Abstract
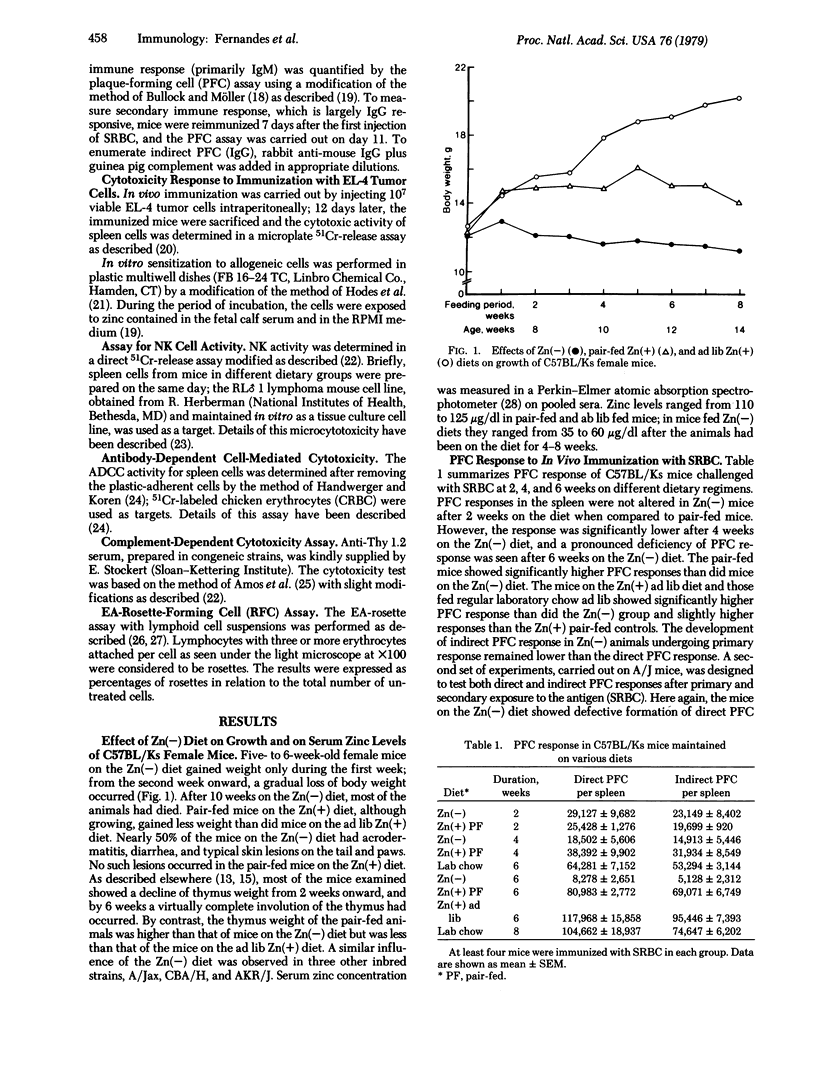
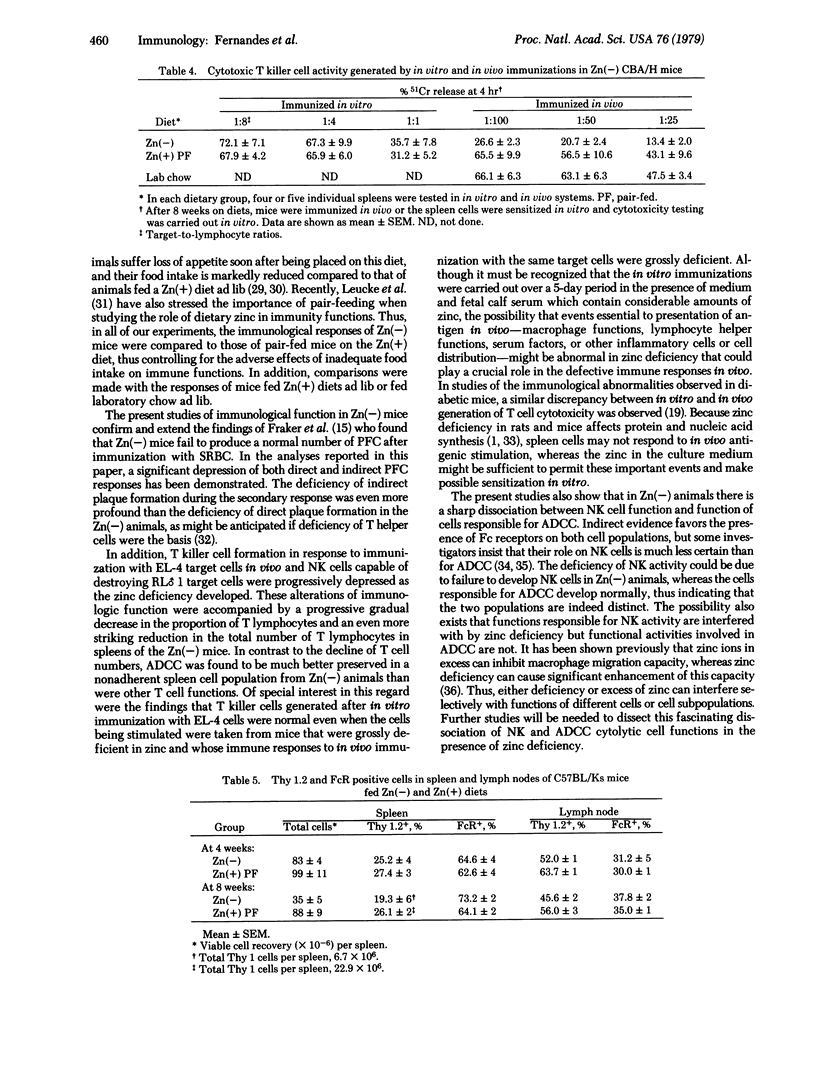
Several immunologic features were analyzed in mice on a zinc-deficient diet [Zn(-)], in mice pair-fed a diet containing zinc [Zn(+)], in mice fed a Zn(+) diet ad lib, and in mice fed laboratory chow ad lib. When placed on a Zn(-) diet, 6- to 8-week-old A/Jax, C57BL/Ks, and CBA/H mice showed loss of body weight, low lymphoid tissue weight, and profound involution of the thymus within 4-8 weeks after initiation of the regimen. Approximately 50% of the mice on the Zn(-) diet developed severe acrodermatitis enteropathica (lesions on tail and paws) and diarrhea. Pair-fed mice on the Zn(+) diet did not show any of these symptoms. Mice on the Zn(-) diet showed the following immune deficiencies: (i) depressed plaque-forming cells against sheep erythrocytes after in vivo immunization; (ii) depressed T killer cell activity against EL-4 tumor cells after in vivo immunization; and (iii) low natural killer cell activity. However, antibody-dependent cell-mediated cytotoxicity against chicken erythrocytes was normal in the mice on the Zn(-) diet. Deficiency of T killer cell activity was not observed when immunization with EL-4 allogeneic lymphoma cells was carried out in vitro. Progressive loss of relative and absolute number of Thy 1.2+ cells and a proportionate relative increase in cells bearing Fc receptors was seen in spleen and lymph nodes of Zn(-) animals. It appears that zinc is an essential element for maintenance of normal T cell and other immune functions in vivo.
Keywords: thymic atrophy, T killer cells, natural killer cells
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos D. B., Bashir H., Boyle W., MacQueen M., Tiilikainen A. A simple micro cytotoxicity test. Transplantation. 1969 Mar;7(3):220–223. doi: 10.1097/00007890-196903000-00023. [DOI] [PubMed] [Google Scholar]
- Brummerstedt E., Flagstad T., Basse A., Andresen E. The effect of zinc on calves with hereditary thymus hypoplasia (lethal trait A 46). Acta Pathol Microbiol Scand A. 1971;79(6):686–687. doi: 10.1111/j.1699-0463.1971.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. W., Möller E. "Spontaneous" B cell activation due to loss of normal mouse serum suppressor. Eur J Immunol. 1972 Dec;2(6):514–517. doi: 10.1002/eji.1830020609. [DOI] [PubMed] [Google Scholar]
- Caggiano V., Schnitzler R., Strauss W., Baker R. K., Carter A. C., Josephson A. S., Wallach S. Zinc deficiency in a patient with retarded growth, hypogonadism, hypogammaglobulinemia and chronic infection. Am J Med Sci. 1969 May;257(5):305–319. doi: 10.1097/00000441-196905000-00003. [DOI] [PubMed] [Google Scholar]
- Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci. 1973 Oct 16;13(8):1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Halberg F., Yunis E. J., Good R. A. Circadian rhythmic plaque-forming cell response of spleens from mice immunized with SRBC. J Immunol. 1976 Sep;117(3):962–966. [PubMed] [Google Scholar]
- Fernandes G., Handwerger B. S., Yunis E. J., Brown D. M. Immune response in the mutant diabetic C57BL/Ks-dt+ mouse. Discrepancies between in vitro and in vivo immunological assays. J Clin Invest. 1978 Feb;61(2):243–250. doi: 10.1172/JCI108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Age and genetic influence on immunity in NZB and autoimmune-resistant mice. Clin Immunol Immunopathol. 1976 Nov;6(3):318–333. doi: 10.1016/0090-1229(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Haas S. M., Luecke R. W. Effect of zinc deficiency on the immune response of the young adult A/J mouse. J Nutr. 1977 Oct;107(10):1889–1895. doi: 10.1093/jn/107.10.1889. [DOI] [PubMed] [Google Scholar]
- Golden M. H., Harland P. S., Golden B. E., Jackson A. A. Zinc and immunocompetence in protein-energy malnutrition. Lancet. 1978 Jun 10;1(8076):1226–1228. doi: 10.1016/s0140-6736(78)92463-7. [DOI] [PubMed] [Google Scholar]
- Golden M. H., Jackson A. A., Golden B. E. Effect of zinc on thymus of recently malnourished children. Lancet. 1977 Nov 19;2(8047):1057–1059. doi: 10.1016/s0140-6736(77)91888-8. [DOI] [PubMed] [Google Scholar]
- Greaves M., Boyde T. R. Plasma-zinc concentrations in patients with psoriasis, other dermatoses, and venous leg ulceration. Lancet. 1967 Nov 11;2(7524):1019–1020. doi: 10.1016/s0140-6736(67)90290-5. [DOI] [PubMed] [Google Scholar]
- Gupta S., Fernandes G., Nair M., Good R. A. Spontaneous and antibody-dependent cell-mediated cytotoxicity by human T cell subpopulations. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5137–5141. doi: 10.1073/pnas.75.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsted J. A., Smith J. C., Jr Plasma-zinc in health and disease. Lancet. 1970 Feb 14;1(7642):322–324. doi: 10.1016/s0140-6736(70)90701-4. [DOI] [PubMed] [Google Scholar]
- Hambidge K. M., Hambidge C., Jacobs M., Baum J. D. Low levels of zinc in hair, anorexia, poor growth, and hypogeusia in children. Pediatr Res. 1972 Dec;6(12):868–874. doi: 10.1203/00006450-197212000-00003. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Koren H. S. The nature of the effector cell in antibody-dependent, cell-mediated cytolysis (ADCC): the cytotoxic activity of murine tumor cells and peritoneal macrophages. Clin Immunol Immunopathol. 1976 Mar;5(2):272–281. doi: 10.1016/0090-1229(76)90032-5. [DOI] [PubMed] [Google Scholar]
- Henkin R. I. New aspects in the control of food intake and appetite. Ann N Y Acad Sci. 1977 Nov 30;300:321–334. doi: 10.1111/j.1749-6632.1977.tb19332.x. [DOI] [PubMed] [Google Scholar]
- Henkin R. I. Trace metals in endocrinology. Med Clin North Am. 1976 Jul;60(4):779–797. doi: 10.1016/s0025-7125(16)31861-2. [DOI] [PubMed] [Google Scholar]
- Hodes R. J., Handwerger B. S., Terry W. D. Synergy between subpopulations of mouse spleen cells in the in vitro generation of cell-mediated cytotoxicity: evidence for the involvement of a non-T cell. J Exp Med. 1974 Dec 1;140(6):1646–1659. doi: 10.1084/jem.140.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M., Kabiel A., el-Khateeb S., Aref K., el-Lozy M., Jahin S., Nasr F. Plasma and red cell water and elements in protein-calorie malnutrition. Am J Clin Nutr. 1974 Mar;27(3):260–267. doi: 10.1093/ajcn/27.3.260. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Haller O. Natural killer cells in the mouse: an alternative immune surveillance mechanism? Contemp Top Immunobiol. 1978;8:171–201. doi: 10.1007/978-1-4684-0922-2_6. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Hudson L., Sprent J. Fc-receptors, Ia-antigens, and immunoglobulin on normal and activated mouse T lymphocytes. J Exp Med. 1975 Dec 1;142(6):1403–1415. doi: 10.1084/jem.142.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGG S. P., SEARS L. Zinc sulphate treatment of parakeratosis in cattle. Nature. 1960 Jun 25;186:1061–1062. doi: 10.1038/1861061a0. [DOI] [PubMed] [Google Scholar]
- Luecke R. W., Olman M. E., Baltzer B. V. Zinc deficiency in the rat: effect on serum and intestinal alkaline phosphatase activities. J Nutr. 1968 Mar;94(3):344–350. doi: 10.1093/jn/94.3.344. [DOI] [PubMed] [Google Scholar]
- Luecke R. W., Simonel C. E., Fraker P. J. The effect of restricted dietary intake on the antibody mediated response of the zinc deficient A/J mouse. J Nutr. 1978 May;108(5):881–887. doi: 10.1093/jn/108.5.881. [DOI] [PubMed] [Google Scholar]
- MacMahon R. A., Parker M. L., McKinnon M. C. Zinc treatment in malabsorption. Med J Aust. 1968 Aug 3;2(5):210–212. doi: 10.5694/j.1326-5377.1968.tb82733.x. [DOI] [PubMed] [Google Scholar]
- McConnell S. D., Henkin R. I. Altered preference for sodium chloride, anorexia, and changes in plasma and uninary zinc in rats fed a zinc-deficient diet. J Nutr. 1974 Sep;104(9):1108–1114. doi: 10.1093/jn/104.9.1108. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Sprent J. Cell-to-cell interaction in the immune response. VI. Contribution of thymus-derived cells and antibody-forming cell precursors to immunological memory. J Exp Med. 1971 Jul 1;134(1):66–82. doi: 10.1084/jem.134.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan E. J. Letter: Acrodermatitis enteropathica: a lethal inherited human zinc-deficiency disorder. Lancet. 1974 Aug 17;2(7877):399–400. doi: 10.1016/s0140-6736(74)91772-3. [DOI] [PubMed] [Google Scholar]
- Moynahan E. J. Letter: Zinc deficiency and cellular immune deficiency in acrodermatitis enteropathica in man and zinc deficiency with thymic hypoplasia in fresian calves: a possible genetic link. Lancet. 1975 Oct 11;2(7937):710–710. doi: 10.1016/s0140-6736(75)90810-7. [DOI] [PubMed] [Google Scholar]
- Swenerton H., Hurley L. S. Severe zinc deficiency in male and female rats. J Nutr. 1968 May;95(1):8–18. doi: 10.1093/jn/95.1.8. [DOI] [PubMed] [Google Scholar]
- TUCKER H. F., SALMON W. D. Parakeratosis or zinc deficiency disease in the pig. Proc Soc Exp Biol Med. 1955 Apr;88(4):613–616. doi: 10.3181/00379727-88-21670. [DOI] [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]



