Abstract
Background
Resection of gliomas located in eloquent brain areas remains a neurosurgical challenge. The reported incidence of transient or permanent neurological deficits after microsurgery in eloquent brain ranges 20%–100%, or 0%–47% among contemporary neurosurgical series. The aim of this study was to assess the feasibility of stereotactic brachytherapy (SBT) as a local treatment alternative to microsurgical resection for patients with gliomas in highly eloquent areas, located in the central sulcus region (CSR).
Method
Between 1997 and 2010, 60 patients with World Health Organization (WHO) grades II and III gliomas located in the CSR were treated with SBT (iodine-125 seeds; cumulative therapeutic dose, 50–65 Gy). Following SBT, WHO grade III glioma patients additionally received percutaneous radiotherapy (median boost dose, 25.2 Gy). We evaluated procedure-related complications, clinical outcome, and progression-free survival.
Results
Procedure-related mortality was zero. Within 30 days of SBT, 3 patients (5%) had transient neurological deficits, and 8 patients (13%) had temporarily increased seizure activity. One patient (1.6%) deteriorated permanently. Space-occupying cysts (6 patients) and radiation necrosis (1 patient) developed after a median of 38 months and required surgical intervention. Seizure activity, rated 12 months following SBT, decreased in 82% of patients (Engel classes I–III). Median progression-free survivals were 62.2 ± 19.7 months (grade II gliomas) and 26.1 ± 17.9 months (grade III gliomas).
Conclusions
Compared with microsurgical resection, SBT harbors a low risk of procedural complications, is minimally invasive, and seems to be an effective local treatment option for patients with inoperable, eloquent WHO grade II and III gliomas in the CSR. However, the value of SBT for treating gliomas still needs to be determined in prospective, randomized studies.
Keywords: central sulcus region; eloquent gliomas; stereotactic iodine-125 brachytherapy, SBT
Microsurgical resection is considered a critical therapeutic modality for patients diagnosed with gliomas, and growing evidence suggests that patients' survival correlates with the extent of resection.1,2 However, these tumors are frequently located in or involve eloquent brain (eg, sensory-motor or language areas, basal ganglia, or subcortical pathways),3 limiting the neurosurgical attempt of a complete or gross total resection of these tumors.4 In this context, contemporary neurosurgical series report transient and permanent neurological deficits ranging 20%–100%5,6 and 0%–47%,6,7 respectively, after resections in eloquent brain.
Stereotactic brachytherapy (SBT) with implantation of iodine-125 (125I) seeds represents a safe and effective local treatment option for selected patients, including those with circumscribed tumors located in eloquent brain.8–10 Histological evaluation within the same operative session, precise treatment of the tumor, maximal sparing of surrounding healthy tissue, minimal rate of long-term complications, and preservation of the whole therapeutic spectrum in case of tumor progression (eg, re-implantation, external beam radiotherapy [EBRT]) are described hallmarks of SBT.9–11
In the present study, we focused on the application of SBT for gliomas located in sensory-motor areas, which are primarily localized at the pre- and postcentral gyrus, also known as the central sulcus region (CSR).12 We assessed the feasibility of SBT as a local treatment regimen by evaluating the patient's clinical and oncological outcome, and compared our results with those in the literature reported for microsurgical series in the CSR.
Material and Methods
Indications for SBT
Treatment was carried out according to a prospective protocol.9,10 In brief, patients were eligible (i) when tumors were considered inoperable due to localization in eloquent brain; (ii) for treatment of residual tumors after subtotal resection; and/or (iii) with relapse of previously treated tumors. In addition, a KPS score ≥60 and a well-circumscribed tumor of ≤40 mm were requested. Tumors displaying contrast enhancement were well circumscribed if their calculated size was the same or nearly the same in contrast enhanced T1- and T2-weighted MRI.11,13 Non-enhancing tumors were defined as well circumscribed based exclusively on T2-weighted MRI. An interdisciplinary tumor board confirmed every indication. Either parents or the patients themselves if ≥18 years old signed informed consent after detailed explanation of procedure-related risks and agreed that their data could be used for scientific evaluation.
Selection of Study Population
For the present study, all patients who had undergone SBT for inoperable World Health Organization (WHO) grades II and III gliomas in the time period 1997–2010 were reviewed; almost 500 patients were identified. In a second step, preoperative MR images (T1- and T2-weighted sequences) were reviewed to identify the tumor location within the CSR. The central sulcus was used as the anatomical reference, either identified directly or by the ascending cingulate sulcus visible in the midline sagittal image to define the pre- and postcentral gyrus from this point. This analysis was independently performed by 2 investigators with experience in neurosurgical oncology (M.I.R., S.G.). Only those cases where both investigators confirmed the tumor location within the CSR were included in the present study.
Treatment Planning and Surgical Procedure
Surgical procedures were carried out as described previously.9,10,14,15 In brief, under general anesthesia a modified Riechert–Mundinger stereotactic frame was fixed on the patient's head and an intraoperative stereotactic CT was performed. Thereafter, CT scans were fused with preoperative contrast enhanced axial T1- and T2-weighted MR images. For image fusion and planning of the trajectory and irradiation treatment, we used specialized software (STP3, Stryker Leibinger). The radiation dose prescribed at the target volume surface (median dose, 50 Gy) was delivered as low-dose irradiation with an initial dose rate of 0.75 Gy/d. Permanent implants were used, meaning that the catheters were left in situ even after the decay of activity. Patients with WHO grade III gliomas additionally received EBRT (median boost dose, 25.2 Gy in fractions of 1.8 Gy) during the first 3 weeks following SBT.16 The rationale for this treatment protocol was to increase the effectiveness of SBT using a daily boost of external irradiation to inhibit proliferation of tumor cells during the protracted low-dose irradiation.17,18
Follow-up
Follow-up examinations were carried out as described previously.9,10 In brief, clinical and neuroradiological follow-up examinations were performed 3 months after SBT and then at 6- to 12-month intervals.
The treatment response after SBT was estimated similar to the Revised Assessment in Neuro-Oncology criteria19 at the time of the last follow-up or at the time of an assumed tumor progression. In detail, we colocalized follow-up MRIs with treatment planning data to colocalize image changes with tumor extension prior to SBT and dose distribution. Briefly, tumor size was determined by the product of the 2 largest perpendicular diameters of the T2 hyperintense lesion in non-enhancing tumors. In tumors displaying contrast enhancement, response criteria considered both the size of the T2 hyperintense lesion and any change of the enhanced T1-weighted images. A complete disappearance of the lesion on MRI (contrast enhanced T1-weighted images and T2-weighted images) was defined as complete response; a tumor volume reduction >50% in both non-enhancing and enhancing lesions (compared with baseline) was considered a partial response. A tumor volume increase >25% (T2-weighted images or contrast enhanced T1-weighted images) outside the volume enclosed by the therapeutic isodose was defined as tumor progression, whereas a tumor volume increase (T2-weighted images or contrast enhanced T1-weighted images) inside the therapeutic isodose was defined as a breakdown of the blood–brain barrier caused by SBT if these tumors did not progress within 6 months after this finding. Otherwise, treatment response was classified as tumor control (stable disease). The biological significance of radiological susceptive tumor progression was determined noninvasively by PET imaging20,21 or close MRI follow-up, or by stereotactic biopsy (or resection).
The patients’ functional outcome was rated 12 months after SBT by using the modified Rankin Scale.9,10,22 In addition, seizure frequency outcome was evaluated according to Engel classification.23
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 20.0. Kaplan–Meier estimates were used to assess progression-free survival (PFS) and overall survival (OS). Patients not experiencing progression were censored at their last follow-up. The chi-square test was applied to identify differences in baseline clinical and epidemiological characteristics between the WHO grades II and III glioma subgroups. Univariate analysis (log-rank test) was performed to identify possible covariates with an influence on PFS. The following covariates were included in the analysis: sex, KPS (<90 vs ≥90), tumor volume (≤ 10 vs >10 mL), WHO grade (II vs III), histology (astrocytoma vs oligoastrocytoma or oligodendroglioma), and previous treatment (yes/no). In the next step, factors identified as significant were included in a multivariate analysis (Cox proportional hazards model). The chi-square test was used to identify predictive factors for patients developing radiation-induced cysts. P < .05 was considered significant. In detail, the factors evaluated regarding the occurrence of radiation-induced cysts were WHO grade, tumor volume, number of implanted seeds/catheters, and seed activity.
Results
Sixty consecutive patients (n = 30 with WHO grade II and n = 30 with WHO grade III gliomas) fulfilled the selection criteria of this study. Baseline epidemiological and clinical parameters are outlined in Table 1. None of these parameters differed significantly between the grade II and grade III subgroups. Most of the patients (77%) received SBT as primary treatment. For (re)evaluation of histology, the majority of patients (92%, n = 55) underwent stereotactic biopsy and SBT within the same surgical procedure. The remaining 5 patients (8%) received a combined procedure of partial tumor resection and SBT, where histological evaluation was performed at the time of surgery.
Table 1.
Baseline epidemiological and clinical characteristics
| Parameter | Grade II Gliomas | Grade III Gliomas |
|---|---|---|
| Number of patients | 30 | 30 |
| Sex, n (%) | ||
| Female | 10 (33) | 10 (33) |
| Male | 20 (67) | 20 (67) |
| Age, y | ||
| Median (range) | 36 (16–77) | 42 (9–75) |
| KPS | ||
| Median (range) | 90 (60–100) | 90 (70–100) |
| Symptoms prior to SBT, n (%) | ||
| Seizures | 25 (83) | 25 (83) |
| Motor deficits | 5 (17) | 7 (23) |
| Sensory deficits | 3 (10) | 3 (10) |
| Speech disorder | 2 (7) | 2 (7) |
| Histology, n (%) | ||
| Astrocytoma | 27 (90) | 22 (73) |
| Oligoastrocytoma | 1 (3) | 5 (17) |
| Oligodendroglioma | 2 (7) | 3 (10) |
| Indication for SBT, n (%) | ||
| Primary treatment | 23 (77) | 23 (77) |
| Stand-alone | 21 (70) | 20 (67) |
| Combined with partial Rx | 2 (7) | 3 (10) |
| Progression after Rx | 7 (23) | 7 (23) |
| Volume of tumor, mL | ||
| Median (range) | 21 (3–50) | 16 (8–56) |
Abbreviation: Rx, resection.
Irradiation Parameters
All patients received permanent implantation of 125I seeds with a median surface dose of 50 Gy (range, 50–65 Gy). The median calculated coverage was 97.01% (mean, 96.03 ± 3.83%; range, 79.77%–100.00%). The median activity of the implanted seeds was 8.7 ± 4.1 mCi (range, 3.0–26.9 mCi). On average we inserted 4.0 seeds (SD 2.0; range, 1–10) and 1.8 catheters (SD 0.8; range, 1–3) in order to use as few catheters as possible. Following our protocol, 29 of 30 patients with WHO grade III gliomas received concurrent percutaneous radiotherapy (median boost dose, 25.2 Gy; range, 21.6–30.6 Gy, in fractions of 1.8 Gy) within 3 weeks after SBT. One patient died prior to scheduled percutaneous radiotherapy due to rapidly developing erysipelas of the leg.
Oncological and Clinical Outcome
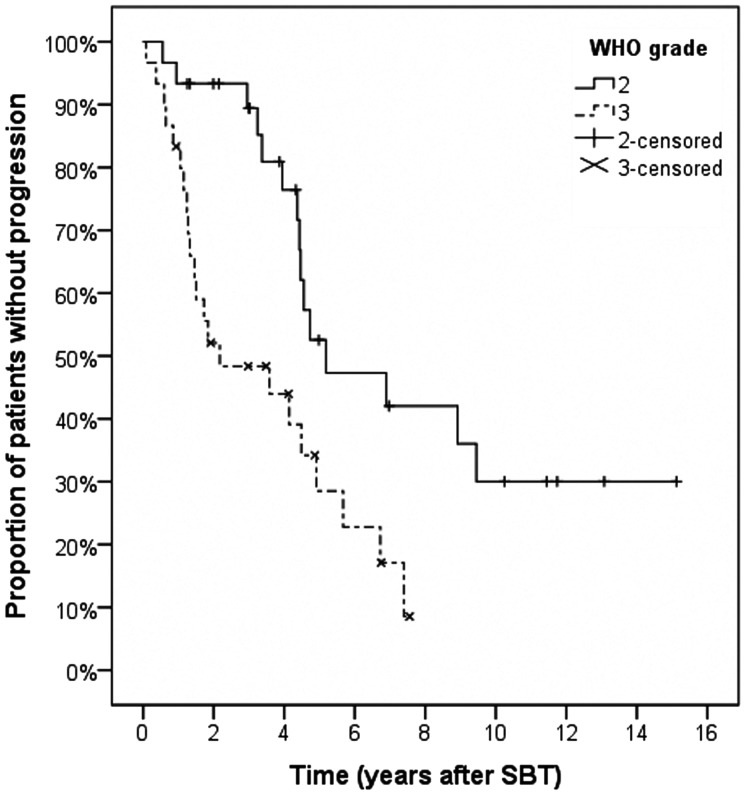
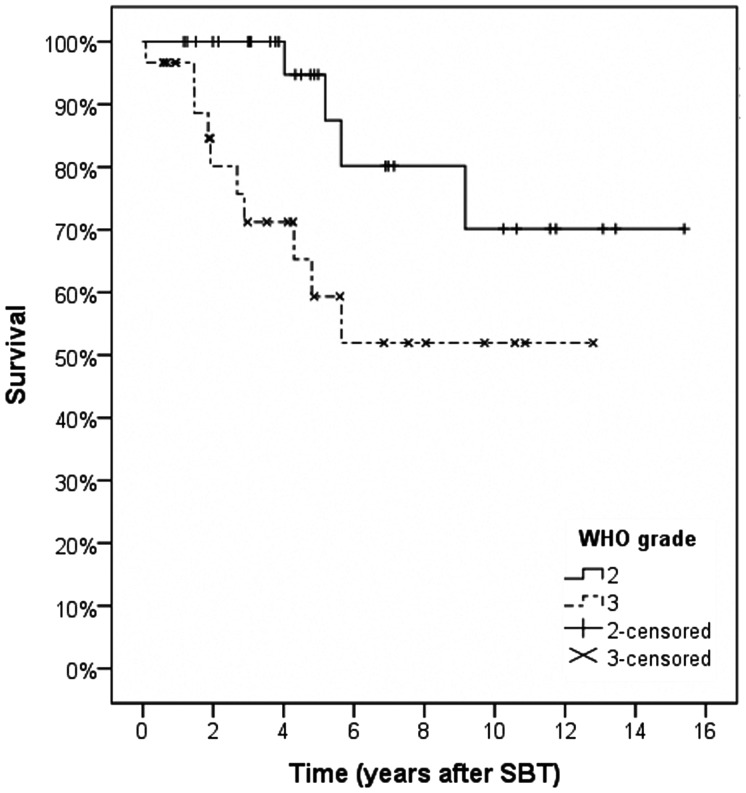
Outcome characteristics, including details of procedure-related complications, radiological and neurological outcomes, and salvage treatment procedures, are listed in Table 2. Patients were followed for a median of 58 months (grade II gliomas) or 39 months (grade III). Median PFS for grade II gliomas was 62.2 ± 19.7 months; actuarial PFS rates were 93.3 ± 4.6% (1 and 2 y) and 52.5 ± 10.6% (5 y). In contrast, patients with grade III gliomas achieved a median PFS of 26.1 ± 17.9 months; actuarial PFS rates were 83.3 ± 6.8%, 52.1 ± 9.3%, and 34.2 ± 9.6% at 1, 2, and 5 years (Fig. 1). The corresponding actuarial OS rates after 1, 2, and 5 years were 100.0 ± 0.0%, 100.0 ± 0.0%, and 94.7 ± 5.1% for grade II gliomas and 96.7 ± 3.3%, 80.1 ± 8.0%, and 59.4 ± 10.9% for grade III gliomas (Fig. 2).
Table 2.
Details on procedural morbidity and radiological and neurological outcomes
| Parameter | Grade II Gliomas | Grade III Gliomas |
|---|---|---|
| Radiological response at last follow-upA1 | ||
| Complete remission, n (%) | 1 (3) | 0 (0) |
| Partial remission, n (%) | 6 (20) | 2 (7) |
| Stable disease, n (%) | 9 (30) | 8 (27) |
| Progressive disease, n (%) | 14 (47) | 20 (67) |
| Time to progression, mo Median | 52.8 ± 30.4 | 19.4 ± 26.1 |
| Range | 6.6–113.4 | 4.4–88.7 |
| Procedure-related morbidity | ||
| Early morbidity (30-day postop), n (%) | 8 (27) | 3 (10) |
| Transient | 1 (3) | 1 (3) |
| Motor deficits | 0 (0) | 1 (3) |
| Language deficits | 7 (23) | 1 (3) |
| Increased seizure activity | 0 (0) | 1 (3) |
| Permanent | 0 (0) | 1 (3) |
| Motor deficits | 4 (13) | 1 (3) |
| Delayed morbidity after SBT, n (%) | 1 (3) | 1 (3) |
| Due to radiation-induced cysts | 3 (10) | 0 (0) |
| Transient | 1 (3) | 0 (0) |
| Increased seizure activity | 1 (3) | 0 (0) |
| Motor deficits | 0 (0) | 1 (3) |
| Permanent | 0 (0) | 1 (3) |
| Motor deficits | ||
| Due to radiation necrosisA2 | ||
| Transient | ||
| Increased seizure activity | ||
| Clinical outcome, rated 12 mo after SBT | ||
| Neurological outcomeA3,4 | 14 (47) | 11 (38) |
| Improved | 12 (40) | 12 (40) |
| Stable | 4 (13) | 6 (20) |
| DeterioratedA5 | 12 (48) | 11 (44) |
| Seizure outcome (Engel classification2)A6 | 4 (16) | 4 (16) |
| I, free of disabling seizures | 4 (16) | 6 (24) |
| II, rare disabling seizures | 5 (20) | 4 (16) |
| III, meaningful seizure improvement | ||
| IV, no improvement or worseningA7 | ||
| Salvage treatment after progression, n (%) | ||
| Microsurgery | 9 (30) | 4 (13) |
| Stand-alone tumor resectionA8 | 6 (20) | 0 (0) |
| Combined with adjuvant RT and/or chemotherapyA9 | 3 (10) | 3 (10) |
| Combined with SBT | 0 (0) | 1 (3) |
| RT and/or chemotherapy | 3 (10) | 10 (33) |
| SBT | 1 (3) | 2 (7) |
Annotations: A1 = estimated similar to the Revised Assessment in Neuro-Oncology criteria.19 A2 = developed 19 mo after receiving SBT (50 Gy) + EBRT boost (25.2 Gy) as primary treatment for grade III glioma. Followed for a period of 73 months, the patient is still alive; follow-up MRI showed tumor control not requiring any further treatment. A3 = rated in 59/60 patients. One patient (grade III subgroup) died within the first month after SBT due to rapidly developing erysipelas (non-procedure-related mortality) and was therefore excluded from this analysis. A4 = including 5 patients with a follow-up period of <12 mo, where outcome was assessed at the latest follow-up. A5 = due to tumor progression (n = 8), early postoperative deterioration (n = 1), radiation-induced cysts (n = 1). A6 = rated in 50/60 patients; 10 patients (5 each from the grade II and III subgroups) were excluded, as they did not show seizures. A7 = worsening was related to tumor progression (n = 4) or (transient) peritumoral edema (n = 2); A8 = (sub)total tumor resection in each case; postoperative hemiparesis reported for 3 patients; A9 = partial tumor resection in each case.
Fig. 1.
Kaplan–Meier estimates for progression-free survival.
Fig. 2.
Kaplan–Meier estimates for overall survival.
Procedure-related mortality was zero. Within 30 days after SBT, 11 patients (18%) showed transient deterioration of clinical status and 1 patient (2%) showed permanent deterioration of clinical status. Space-occupying cysts occurred in 6 patients after a median of 38 months (range, 12–97 mo) and required surgical intervention. Following intervention, the neurological status improved to baseline in 5 patients, while 1 patient remained unchanged regarding his motor deficit. One grade III glioma patient developed a symptomatic radiation necrosis 19 months after SBT (50 Gy) + EBRT boost (25.2 Gy) and required microsurgical intervention.
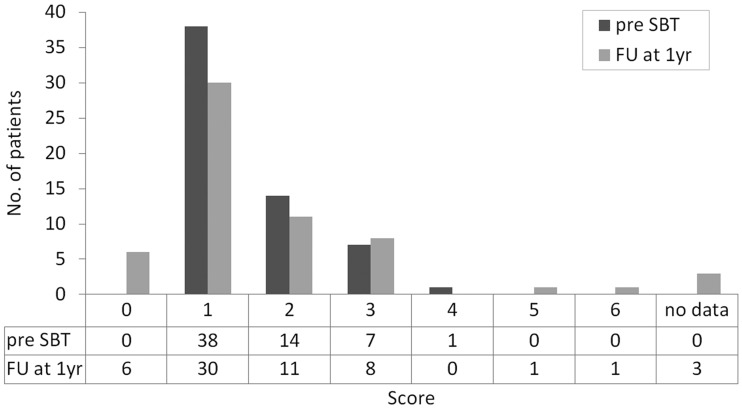
Neurological status improved or remained stable for the majority of patients (83%). Worsening (17%) was related to tumor progression (n = 8), early postoperative deterioration (n = 1), and radiation-induced cysts (n = 1). These results were reflected by the modified Rankin Scale displaying the patients’ functional outcomes (Fig. 3). Seizure activity, rated 12 months following SBT, decreased in 82% of patients (Engel classes I–III).
Fig. 3.
Improved functional outcome rated 12 mo following SBT using the modified Rankin Scale—0: no symptoms at all; 1: no significant disability despite symptoms, able to carry out all usual activities; 2: slight disability, unable to carry out all previous activities but able to look after own affairs without assistance; 3: moderate disability, requiring some help but able to walk without assistance; 4: moderate severe disability, unable to walk without assistance and unable to attend to own bodily needs without assistance; 5: severe disability, bedridden, incontinent, and requiring constant nursing care and attention; 6: dead. FU, follow-up.
Prognostic Factors
Univariate analysis of covariates showed that neither gender, age, KPS, previous treatment, treatment date, nor treatment volume proved to be statistically significant for PFS. The variables of grade III and histological diagnosis of “astrocytoma” were significantly associated with reduced PFS (P = .003 and P = .015). On multivariate analysis, both variables retained independent prognostic significance. There was no association between the risk for developing space-occupying cysts and the variables of WHO grade, tumor volume, number of implanted seeds/catheters, and seed activity.
Discussion
Here we present a unique series of 60 patients treated with SBT for grades II and III gliomas located in the CSR. We focused on feasibility, procedural safety, and oncological efficacy of SBT applied in a highly eloquent area of the brain. SBT represents a strictly localized treatment option for well-circumscribed tumors; its objective, as well as that of microsurgical resection, is devitalization/removal of the dense (visible) portion of tumor cells by delivering a lethal irradiation dose (≥200 Gy in the vicinity of the implanted source) from within the tumor while optimally sparing the surrounding tissue. Thus, we compared our treatment results with those reported for microsurgical resection.
The neurosurgical management of gliomas located in eloquent brain remains a controversial issue for specific reasons: (i) radical resection (complete or gross total) seems to have an oncological impact1,2 but (ii) also harbors a high risk for neurological disability, which in turn may have a significant impact on the patient's further oncological treatment course. We therefore performed a literature review to determine an accurate risk profile for microsurgical tumor resection in eloquent brain (focusing on tumors in the CSR). Eleven studies covering 943 patients were identified (Table 3). A gross total resection was achieved in 61% of cases. The cumulative overall risk for postoperative deficits was 32%, and in 9% of patients these were permanent. In the present study, the 30-day postoperative morbidity was 20%. Taking into account both early postoperative deterioration and deterioration over the long term, permanent neurological deficits occurred in only 3% of our patients, which is substantially lower than the reported incidence from surgical groups (permanent neurological deficits in 9% of cases). These data clearly underline the safety of SBT for gliomas in highly eloquent brain such as the CSR and are in line with previous data on SBT for eloquent brain tumors.9,10,13,14 Besides its safety, strong indicators for the efficacy of this treatment are oncological outcome data (median PFS, 62 mo for grade II gliomas, 26 mo for grade III gliomas), as well as clinical outcome data (improvement or stabilization of the neurological status in 83% of patients, decreased seizure activity in 82%) (Fig. 4).
Table 3.
Resection of gliomas located in the CSR, review of literature
| Study | N | Histology | Location | GTR | Postoperative Neurological Deficits | Oncological Outcome |
|---|---|---|---|---|---|---|
| Cedzich et al36 | 99 | 55% gliomas, 19% angiomas/cavernomas, 12% metastases, 14% others | 100% CSR | 71% | Postop: n.r.; permanent: 17% (impairment of motor function) | n.r. |
| Duffau et al37 | 60 | 52% LGG, 22% HGG, 15% metastases, 12% others | 70% CSR, 23% perisylvian (dominant), 7% deep-seated | 51% | Postop: 52%; permanent: 5% | n.r. |
| Duffau et al4 | 6 | 100% LGG | 100% CSR (100% primary somatosensory area) | 83% | Transient: 100%; permanent: 0% | n.r. |
| Duffau et al38 | 8 (SP) | 100% LGG | 100% CSR | 25% | Postop: 63%; permanent: 13% (severe motor deficit after 3 mo) | n.r. |
| Duffau et al4 | 12 (SP) | 100% LGG | 100% CSR (67% primary somatosensory area, 33% primary motor area) | n.r. | Postop: 100% (sensory loss: 67%/central facial palsy: 25%/hypophonia: 8%); permanent: 17% (mild residual dysesthesias) | n.r. |
| Keles et al5 | 294 | 59% HGG, 41% LGG | Rolandic (CSR) and perirolandic lesions (within 2 cm of the rolandic cortex) | n.r. | Postop: 20% (new motor deficit); permanent: 5% (persistent motor deficit after 3 mo) | n.r. |
| Carrabba et al39 | 146 | 60% LGG, 40% HGG | 30% CSR; 70% also involving language areas or pathways | 65% | Postop: 42% (new motor deficits); permanent: 5% (new motor deficits after 1 mo) | n.r. |
| Shinoura et al40 | 18 | 61% metastases, 33% gliomas, 6% others | 100% CSR (primary motor area) | 56% | Postop: 50%; permanent: 6% | n.r. |
| González-Darder et al7 | 17 | 41% LGG, 41% HGG, 18% metastases | 41% CSR, 18% superior frontal gyrus, 12% frontal operculum, 29% subcortical (60% in the corona radiata; 40% in the insular white matter) | 47% | Postop: 71% (new motor deficit); permanent: 47% (persistent motor deficit after 1 mo) | n.r. |
| Talacchi et al41 | 171 | 100% HGG | 55% CSR, 45% insular | 54% | Postop: 24% (worsened motor function); permanent: 8% (worsened motor function after 4 mo) | n.r. |
| Krieg et al42 | 112 | 82% HGG, 18% LGG | 34% CSR, 34% adjacent to the corticospinal tract, 32% frontodorsal (close to the anterior part of the internal capsule) | 69% | Postop: 30% (new motor deficit); permanent: 13% (new permanent deterioration of motor function), stratified by histology: LGG: 0%, HGG: 14%; stratified by location: precentral gyrus: 39%, postcentral gyrus: 5%, frontal to precentral gyrus: 5%, corticospinal tract: 8% | Mean PF-FU: 9.7 mo |
| Cumulative rates | 943 | 61% (390/637) | Postop: 32% (270/844); permanent: 9% (82/943) | |||
| This series (SBT) | 60 | 50% LGG, 50% HGG | 100% CSR | n.a. | Postop: 18% (11/60); permanent: 3% (2/60)* | Median PFS: 62 mo (LGG), 26 mo (HGG) |
Abbreviations: HGG, high-grade glioma; LGG, low-grade glioma; n.a., not applicable; n.r., not reported; PF-FU: progression-free follow-up; SP, data extracted/calculated from a subpopulation.
* = permanent deterioration in one patient immediately postoperative, in the other patient due to radiation-induced cysts 38 mo after SBT.
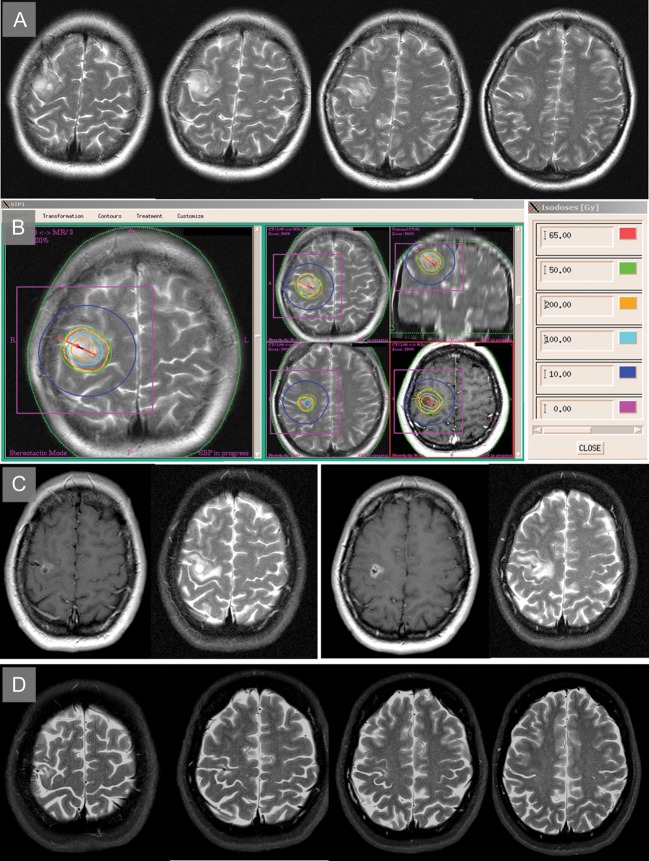
Fig. 4.
T2-weighted images of a 36-year-old neurologically intact female patient presenting in 2002 with secondary generalized motor seizures, showing a non–contrast enhancing tumor involving the precentral gyrus. A stereotactic biopsy revealed an astrocytoma of WHO grade II. (B) After outlining the outer boundary of the visible tumor (yellow dotted line), one catheter containing 3 iodine-125 seeds (red line) was inserted using a precentral stereotactic approach. The green line represents the therapeutic isodose (50 Gy, permanent implantation, coverage 98.7%). In the following 3 months, she suffered an increased frequency of focal motoric seizures, which could then be controlled with anticonvulsive medication. (C) Two years later, MRI showed partial response of the visible tumor on T2-weighted images and the typical temporary blood–brain barrier breakdown resulting in contrast enhancement in the T1-weighted images. (D) In June 2013, the patient was without any neurological deficit, was free from seizures, and showed complete remission of the tumor.
Is There a Place for SBT in the Management of Grade II Gliomas?
According to the guidelines from the European Federation of Neurological Societies and the European Association of Neuro-Oncology, surgical resection represents the first treatment option for grade II gliomas, with the goal of maximally resecting the tumor mass whenever possible while minimizing postoperative morbidity.24 When surgery is not feasible due to the location in eloquent brain, a biopsy should be performed to obtain histological proof of malignancy. For these patients, as well as those with incompletely resected tumors, adjuvant treatment is indicated, usually in the form of EBRT.
Based on these guidelines, a subpopulation of patients can be identified: those with tumors located in eloquent brain receiving incomplete tumor resection or biopsy followed by postoperative EBRT. However, the following points should be considered when applying this treatment regimen in this specific subgroup:
-
Surgery is a critical therapeutic modality for patients with grade II gliomas, where the extent of tumor resection is a strong prognostic factor, determining the patient's oncological outcome.1 Today, the aim of surgery should be complete resection of the preoperative T2/fluid attenuated inversion recovery signal abnormality while simultaneously avoiding operation-related neurological deficits in order to improve survival and quality of life. However, particularly those patients with tumors located in eloquent brain (eg, the CSR) should undergo critical preoperative evaluation, since they are at high risk for both incomplete tumor resection and postoperative neurological deficits. This is underlined by our literature review (Table 3), revealing that only 61% of tumors located in eloquent areas are amenable to complete resection and that postoperative neurological deficits occur in 32% (transient) and 9% (permanent) of cases. SBT, as well as surgery, is a strictly local treatment regimen that has been shown to be associated with only minimal postoperative morbidity, even for tumors located in highly eloquent brain.9,10,13 Furthermore, from an oncological point of view, SBT has been reported to be as effective as surgery in patients with grade II gliomas.11,25 Kreth et al11 compared grade II patients receiving either SBT (n = 239) or microsurgery (n = 108) as primary treatment. The authors observed no difference in terms of PFS or time to malignant transformation between the 2 treatment groups. Moreover, a comparison of survival curves after SBT (n = 239) with those of surgery + EBRT (n = 150 from the European Organisation for Research and Treatment of Cancer [EORTC] study 2284426) in patients with grade II gliomas showed nearly identical results.11,26 The results from our present study (median PFS, 62 mo) compare well with those reported for grade II gliomas treated with surgery + EBRT (eg, median PFS in the EORTC 22844 and 22845 trials: 60 and 64 mo, respectively, for surgery + EBRT27,28). Interestingly, the reported median PFS of 41 months in the EORTC 22845 trial for patients undergoing surgery without EBRT is far below the median PFS in our study (62 mo). However, whether the improved PFS in our study was based simply on favorable selection criteria for SBT (ie, its limitation for well-circumscribed tumors ≤4 cm in diameter) or by the treatment itself remains to be determined in future studies.
Despite its efficacy and safety, the application of SBT is, however, limited to well-circumscribed tumors ≤4 cm, since tumors beyond this threshold pose a significantly increased risk for radiation-induced side effects.29 In contrast, the extent of surgery is usually determined and limited by the tumor location itself, with joint goals of maximal tumor resection and preserving the patient's neurological function. Taking into account limitations of both SBT and surgery, a planned combined approach using these treatment modalities (low-risk partial tumor resection followed by SBT for the residual tumor) seems logical for large tumors (>4 cm) located in eloquent brain. This approach has already been reported as a feasible and safe treatment concept for patients with large, grade II gliomas located in eloquent areas.12,14,29 In the present study, 5/60 patients (8%) were successfully treated with this combined concept.
Given the long course of the disease, with more than one third of grade II glioma patients surviving at least 10 years,30 timing and application of EBRT need to be planned carefully, since there is a substantial risk of acquiring late or delayed radiation injuries. This is underlined by a recent study by Douw et al,31 who evaluated the cognitive abnormalities in survivors of grade II gliomas at a mean of 12 years after first diagnosis. They reported that deterioration of cognitive function in patients receiving EBRT was twice as high compared with those who were EBRT naive (53% vs 27%). The authors recommended that deferring EBRT might be the strategy most beneficial to cognitive status and quality of life. We therefore suggest that patients with grade II gliomas located in eloquent brain be considered as candidates for SBT instead of receiving incomplete tumor resection/biopsy followed by upfront EBRT. Although structures involved in memory and cognition (eg, the hippocampus) are less likely to be exposed to EBRT for gliomas located in the CSR, the occurrence of late or delayed radiation injuries for brain tumors in general seems—despite only limited available data9,10—less likely for SBT compared with EBRT. Since the irradiation source is directly placed into the tumors, this results in a very steep dose fall-off toward the periphery within a millimeter range, which substantially reduces the radiation burden on surrounding tissue.7,8 Therefore, late or delayed radiation injuries may be avoided for those patients receiving SBT. It furthermore allows deferring EBRT until the time of tumor progression, since previous SBT does not limit or hinder the application of EBRT.8,9,12,14
Is There a Place for SBT in the Management of Grade III Gliomas?
According to the German Neuro-oncology Working Group NOA-04 trial, the standard of care for newly diagnosed grade III gliomas is surgery followed by EBRT or chemotherapy.32 This phase III study showed no difference between the 2 treatment arms (postoperative EBRT vs postoperative chemotherapy) regarding PFS (median PFS, 30.6 mo for EBRT and 31.9 mo for chemotherapy) or OS (4-y OS, 72.6% for EBRT and 64.6% for chemotherapy). In comparison with the NOA-04 trial, our treatment (SBT + EBRT boost) achieved similar results, with a median PFS of 26.2 months and a 4-year OS rate of 71.2%. Although no definitive conclusion can be drawn from this comparison—since different patient selection criteria, as well as a different distribution of prognostic relevant factors (eg, mutation status of isocitrate dehydrogenase 1,33 which was not assessed in the present study), may have influenced the oncological outcome—we could, however, show that a combined treatment with SBT + EBRT boost is a safe and effective treatment option for highly eloquent grade III gliomas not amenable to (a low-risk) complete tumor resection. Furthermore, the oncological outcome in the present study has to be seen in the context that in the NOA-04 trial, incomplete tumor resection and biopsy were—compared with complete resection—associated with a significantly increased risk for treatment failure (1.6 and 3.5 times [hazard ratios]).
In comparison with previous studies on SBT for grade III gliomas, applying a combined treatment concept of SBT + EBRT boost, the risk for development of radiation necrosis in our study was substantially lower (1/30 [3%] grade III patients compared with 19% in a study by Fernandez et al17). Although the basic treatment concepts (low-dose SBT + EBRT boost) were identical, other groups applied cumulative SBT doses of more than 100 Gy followed by an EBRT boost of up to 50 Gy.17 In contrast, we reduced irradiation to a cumulative SBT dose of 50 Gy followed by an EBRT boost of 25 Gy. The rationale for this treatment protocol was to decrease the risk for radiation necrosis while simultaneously preserving the oncological efficacy.34
Based on the results from the present study, we were able to demonstrate that SBT in combination with an EBRT boost is safe and efficient for treating inoperable grade III gliomas within eloquent brain. The combination of 2 irradiation modalities (SBT + EBRT) was originally introduced to improve local tumor control rates for patients with grade III gliomas.17 However, validation of this hypothesis is still pending, since convincing data (from prospective, randomized trials) are still not available. Therefore, the place of SBT (in combination with EBRT boost) within a multimodal treatment concept for grade III gliomas is still unclear and remains to be determined in future studies.
Conclusions
The application of SBT in patients with tumors located in highly eloquent brain, such as the CSR, is safe and—compared with surgery—associated with only minimal postoperative morbidity. The oncological efficacy of SBT for patients with grade II gliomas seems to be at least equal to surgery ± EBRT. In addition, the application of SBT as a primary treatment for grade II gliomas allows deferring EBRT until the time of progression in those patients whose tumors in eloquent brain would likely have been treated with incomplete tumor resection or biopsy followed by EBRT. Hence, late or delayed radiation injuries from EBRT can be avoided. Oncological outcomes of grade III glioma patients receiving a combination of SBT followed by EBRT boost were promising and comparable to those from the NOA-04 trial. However, the value of this treatment concept remains to be determined in future studies.
Conflict of interest statement. None declared.
Acknowledgments
We thank Manuel Fuetsch and Christina Hamisch for their assistance in data acquisition.
References
- 1.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764. doi: 10.1227/01.neu.0000318159.21731.cf. discussion 264–266. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N, Berger MS. Extent of resection influences outcomes for patients with gliomas. Rev Neurol (Paris) 2011;167(10):648–654. doi: 10.1016/j.neurol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Chang EF, Clark A, Smith JS, et al. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. Clinical article. J Neurosurg. 2011;114(3):566–573. doi: 10.3171/2010.6.JNS091246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffau H, Capelle L, Denvil D, et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74(7):901–907. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100(3):369–375. doi: 10.3171/jns.2004.100.3.0369. [DOI] [PubMed] [Google Scholar]
- 6.Duffau H, Capelle L. [Functional recuperation after resection of gliomas infiltrating primary somatosensory fields. Study of perioperative electric stimulation] Neurochirurgie. 2001;47(6):534–541. [PubMed] [Google Scholar]
- 7.Gonzalez-Darder JM, Gonzalez-Lopez P, Talamantes F, et al. Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus. 2010;28(2):E5. doi: 10.3171/2009.11.FOCUS09234. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz SB, Thon N, Nikolajek K, et al. Iodine-125 brachytherapy for brain tumours—a review. Radiat Oncol. 2012;7(1):30. doi: 10.1186/1748-717X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruge MI, Kickingereder P, Simon T, Treuer H, Sturm V. Stereotactic iodine-125 brachytherapy for treatment of inoperable focal brainstem gliomas of WHO grades I and II: feasibility and long-term outcome. J Neurooncol. 2012;109(2):273–283. doi: 10.1007/s11060-012-0889-1. [DOI] [PubMed] [Google Scholar]
- 10.Ruge MI, Simon T, Suchorska B, et al. Stereotactic brachytherapy with iodine-125 seeds for the treatment of inoperable low-grade gliomas in children: long-term outcome. J Clin Oncol. 2011;29(31):4151–4159. doi: 10.1200/JCO.2011.37.3381. [DOI] [PubMed] [Google Scholar]
- 11.Kreth FW, Thon N, Siefert A, Tonn JC. The place of interstitial brachytherapy and radiosurgery for low-grade gliomas. Adv Tech Stand Neurosurg. 2010;35:183–212. doi: 10.1007/978-3-211-99481-8_7. [DOI] [PubMed] [Google Scholar]
- 12.Wengenroth M, Blatow M, Guenther J, Akbar M, Tronnier VM, Stippich C. Diagnostic benefits of presurgical fMRI in patients with brain tumours in the primary sensorimotor cortex. Eur Radiol. 2011;21(7):1517–1525. doi: 10.1007/s00330-011-2067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnell O, Scholler K, Ruge M, Siefert A, Tonn J, Kreth F. Surgical resection plus stereotactic I-125 brachytherapy in adult patients with eloquently located supratentorial WHO grade II glioma—feasibility and outcome of a combined local treatment concept. J Neurol. 2008;255(10):1495–1502. doi: 10.1007/s00415-008-0948-x. [DOI] [PubMed] [Google Scholar]
- 14.Ruge MI, Suchorska B, Maarouf M, et al. Stereotactic 125iodine brachytherapy for the treatment of singular brain metastases: closing a gap? Neurosurgery. 2011;68(5):1209–1218. doi: 10.1227/NEU.0b013e31820b526a. discussion 1218–1219. [DOI] [PubMed] [Google Scholar]
- 15.Suchorska B, Ruge M, Treuer H, Sturm V, Voges J. Stereotactic brachytherapy of low-grade cerebral glioma after tumor resection. Neuro Oncol. 2011;13(10):1133–1142. doi: 10.1093/neuonc/nor100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voges J, Treuer H, Schlegel W, Pastyr O, Sturm V. Interstitial irradiation of cerebral gliomas with stereotactically implanted iodine-125 seeds. Acta Neurochir Suppl (Wien) 1993;58:108–111. doi: 10.1007/978-3-7091-9297-9_25. [DOI] [PubMed] [Google Scholar]
- 17.Zamorano L, Yakar D, Dujovny M, Sheehan M, Kim J. Permanent iodine-125 implant and external beam radiation therapy for the treatment of malignant brain tumors. Stereotact Funct Neurosurg. 1992;59(1–4):183–192. doi: 10.1159/000098940. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez PM, Zamorano L, Yakar D, Gaspar L, Warmelink C. Permanent iodine-125 implants in the up-front treatment of malignant gliomas. Neurosurgery. 1995;36(3):467–473. doi: 10.1227/00006123-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 20.Galldiks N, Langen K-J, Holy R, et al. Assessment of treatment response in patients with glioblastoma using O-(2–18F-fluoroethyl)-l-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. doi: 10.2967/jnumed.111.098590. [DOI] [PubMed] [Google Scholar]
- 21.Jansen NL, Suchorska B, Schwarz SB, et al. [18F]fluoroethyltyrosine-positron emission tomography–based therapy monitoring after stereotactic iodine-125 brachytherapy in patients with recurrent high-grade glioma. Mol Imaging. 2013;12(3):137–147. [PubMed] [Google Scholar]
- 22.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 23.Engel J, Van Ness P, Rasmussen T, Ojemann L. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical Treatment of the Epilepsies. ed 2. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 24.Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO task force. Eur J Neurol Sep. 2010;17(9):1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- 25.Kreth FW, Mehrkens JH. Stereotactic brachytherapy in low-grade gliomas. In: Tonn J-C, Westphal M, Rutka JT, editors. Oncology of CNS Tumors. Berlin:Springer: 2010. pp. 135–145. [Google Scholar]
- 26.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 27.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 28.Kreth FW, Faist M, Rossner R, Birg W, Volk B, Ostertag CB. The risk of interstitial radiotherapy of low-grade gliomas. Radiother Oncol. 1997;43(3):253–260. doi: 10.1016/s0167-8140(97)01948-8. [DOI] [PubMed] [Google Scholar]
- 29.Peraud A, Goetz C, Siefert A, Tonn JC, Kreth FW. Interstitial iodine-125 radiosurgery alone or in combination with microsurgery for pediatric patients with eloquently located low-grade glioma: a pilot study. Childs Nerv Syst. 2007;23(1):39–46. doi: 10.1007/s00381-006-0203-7. [DOI] [PubMed] [Google Scholar]
- 30.Schomas DA, Laack NN, Rao RD, et al. Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol. 2009;11(4):437–445. doi: 10.1215/15228517-2008-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 32.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 34.Sturm V. Comment on Fernandez et al: permanent iodine-125 implants in the up-front treatment of malignant gliomas. Neurosurgery. 1994;36(3):467–473. doi: 10.1227/00006123-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 36.Cedzich C, Taniguchi M, Schafer S, Schramm J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38(5):962–970. doi: 10.1097/00006123-199605000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Duffau H, Capelle L, Sichez J, et al. Intra-operative direct electrical stimulations of the central nervous system: the Salpetriere experience with 60 patients. Acta Neurochir (Wien) 1999;141(11):1157–1167. doi: 10.1007/s007010050413. [DOI] [PubMed] [Google Scholar]
- 38.Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98(4):764–778. doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- 39.Carrabba G, Fava E, Giussani C, et al. Cortical and subcortical motor mapping in rolandic and perirolandic glioma surgery: impact on postoperative morbidity and extent of resection. J Neurosurg Sci. 2007;51(2):45–51. [PubMed] [Google Scholar]
- 40.Shinoura N, Yoshida M, Yamada R, et al. Awake surgery with continuous motor testing for resection of brain tumors in the primary motor area. J Clin Neurosci. 2009;16(2):188–194. doi: 10.1016/j.jocn.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Talacchi A, Turazzi S, Locatelli F, et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol. 2010;100(3):417–426. doi: 10.1007/s11060-010-0193-x. [DOI] [PubMed] [Google Scholar]
- 42.Krieg SM, Shiban E, Droese D, et al. Predictive value and safety of intraoperative neurophysiological monitoring with motor evoked potentials in glioma surgery. Neurosurgery. 2012;70(5):1060–1070. doi: 10.1227/NEU.0b013e31823f5ade. discussion 1070–1071. [DOI] [PubMed] [Google Scholar]