomas, radiotherapy, seizure control
Seizures are the most common presenting symptom of slowly growing diffuse gliomas, especially low-grade gliomas.1–3 Gross total resection is strongly associated with tumor-related seizure control.4–7 However, despite the favorable effect of surgery and the best treatment with antiepileptic drugs (AEDs),8,9 a number of patients still have seizures over the course of the disease. The persistence of seizures and the use of AEDs may negatively influence quality of life and cognitive functions.10,11 Radiation delivered via different modalities, such as interstitial brachytherapy,12,13 Gamma Knife radiosurgery,14 or conventional external radiotherapy,15,16 can improve seizure control in both low- and high-grade gliomas. In this regard, the efficacy of conventional radiotherapy has been supported by the results of the European Organisation for Research and Treatment of Cancer phase III trial 22845 in low-grade gliomas, showing that at 1 year after surgery 25% of patients who received adjuvant radiotherapy had seizures compared with 41% of those who had observation alone.17 Moreover, a recent retrospective study found radiotherapy to be a positive prognostic factor in terms of seizure control.18
Our aim in this current retrospective study, performed on a cohort of patients with diffuse gliomas of the adult treated at a single institution, was 2-fold: to describe seizure reduction and outcome after conventional radiotherapy and to explore the factors associated with seizure control.
Materials and Methods
Patient Selection and Data Collection
Patient information was collected from the database of the Department of Neuro-Oncology, University Hospital of Torino, Italy, for patients treated between 1989 and 2009. The patients who met the following criteria were included in the study: (i) histologically verified hemispheric World Health Organization (WHO) grade II astrocytoma, oligodendroglioma, or oligoastrocytoma or histologically verified hemispheric WHO grade III astrocytoma, oligodendroglioma, or oligoastrocytoma with a preexisting history of low-grade glioma (duration of seizures before surgery >1 year and nonenhancing lesion on MRI); (ii) medically intractable epilepsy (seizure frequency >1 per month despite adequate serum concentrations of AEDs) and absence of any neurological deficit; (iii) focal radiotherapy in a conventional fractionation (1.8–2 Gy per fraction, 5 d/wk), delivered either early (within 8 wk of surgery) or late at tumor progression; (iv) no use of chemotherapy during radiotherapy and up to 12 months after the end of radiotherapy; (v) availability of MRI scans before and after radiotherapy with measurable disease and absent or mild contrast enhancement; (vi) Karnofsky performance status score ≥70; (vii) age ≥18 years.
We excluded from the study those patients who displayed a reduction of seizures during and up to 12 months after the end of radiotherapy and had a concurrent change of regimen or increase in AED dosage because the reduction of seizures could not be attributed solely to radiotherapy. Conversely, we did not exclude patients whose seizure status worsened and who needed a change of medication during and after radiotherapy; in the analysis, these patients were not considered to have a reduction of seizures following radiotherapy.
Data pertaining to patient demographics, seizure characteristics, therapeutic management, and outcome were retrieved from the database as well as from outpatient clinical notes of follow-up visits or telephone calls. The local institutional review board approved this retrospective study.
Assessment of Seizure Reduction and Response on MRI
The seizure frequency was reported by the patients based on a seizure diary, without confirmatory electrophysiological testing.
We analyzed the seizure frequency before radiotherapy (baseline evaluation) and at 3, 6, and 12 months after radiotherapy. Seizure reduction was considered significant when a ≥50% reduction in seizure frequency compared with baseline was observed, with concomitant AEDs being unchanged. Any change (decrease or increase) of seizure frequency <50% was considered as no change, while an increase in seizure frequency ≥50% was considered as a significant increase.
Response of tumor on MRI was evaluated at 3, 6, and 12 months after radiotherapy according to Macdonald criteria adapted to low-grade gliomas.19,20
These criteria are based on changes in tumor size defined as the product of the two largest perpendicular diameters of the T2 or fluid attenuated inversion recovery (FLAIR) hypersignal lesion in nonenhancing tumors. In tumors displaying contrast enhancement, response criteria took into consideration the size of the T1 postcontrast enhancement as well. In brief, a complete response (CR) was defined as complete disappearance of all T2/FLAIR hypersignal and T1 postcontrast enhancing lesions. A partial response (PR) was defined as >50% reduction in size in both nonenhancing and enhancing (when present) lesions from baseline. Minor response (MR) was defined as a 25% to 50% reduction in the size of nonenhancing tumors; in patients with enhancing tumors, disappearance of all contrast enhancement and stable T2/FLAIR hypersignal lesion size were also considered to be MR. Stability or a reduction in the corticosteroid dose and stable neurological status were required to qualify for CR, PR, and MR. Progressive disease (PD) was defined as a >25% increase in the size of the T2/FLAIR hypersignal or contrast enhancement, any new tumor on MRI scans, or tumor-related neurological deterioration in patients on stable or increased doses of corticosteroids. Stable disease was defined as any other clinical status not meeting the criteria for CR, PR, MR, or PD. The radiographic responses were reviewed independently by 2 investigators who were kept unaware of seizure response. Because many patients had MRI examinations with different MRI machines during the long time interval covered by the study and because the distinction between MR and PR was often difficult, we grouped together MRs and PRs into the category of objective responses.
Assessment of Seizure Outcome
Seizure outcome at 3, 6, and 12 months after radiotherapy was evaluated using the Engel classification of seizures21: class I, seizure free; class II, rare seizures; class III, meaningful seizure improvement; class IV, no seizure improvement or worsening.
Statistical Analysis
Baseline demographics and clinical characteristics of the cohort were described using either mean and standard deviation (SD) or median and interquartile range (IQR) for the continuous variables and using percentage frequencies for the categorical variables.
We selected a priori the following factors as potentially associated with seizure control: age, sex, tumor type (astrocytoma vs oligodendroglioma or oligoastrocytoma), tumor grade (WHO grade III vs II), tumor location (temporal vs extratemporal), tumor enhancement on MRI (present vs absent), extent of surgery (partial or subtotal resection vs biopsy), timing of radiotherapy (early or late), seizure type (simple partial vs complex partial vs other), seizure frequency (≥1 per wk vs <1 per wk), duration of seizures before radiotherapy, AEDs (polytherapy vs monotherapy), and steroid use (yes vs no). Age and duration of seizures were considered both as continuous and as categorical variables.
To obtain estimates not influenced by extreme values, the duration of seizures was logarithmically transformed.
For outcome analyses, Engel classification was dichotomized as class I (seizure freedom) versus classes II–IV.
To identify those variables that might be associated with seizure control in terms of both reduction of seizures and seizure freedom, we estimated crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) using univariate and multivariate logistic regression models. In the multivariate model, we included the variables that showed stronger association in univariate analysis along with the few known prognostic factors, to take into account the most important confounders.
Because timing to radiotherapy and duration of seizures were strongly associated, we used 2 different models.
Owing to the reduction in the sample size after 3 months, we performed only univariate analyses to explore factors associated with seizure control at 6 and 12 months.
All analyses were also repeated, as sensitivity analyses, for patients with a histologic diagnosis of grade II glioma only.
To evaluate the association between radiological and seizure response, we used the chi-square test.
All analyses were performed using Stata 11.2.
Results
Population Characteristics
We found 43 patients with histologic diagnoses of grade II (n = 33) or III (n = 10) glioma and medically intractable epilepsy who received focal radiotherapy and who were eligible for the study. Patient characteristics are reported in Table 1; their distribution among the whole population did not significantly differ in comparison with grade II tumors alone. Nine of these patients had incomplete data at 6 and 12 months and were excluded from the analysis for these time points.
Table 1.
Baseline characteristics of the study population
| Frequency | % | |
|---|---|---|
| Age, y (at radiotherapy) | ||
| 20–29 | 8 | 18.6 |
| 30–39 | 12 | 27.9 |
| 40–49 | 14 | 32.6 |
| >50 | 9 | 20.9 |
| Age y (mean, standard deviation) | 40.6 | 11.5 |
| Sex | ||
| Male | 28 | 65.1 |
| Female | 15 | 34.9 |
| Tumor type | ||
| Astrocytoma | 28 | 65.1 |
| Oligodendroglioma | 10 | 23.3 |
| Oligoastrocytoma | 5 | 11.6 |
| Tumor grade, WHO | ||
| II | 33 | 76.7 |
| III | 10 | 23.3 |
| Tumor location | ||
| Temporal lobe | 21 | 48.8 |
| Other | 22 | 51.2 |
| Tumor enhancement | ||
| Absent | 22 | 51.2 |
| Present | 21 | 48.8 |
| Extent of surgery | ||
| Biopsy | 14 | 32.6 |
| Partial/subtotal resection | 29 | 67.4 |
| Timing of radiotherapy | ||
| Early | 19 | 44.2 |
| Late | 24 | 55.8 |
| Seizure type | ||
| Partial simple | 25 | 58.1 |
| Partial complex | 10 | 23.3 |
| Secondary generalized | 4 | 9.3 |
| Generalized | 4 | 9.3 |
| Seizure frequency | ||
| Daily | 11 | 25.6 |
| Weekly | 14 | 32.6 |
| Monthly | 18 | 41.9 |
| Duration of seizures | ||
| ≤1 y | 22 | 51.2 |
| >1 y | 21 | 48.8 |
| Duration of seizures (median/IQR) | 12 | 2–5 |
| AED | ||
| Monotherapy | 20 | 46.5 |
| Polytherapy | 23 | 53.5 |
| Steroid | ||
| No | 32 | 74.4 |
| Yes | 11 | 25.6 |
| Total | 43 | 100 |
Forty-one patients received a total radiation dose of 50–60 Gy, whereas 2 patients received totals of 39.6 and 46.8 Gy because of side effects (headache, nausea, vomiting) during treatment. Nineteen patients (14 with grade II gliomas, 5 with grade III gliomas) received early radiotherapy after surgery, and 24 patients (19 with grade II gliomas, 5 with grade III tumors) received late radiotherapy at tumor progression after either observation (grade II tumors) or upfront chemotherapy (grade III tumors).
During the long period covered by this retrospective study (20 y), the opinions of the different physicians regarding the optimal timing of radiotherapy in low-grade gliomas, in relation to the patients' characteristics, were not uniform; therefore the choice between early and late radiotherapy was individually based and quite variable. In general, intractable epilepsy in this series was not the sole reason to start radiotherapy.
Twenty-five patients had simple partial seizures (23 motor and 2 sensory), 10 complex partial seizures, 4 secondary generalized seizures, and 4 generalized tonic-clonic seizures. Seizure frequency varied greatly: 11 patients had ≥1 seizure per day, 14 patients had ≥1 seizure per week, and 18 patients had ≥1 seizure per month. The median duration of seizures before radiotherapy was 12 months (range 3 mo to 12 y). All patients had an increase in seizure frequency over time prior to radiotherapy.
Regarding AED use, 20 patients received monotherapy with oxcarbazepine (7 patients), carbamazepine (6 patients), phenobarbital (5 patients), lamotrigine (1 patient), and levetiracetam (1 patient). Twenty-three patients received a combination of AEDs: 16 patients received 2 AEDs, 6 patients received 3 AEDs, and 1 patient received 4 AEDs.
Eleven of the 43 patients were receiving steroids at the start of radiotherapy. Twenty-four patients received chemotherapy (procarbazine/lomustine/vincristine [PCV] or temozolomide) at some point in their disease course, but never concurrently or after radiotherapy for the time period covered by the study (12 mo). The median duration of follow-up was 12 months (range, 3–120 mo).
Seizure Reduction After Radiotherapy
When considering the whole population, at 3 months after radiotherapy, seizure frequency was significantly reduced in 31/43 patients (72.1%; 95% CI: 56.3–84.7%). The reduction of seizures began during radiation therapy (more commonly 2–3 wk after start) in 23/43 (53.5%) patients. Because between 6 and 12 months no patients were lost or changed seizure status, we reported the evaluation at 12 months only. At 12 months after radiotherapy, seizure frequency was significantly reduced in 26/34 patients (76.5%; 95% CI: 58.8–89.3%).
All patients who had a reduction of seizure frequency at 3 months maintained the reduction at 6 and 12 months.
The results did not change when we considered the 33 patients with grade II gliomas: at 3 months after radiotherapy, seizure frequency was significantly reduced in 25/33 patients (75.8%; 95% CI: 57.7–88.9%), whereas at 12 months after radiotherapy, seizure frequency was significantly reduced in 19/25 patients (76%; 95% CI: 54.9–90.6%).
Among patients with grade III tumors, 6/10 (60%; 95% CI: 26.2–87.8%) and 6/9 (66.7%; 95% CI: 40.0–97.2%) patients had a significant reduction of seizures at 3 and 12 months, respectively.
Relationships Between Response on MRI and Seizure Reduction
We did not observe any CR. At 3, 6, and 12 months, numbers of patients showing objective responses on MRI were 18/43 (41.9%; 95% CI: 27.0–57.9%), 14/34 (41.2%, 95% CI: 24.6–59.3%), and 8/34 (23.5%, 95% CI: 12.9–44.4%), respectively. Considering grade II tumors only, response rates were 45.5% (95% CI: 28.1–63.6%), 44.0% (95% CI: 24.4–65.1%), and 32% (95% CI: 14.9–53.5%) at 3, 6, and 12 months, respectively.
Overall, at 3 months after radiotherapy, 15 (83.3%) of 18 patients who had a response on MRI had also a seizure reduction compared with 16 (64%) of 25 patients who did not have a response on MRI but had a seizure reduction (P = .163). At 6 months after radiotherapy, 12/14 patients (85.7%) who had a response on MRI had also a seizure reduction compared with 14/20 (70%) who did not have a response on MRI but had a seizure reduction (P = .288). At 12 months after radiotherapy, the seizure response rate was 77.8% in patients who had a response on MRI and 76% in patients without a response on MRI (P = .914).
When considering grade II tumors only, at 3 months after radiotherapy 12 (80%) of 15 patients who had a response on MRI had a seizure reduction compared with 13 (72.2%) of 18 patients who did not have a radiological response but had a seizure reduction (P = .604). At 6 months after radiotherapy, 9/11 patients (81.8%) with a response on MRI had a reduction of seizures compared with 10/14 patients (71.4%) who did not have a radiological response but had a seizure reduction (P = .546). At 12 months after radiotherapy, 6/8 patients (75%) with a response on MRI had a seizure reduction compared with 13/17 patients (76.4%) without a radiological response who had a seizure reduction (P = .936).
Seizure Outcome After Radiotherapy
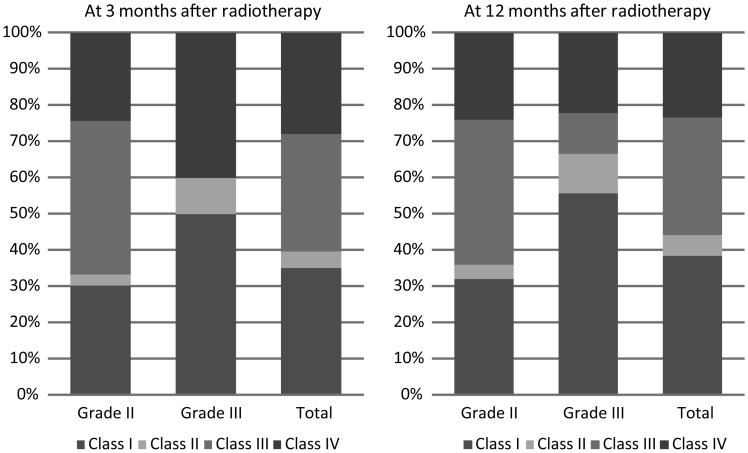
The seizure status was the same at 6 and 12 months after radiotherapy; thus, in the text and in Fig. 1 the seizure outcome is reported at 3 and 12 months after radiotherapy.
Fig. 1.
Graph illustrating seizure outcome (according to the Engel classification) at 3 and 12 months.
When considering the whole population, at 3 months after radiotherapy 15/43 patients (35%) were seizure free (class I), 2/43 (5%) had rare seizures (class II), 14/43 (32%) had a meaningful improvement of seizures (class III), and 12/43 (28%) had no improvement or worsening (class IV). At 12 months after radiotherapy, 13/34 patients (38%) were seizure free (class I), 2/34 (6%) had rare seizures (class II), 11/34 (32%) had a meaningful improvement of seizures (class III), and 8/34 (24%) had no improvement or worsening (class IV).
When considering grade II tumors, at 3 months after radiotherapy 10/33 patients (30%) were seizure free (class I), 1/33 (3%) had rare seizures (class II), 14/33 (43%) had a meaningful improvement in seizures (class III), and 8/33 (24%) had no improvement or worsening (class IV). At 12 months after radiotherapy, 8/25 patients (32%) were seizure free (class I), 1/25 (4%) had rare seizures (class II), 10/25 (40%) had a meaningful improvement of seizures (class III), and 6/25 (24%) had no improvement or worsening (class IV).
Among the 13 patients who were seizure free at 12 months, none was able to discontinue AEDs, whereas in 6 patients (5 grade II and 1 grade III) AEDs were significantly tapered (from polytherapy to monotherapy), and 4 patients were seizure free at 12 months.
None of the clinicopathological factors analyzed predicted seizure control at 12 months.
Factors Influencing Seizure Reduction and Seizure Freedom After Radiotherapy
The distribution of clinicopathological factors in relation to seizure reduction and seizure freedom at 3 months after radiotherapy is reported in Table 2. Univariate analysis showed that seizure reduction was significantly associated with duration of seizures prior to radiotherapy (OR 7.92, 95% CI: 1.47–42.54, P = .016) and timing of radiotherapy (OR 6.3, 95% CI: 1.39–28.46, P = .017). In particular, the proportion of patients with seizure reduction at 3 months after radiotherapy was 52.6% in the early radiotherapy group compared with 87.5% in the late radiotherapy group.
Table 2.
Factors influencing seizure reduction and seizure freedom at 3 months after radiotherapy
| Total | Seizure Freedom |
Seizure Reduction |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Freq | % | OR | 95% CI | P | % | OR | 95% CI | P | |
| Age, y | |||||||||
| ≤40 | 21 | 28.6 | 1 | 66.7 | 1 | ||||
| >40 | 22 | 40.9 | 1.73 | [0.48, 6.18] | .398 | 77.3 | 1.7 | [0.44, 6.55] | .44 |
| Sex | |||||||||
| Female | 15 | 20 | 1 | 80 | 1 | ||||
| Male | 28 | 42.9 | 3 | [0.69, 13.05] | .143 | 67.9 | 0.53 | [0.12, 2.35] | .402 |
| Tumor type | |||||||||
| Oligodendroglioma or oligoastrocytoma | 15 | 26.7 | 1 | 66.7 | 1 | ||||
| Astrocytoma | 28 | 39.3 | 1.78 | [0.45, 7.02] | .411 | 75 | 1.5 | [0.38, 5.92] | .563 |
| Tumor grade, WHO | |||||||||
| II | 33 | 30.3 | 1 | 75.8 | 1 | ||||
| III | 10 | 50 | 2.3 | [0.54, 9.76] | .259 | 60 | 0.48 | [0.11, 2.14] | .336 |
| Tumor location | |||||||||
| Temporal lobe | 21 | 33.3 | 1 | 71.4 | 1 | ||||
| Other | 22 | 36.4 | 1.14 | [0.33, 4.01] | .835 | 72.7 | 1.07 | [0.28, 4.04] | .924 |
| Tumor enhancement | |||||||||
| No | 22 | 27.3 | 1 | 68.2 | 1 | ||||
| Yes | 21 | 42.9 | 2 | [0.56, 7.16] | .287 | 76.2 | 1.49 | [0.39, 5.74] | .559 |
| Extent of surgery | |||||||||
| Biopsy | 14 | 28.6 | 1 | 57.1 | 1 | ||||
| Partial/subtotal resection | 29 | 37.9 | 1.53 | [0.38, 6.08] | .548 | 79.3 | 2.87 | [0.72, 11.52] | .136 |
| Timing of radiotherapy | |||||||||
| Early | 19 | 26.3 | 1 | 52.6 | 1 | ||||
| Late | 24 | 41.7 | 2 | [0.54, 7.37] | .298 | 87.5 | 6.3 | [1.39, 28.46] | .017 |
| Seizure type | |||||||||
| Partial simple | 25 | 32 | 1 | 72 | 1 | ||||
| Partial complex | 10 | 40 | 1.42 | [0.31, 6.47] | .653 | 70 | 0.91 | [0.18, 4.54] | .906 |
| Other | 8 | 37.5 | 1.27 | [0.24, 6.70] | .774 | 75 | 1.17 | [0.19, 7.22] | .868 |
| Seizure frequency | |||||||||
| <1 wk | 18 | 33.3 | 1 | 72.2 | 1 | ||||
| ≥1 wk | 25 | 36 | 1.12 | [0.31, 4.03] | .856 | 72 | 0.99 | [0.26, 3.82] | .987 |
| Duration of seizures* | |||||||||
| ≤1 y | 22 | 22.7 | 1 | 54.5 | 1 | ||||
| >1 y | 21 | 47.6 | 3.09 | [0.83, 11.51] | .092 | 90.5 | 7.92 | [1.47, 42.54] | .016 |
| AED | |||||||||
| Monotherapy | 20 | 45 | 1 | 80 | 1 | ||||
| Polytherapy | 23 | 26.1 | 0.43 | [0.12, 1.55] | .198 | 65.2 | 0.47 | [0.12, 1.89] | .286 |
| Steroid | |||||||||
| No | 32 | 34.4 | 1 | 75 | 1 | ||||
| Yes | 11 | 36.4 | 1.09 | [0.26, 4.55] | .905 | 63.6 | 0.58 | [0.13, 2.53] | .471 |
*If we insert in the model the log-transformation of duration of seizure, the OR estimate of seizure freedom is 1.59 (95% CI: 1.04–2.44, P = .033) and the OR estimate of seizure reduction is 1.77 (95% CI: 1.09–2.88, P = .021).
Duration of seizures prior to radiotherapy and timing of radiotherapy were associated, and multivariate analysis showed that each variable maintained statistical significance when the other one was removed from the model (Table 3). Because the difference in duration of seizures seemed to reflect the differences between early and late radiotherapy, we analyzed the distribution of clinicopathological factors according to the timing of radiotherapy (Table 4). Patients who received early radiotherapy had more biopsies (42% vs 25%), more simple partial seizures (68.4% vs 50%), and more AED polytherapies (58% vs 50%) than did patients who received late radiotherapy; however, these differences did not reach statistical significance.
Table 3.
Multivariate predictors of seizure reduction and seizure freedom at 3 months
| Seizure Freedom |
Seizure Reduction |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Tumor type | ||||||
| Oligodendroglioma or oligoastrocytoma | 1 | 1 | ||||
| Astrocytoma | 5.64 | [0.87, 36.48] | .069 | 3.5 | [0.43, 28.59] | .243 |
| Tumor grade, WHO | ||||||
| II | 1 | 1 | ||||
| III | 5.93 | [0.86, 40.72] | .070 | 0.68 | [0.09, 5.03] | .709 |
| Extent of surgery | ||||||
| Biopsy | 1 | 1 | ||||
| Partial/subtotal resection | 1.45 | [0.32, 6.51] | .624 | 3.05 | [0.60, 15.66] | .181 |
| Timing of radiotherapy | ||||||
| Early | 1 | 1 | ||||
| Late | 3.33 | [0.76, 14.59] | .111 | 8.4 | [1.36, 51.68] | .022 |
| Seizure duration (log)* | 2.27 | [1.25, 4.12] | .007 | 2.77 | [1.21, 6.34] | .016 |
*OR estimated excluding timing of radiotherapy.
Table 4.
Distribution of clinicopathological factors according to timing of radiotherapy
| Timing of Radiotherapy |
Chi-square Test P-value | ||||
|---|---|---|---|---|---|
| Early (n = 19) |
Late (n = 24) |
||||
| Frequency | % | Frequency | % | ||
| Age, y | |||||
| ≤40 | 10 | 52.6 | 11 | 45.8 | |
| >40 | 9 | 47.4 | 13 | 54.2 | .658 |
| Age, y, mean | 39.5 | 11.9 | 41.5 | 11.3 | .571* |
| Sex | |||||
| Female | 6 | 31.6 | 9 | 37.5 | |
| Male | 13 | 68.4 | 15 | 62.5 | .686 |
| Tumor type | |||||
| Astrocytoma | 15 | 78.9 | 13 | 54.2 | |
| Oligodendroglioma or oligoastrocytoma | 4 | 21.1 | 11 | 45.9 | .090 |
| Tumor grade, WHO | |||||
| II | 14 | 73.7 | 19 | 79.2 | |
| III | 5 | 26.3 | 5 | 20.8 | .673 |
| Tumor location | |||||
| Temporal lobe | 9 | 47.4 | 12 | 50.0 | |
| Other | 10 | 52.6 | 12 | 50.0 | .864 |
| Tumor enhancement | |||||
| No | 12 | 63.2 | 10 | 41.7 | |
| Yes | 7 | 36.8 | 14 | 58.3 | .161 |
| Extent of surgery | |||||
| Biopsy | 8 | 42.1 | 6 | 25.0 | |
| Partial/subtotal resection | 11 | 57.9 | 18 | 75.0 | .235 |
| Seizure type | |||||
| Partial simple | 13 | 68.4 | 12 | 50.0 | |
| Partial complex | 2 | 10.5 | 8 | 33.3 | |
| Other | 4 | 21.1 | 4 | 16.7 | .212 |
| Seizure frequency | |||||
| <1 wk | 9 | 47.4 | 9 | 37.5 | |
| ≥1 wk | 10 | 52.6 | 15 | 62.5 | .515 |
| Duration of seizures | |||||
| ≤1 y | 15 | 78.9 | 7 | 29.2 | |
| >1 y | 4 | 21.1 | 17 | 70.8 | .001 |
| AED | |||||
| Monotherapy | 8 | 42.1 | 12 | 50.0 | |
| Polytherapy | 11 | 57.9 | 12 | 50.0 | .606 |
| Steroid | |||||
| No | 13 | 68.4 | 19 | 79.2 | |
| Yes | 6 | 31.6 | 5 | 20.8 | .423 |
*t-test P-value.
Seizure freedom was not associated in univariate analysis with any clinicopathological factor at 3 months; when we performed multivariate analysis, the duration of seizures prior to radiotherapy was significant (P = .007; Table 3).
At 12 months after radiotherapy (Table 5), seizure reduction was significantly correlated with duration of seizures prior to radiotherapy in univariate analysis (OR 13.22, 95% CI: 1.4–124.9, P = .024).
Table 5.
Factors influencing seizure reduction and seizure freedom at 12 months after radiotherapy
| Total | Seizure Freedom |
Seizure Reduction |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Freq | % | OR | 95% CI | P | % | OR | 95% CI | P | |
| Age, y | |||||||||
| ≤40 | 14 | 35.7 | 1 | 78.6 | 1 | [1.00, 1.00] | |||
| >40 | 20 | 40 | 1.2 | [0.29, 4.93] | .800 | 75 | 0.82 | [0.16, 4.17] | .809 |
| Sex | |||||||||
| Female | 13 | 23.1 | 1 | 84.6 | 1 | [1.00, 1.00] | |||
| Male | 21 | 47.6 | 3.03 | [0.64, 14.26] | .161 | 71.4 | 0.45 | [0.08, 2.69] | .385 |
| Tumor type | |||||||||
| Oligodendroglioma or oligoastrocytoma | 13 | 30.8 | 1 | 76.9 | 1 | [1.00, 1.00] | |||
| Astrocytoma | 21 | 42.9 | 1.69 | [0.39, 7.27] | .483 | 76.2 | 0.96 | [0.19, 4.92] | .961 |
| Tumor grade, WHO | |||||||||
| II | 25 | 32 | 1 | 76 | 1 | [1.00, 1.00] | |||
| III | 9 | 55.6 | 2.66 | [0.56, 12.65] | .220 | 77.8 | 1.11 | [0.18, 6.82] | .914 |
| Tumor location | |||||||||
| Temporal lobe | 16 | 37.5 | 1 | 81.3 | 1 | [1.00, 1.00] | |||
| Other | 18 | 38.9 | 1.06 | [0.27, 4.24] | .934 | 72.2 | 0.6 | [0.12, 3.05] | .538 |
| Tumor enhancement | |||||||||
| No | 19 | 31.6 | 1 | 68.4 | 1 | [1.00, 1.00] | |||
| Yes | 15 | 46.7 | 1.9 | [0.47, 7.70] | .371 | 86.7 | 3 | [0.51, 17.71] | .225 |
| Extent of surgery | |||||||||
| Biopsy | 10 | 30 | 1 | 70 | 1 | [1.00, 1.00] | |||
| Partial/subtotal resection | 24 | 41.7 | 1.67 | [0.34, 8.07] | .526 | 79.2 | 1.63 | [0.31, 8.68] | .568 |
| Timing of radiotherapy | |||||||||
| Early | 16 | 31.3 | 1 | 62.5 | 1 | [1.00, 1.00] | |||
| Late | 18 | 44.4 | 1.76 | [0.43, 7.19] | .431 | 88.9 | 4.8 | [0.81, 28.60] | .085 |
| Seizure type | |||||||||
| Partial simple | 22 | 36.4 | 1 | 77.3 | 1 | [1.00, 1.00] | |||
| Partial complex | 5 | 60 | 2.62 | [0.36, 19.18] | .342 | 80 | 1.18 | [0.11, 13.07] | .895 |
| Other | 7 | 28.6 | 0.7 | [0.11, 4.48] | .706 | 71.4 | 0.74 | [0.11, 5.01] | .754 |
| Seizure frequency | |||||||||
| <1 wk | 14 | 35.7 | 1 | 78.6 | 1 | [1.00, 1.00] | |||
| ≥1 wk | 20 | 40 | 1.2 | [0.29, 4.93] | .800 | 75 | 0.82 | [0.16, 4.17] | .809 |
| Duration of seizures* | |||||||||
| ≤1 y | 16 | 25 | 1 | 56.3 | 1 | [1.00, 1.00] | |||
| >1 y | 18 | 50 | 3 | [0.70, 12.93] | .140 | 94.4 | 13.22 | [1.40, 124.91] | .024 |
| AED | |||||||||
| Monotherapy | 17 | 41.2 | 1 | 82.4 | 1 | [1.00, 1.00] | |||
| Polytherapy | 17 | 35.3 | 0.78 | [0.19, 3.12] | .724 | 70.6 | 0.51 | [0.10, 2.61] | .423 |
| Steroid | |||||||||
| No | 27 | 33.3 | 1 | 77.8 | 1 | [1.00, 1.00] | |||
| Yes | 7 | 57.1 | 2.67 | [0.49, 14.56] | .257 | 71.4 | 0.71 | [0.11, 4.65] | .725 |
*If we insert in the model the log-transformation of duration of seizure, the OR estimate of seizure freedom is 1.54 (95% CI: 0.98–2.42, P = .063) and the OR estimate of seizure reduction is 2.07 (95% CI: 1.09–3.89, P = .025).
We obtained similar results when performing the statistical analysis for grade II tumors.
Discussion
Limited data are available regarding the effect of radiotherapy on glioma-related seizures. Stereotactic interstitial irradiation improves seizure control in 40%–100% of unresectable low-grade gliomas,12,13 whereas Gamma Knife radiosurgery is helpful in mesiotemporal tumor–related epilepsy.14 Two small series have suggested that conventional radiotherapy can improve seizure control in both low- and high-grade gliomas with medically intractable epilepsy. The first series reported a reduction of seizure frequency of more than 75% in 4 of 5 patients with unresectable low-grade astrocytomas, and 1 patient became seizure free for as long as 8.2 years.15 The second study evaluated the effects of radiotherapy in 7 patients with malignant gliomas: all subjects had a reduction in seizure frequency, and 4 patients became seizure free for 2–12 months.16 Soon after both interstitial and external irradiation, seizure frequency was reduced.
Our current study analyzed the effect of conventional radiotherapy on seizure control in a larger series of slowly growing gliomas treated in the contemporary MRI era and explored the clinicopathological factors affecting seizure control.
Most of our patients had grade II gliomas, but we also considered some patients with grade III gliomas with a preexisting history of a low-grade tumor (long duration of seizures and nonenhancing pattern on MRI before radiation), because the endpoint of our study was seizure control and not survival parameters such as progression-free or overall survival.
We used 2 different measures to evaluate the effect of radiotherapy on seizures: the reduction in seizure frequency, because it reflects changes in the epilepsy burden, and the Engel classification, because it mainly reflects freedom from seizures.
Regarding the reduction of seizures after radiotherapy, this study confirms and expands the results of the previous studies. Focal radiotherapy allowed a significant reduction of seizure frequency in a high proportion of patients (72% in the whole series and 76% in grade II patients) by 3 months after the end of treatment. The maximum effect of radiation on seizures manifested early and did not increase significantly over time. Because 6 of the 9 patients lost at follow-up already had a response at 3 months, we can hypothesize that this loss has not biased our results at longer time points.
The maximum tumor response on MRI was observed at 3 months both for the whole population (42%) and for patients with grade II tumors (45%). We cannot exclude the possibility that some of the 9 patients lost to follow-up between 3 and 12 months could have had a further tumor reduction on MRI, but this could be the case only for the 3 patients with stable disease at 3 months, because the remaining patients already had either a PR (n = 3) or PD (n = 3). Likewise, an earlier study measuring the response on CT of 21 patients with low-grade gliomas after conventional radiotherapy22 found a median time to maximum radiological response of 2.8 months and a response rate (PR) of 52%. Conversely, when the velocity of diametric expansion was measured over time, a prolonged postradiotherapy tumor volume decrease (median time, 42 months) as shown on MRI was reported in a recent series of 33 low-grade gliomas.23
Seizure reduction was observed more often among patients displaying an objective tumor response on MRI; but as reported in earlier studies,15,22 patients with no change on MRI also had a significant seizure response. The lack of a strict association between seizure reduction and MRI shrinkage is reinforced in this study by the observation that some patients who developed a tumor progression on MRI at either 6 months (n = 2) or 12 months (n = 7) did not have increased seizure frequency; the same finding has been reported in 2 recent series.3,18
The efficacy of chemotherapy with alkylating agents (eg, PCV, temozolomide) in improving seizure control in patients with low-grade gliomas is even better recognized, and the patterns of response seem similar to those reported after radiotherapy. Overall, >50% of patients receiving chemotherapy for progressive low-grade gliomas had a significant reduction in seizure frequency irrespective of radiographic tumor response.19,20,24–30 Recent studies have reported that after chemotherapy, the decrease in tumor volume is progressive, and in some instances it can be prolonged after the interruption of chemotherapy.31,32
Both tumor- and treatment-related factors affecting the response of seizures to radiotherapy have not yet been defined2 except for radiation dose, which does not seem to be important.33 In our study, timing of radiotherapy and duration of seizures prior to radiotherapy were significantly associated with seizure reduction at 3 months. In particular, a lower rate of seizure reduction was observed among patients who received early radiotherapy compared with those who received late radiotherapy (52.6% vs 87.5%). A possible explanation of this difference is that among patients who had early radiotherapy, there was a prevalence of factors negatively affecting seizure control (such as a larger tumor size after surgery) or of factors reflecting a more resistant epilepsy, such as simple partial seizures and need for multiple AEDs. All these factors could not have reached statistical significance owing to the small sample size and the retrospective nature of the study. On the other hand, these findings could suggest that in low-grade and anaplastic gliomas, salvage radiotherapy (ie, postponed at tumor progression) after either observation or chemotherapy is at least as effective as early radiotherapy in terms of seizure control; however, this cannot be interpreted as a factor in favor of delaying radiotherapy as a general strategy, because we did not analyze the correlation between timing of radiotherapy and outcome. In this regard, phase III studies in low-grade17 and anaplastic gliomas34 suggest that postponing radiotherapy at tumor progression is not detrimental for overall survival.
Achieving freedom from seizures after treatment is of utmost importance for patients with low- and intermediate-grade gliomas, in whom an extended survival can be expected, but there are no available data in the literature with regard to radiotherapy. In this study, we found that following radiotherapy, 30%–35% of patients were seizure free at 3 months and 32%–38% at 12 months. This rate is lower than that reported after surgery: a recent systematic literature review of 773 patients with low-grade gliomas across 20 series reported that 71% were completely seizure free (Engel class I) at 12 months, and gross total removal was most predictive of seizure freedom.7 In our series, most patients underwent a biopsy or partial resection due to the extension or critical location of tumors, and thus the large tumor size could have hindered the probability of seizure control after radiotherapy; future studies must address this issue. Among surgical series, in addition to gross total resection, other factors such as preoperative duration of seizures ≤1 year, preoperative seizure control on antiepileptic medication, and nonsimple partial seizures predicted seizure freedom.6,7 In this study, a long seizure duration did not preclude the possibility of achieving seizure control following radiotherapy.
None of our patients was able to discontinue AEDs; perhaps the unwillingness of clinicians to withdraw antiepileptic medication due to fear of subtle tumor progression and subsequent exacerbation of epilepsy could have played some role.
The main limitations of our study were the relatively small number of patients (especially at 12 mo), the short postradiotherapy follow-up, and the long period that was retrospectively analyzed (20 y). We could not assess very precisely the response on MRI over time; for future prospective studies or clinical trials, the recently proposed Revised Assessment in Neuro-Oncology criteria for response assessment35 must be employed, and volumetric measurements of the tumor could show a better correlation between the response of seizures and tumor shrinkage, but these methods need to be standardized and validated. Moreover, we were not able to analyze the duration of medical intractability of seizures prior to radiotherapy and the influence of intractable seizure duration on seizure reduction following radiotherapy. We did not collect information on quality of life and cognitive functions, which can be affected by both epilepsy burden36 and radiotherapy.33 Nonetheless, within the time of follow-up (12 mo), we did not observe any patient with severe cognitive decline and/or leukoencephalopathy on MRI. Another limitation of this study is that the number of patients with grade III tumors was too small to allow a separate analysis with regard to radiological response and clinical factors affecting seizure control; in any event, among these patients, the rate of seizure reduction was of the same order as that observed among patients with grade II tumors.
A last point deserves a comment: both the early appearance of seizure reduction after radiotherapy and the absence of a strict correlation with the response on MRI reinforce the hypothesis that in addition to a direct antitumor effect, ionizing radiation could decrease seizure activity by damaging epileptogenic neurons or inducing changes in the microenvironment within the peritumoral tissue.37 Why the radiation effects on seizures are seen early after treatment, while they are typically delayed with regard to tumor and normal nervous tissue, is unknown.
In conclusion, this study has shown that a high proportion of patients with medically intractable epilepsy from diffuse gliomas of low and intermediate grade of malignancy derive significant and durable benefit from conventional radiotherapy in terms of seizure control and that this positive effect is not strictly correlated with tumor shrinkage on MRI. Moreover, radiotherapy at tumor progression seems as effective as early radiotherapy after surgery.
These preliminary results need to be validated within multicenter randomized studies. Ideally, these studies should prospectively collect information on seizure characteristics and treatment and should better define the correlation between seizure response and survival.
Ultimately, an effective treatment of tumor-related epilepsy needs a multimodality approach including both AEDs and antineoplastic modalities (surgery, radiotherapy, chemotherapy), and probably a “personalized” approach will offer the best results.
Funding
None.
Conflict of interest statement. None declared.
Acknowledgments
None declared.
References
- 1.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 2.Smits A, Duffau H. Seizures and the natural history of World Health Organization grade II gliomas: a review. Neurosurgery. 2011;68:1326–1333. doi: 10.1227/NEU.0b013e31820c3419. [DOI] [PubMed] [Google Scholar]
- 3.You G, Sha ZY, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro Oncol. 2012;14:230–241. doi: 10.1093/neuonc/nor205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffau H, Capelle L, Lopes M, et al. Medically intractable epilepsy from insular low-grade gliomas: improvement after an extended lesionectomy. Acta Neurochir. 2002;144:563–572. doi: 10.1007/s00701-002-0941-6. [DOI] [PubMed] [Google Scholar]
- 5.Zaatreh MM, Firlik KS, Spencer DD, et al. Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology. 2003;61:636–641. doi: 10.1212/01.wnl.0000079374.78589.1b. [DOI] [PubMed] [Google Scholar]
- 6.Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 7.Englot DJ, Berger MS, Barbaro NM, et al. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011;115:240–244. doi: 10.3171/2011.3.JNS1153. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrand J, Lecaille C, Perennes J, et al. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65:212–215. doi: 10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- 9.Rudà R, Trevisan E, Soffietti R. Epilepsy and brain tumors. Curr Opin Oncol. 2010;22:611–620. doi: 10.1097/CCO.0b013e32833de99d. [DOI] [PubMed] [Google Scholar]
- 10.Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54:514–520. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- 11.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 12.Scerrati M, Montemaggi P, Iacoangeli M, et al. Interstitial brachytherapy for low-grade cerebral gliomas: analysis of results in a series of 36 cases. Acta Neurochir. 1994;131:97–105. doi: 10.1007/BF01401459. [DOI] [PubMed] [Google Scholar]
- 13.Warnke PC, Berlis A, Weyerbrock A, et al. Significant reduction of seizure incidence and increase of benzodiazepine receptor density after interstitial radiosurgery in low-grade gliomas. Acta Neurochir. 1997;68:90–92. doi: 10.1007/978-3-7091-6513-3_17. [DOI] [PubMed] [Google Scholar]
- 14.Schröttner O, Unger F, Eder HG, et al. Gamma-Knife radiosurgery of mesiotemporal tumour epilepsy observations and long-term results. Acta Neurochir. 2002;84:49–55. doi: 10.1007/978-3-7091-6117-3_5. [DOI] [PubMed] [Google Scholar]
- 15.Rogers LR, Morris HH, Lupica K. Effect of cranial irradiation on seizure frequency in adults with low-grade astrocytoma and medically intractable epilepsy. Neurology. 1993;43:1599–1601. doi: 10.1212/wnl.43.8.1599. [DOI] [PubMed] [Google Scholar]
- 16.Chalifoux R, Elisevich K. Effect of ionizing radiation on partial seizures attributable to malignant cerebral tumors. Stereotact Funct Neurosurg. 1997;67:169–182. doi: 10.1159/000099446. [DOI] [PubMed] [Google Scholar]
- 17.van den Bent MJ, Afra D, de Witte O, et al. EORTC radiotherapy and brain tumor groups and the UK Medical Research Council. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 18.Kahlenberg CA, Fadul CE, Roberts DW, et al. Seizure prognosis of patients with low-grade tumors. Seizure. 2012;21:540–545. doi: 10.1016/j.seizure.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1 p deletions. J Clin Oncol. 2004;22:3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 20.Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 21.Engel JJ, Van Ness PC, Rasmussen TB, et al. Outcome with respect to epileptic seizures. In: Engels JJ, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 22.Bauman G, Pahapill P, Macdonald D, et al. Low grade glioma: a measuring radiographic response to radiotherapy. Can J Neurol Sci. 1999;26:18–22. [PubMed] [Google Scholar]
- 23.Pallud J, Llitjos JF, Dhermain F, et al. Dynamic imaging response following radiation therapy predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2012;14:496–505. doi: 10.1093/neuonc/nos069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason WP, Krol GS, DeAngelis LM. Low-grade oligodendroglioma responds to chemotherapy. Neurology. 1996;46:203–207. doi: 10.1212/wnl.46.1.203. [DOI] [PubMed] [Google Scholar]
- 25.Soffietti R, Rudà R, Bradac GB, et al. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43:1066–1073. doi: 10.1097/00006123-199811000-00035. [DOI] [PubMed] [Google Scholar]
- 26.Brada M, Viviers L, Abson C, et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14:1715–1721. doi: 10.1093/annonc/mdg371. [DOI] [PubMed] [Google Scholar]
- 27.Pace A, Vidiri A, Galiè E, et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14:1722–1726. doi: 10.1093/annonc/mdg502. [DOI] [PubMed] [Google Scholar]
- 28.Lebrun C, Fontaine D, Bourg V, et al. Treatment of newly diagnosed symptomatic pure low-grade oligodendrogliomas with PCV chemotherapy. Eur J Neurol. 2007;14:391–398. doi: 10.1111/j.1468-1331.2007.01675.x. [DOI] [PubMed] [Google Scholar]
- 29.Taillandier L, Duffau H. Epilepsy and insular grade II gliomas: an interdisciplinary point of view from a retrospective monocentric series of 46 cases. Neurosurg Focus. 2009;27:E8. doi: 10.3171/2009.6.FOCUS09102. [DOI] [PubMed] [Google Scholar]
- 30.Sherman JH, Moldovan K, Yeoh HK, et al. Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg. 2011;114:1617–1621. doi: 10.3171/2010.12.JNS101602. [DOI] [PubMed] [Google Scholar]
- 31.Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61:484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- 32.Peyre M, Cartalat-Carel S, Meyronet D, et al. Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro Oncol. 2010;12:1078–1082. doi: 10.1093/neuonc/noq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiebert GM, Curran D, Aaronson NK, et al. Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). EORTC Radiotherapy Co-operative Group. Eur J Cancer. 1998;34:1902–1909. doi: 10.1016/s0959-8049(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 34.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 35.van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 36.Aaronson NK, Taphoorn MJ, Heimans JJ, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29:4430–4435. doi: 10.1200/JCO.2011.35.5750. [DOI] [PubMed] [Google Scholar]
- 37.Rudà R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis and outcome after treatments. Neuro Oncol. 2012;14:iv55–iv64. doi: 10.1093/neuonc/nos199. [DOI] [PMC free article] [PubMed] [Google Scholar]