Abstract
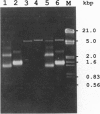
We have developed a system for generation of infectious bursal disease virus (IBDV), a segmented double-stranded RNA virus of the Birnaviridae family, with the use of synthetic transcripts derived from cloned cDNA. Independent full-length cDNA clones were constructed that contained the entire coding and noncoding regions of RNA segments A and B of two distinguishable IBDV strains of serotype I. Segment A encodes all of the structural (VP2, VP4, and VP3) and nonstructural (VP5) proteins, whereas segment B encodes the RNA-dependent RNA polymerase (VP1). Synthetic RNAs of both segments were produced by in vitro transcription of linearized plasmids with T7 RNA polymerase. Transfection of Vero cells with combined plus-sense transcripts of both segments generated infectious virus as early as 36 hr after transfection. The infectivity and specificity of the recovered chimeric virus was ascertained by the appearance of cytopathic effect in chicken embryo cells, by immunofluorescence staining of infected Vero cells with rabbit anti-IBDV serum, and by nucleotide sequence analysis of the recovered virus, respectively. In addition, transfectant viruses containing genetically tagged sequences in either segment A or segment B of IBDV were generated to confirm the feasibility of this system. The development of a reverse genetics system for double-stranded RNA viruses will greatly facilitate studies of the regulation of viral gene expression, pathogenesis, and design of a new generation of live vaccines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzola J. V., Xu Z. K., Asamizu T., Nuss D. L. Segment-specific inverted repeats found adjacent to conserved terminal sequences in wound tumor virus genome and defective interfering RNAs. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8301–8305. doi: 10.1073/pnas.84.23.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A. A., Jagadish M. N., Brown M. A., Hudson P. J. Deletion mapping and expression in Escherichia coli of the large genomic segment of a birnavirus. Virology. 1987 Nov;161(1):145–152. doi: 10.1016/0042-6822(87)90180-2. [DOI] [PubMed] [Google Scholar]
- Becht H. Infectious bursal disease virus. Curr Top Microbiol Immunol. 1980;90:107–121. doi: 10.1007/978-3-642-67717-5_5. [DOI] [PubMed] [Google Scholar]
- Boyer J. C., Haenni A. L. Infectious transcripts and cDNA clones of RNA viruses. Virology. 1994 Feb;198(2):415–426. doi: 10.1006/viro.1994.1053. [DOI] [PubMed] [Google Scholar]
- Chen B., Choi G. H., Nuss D. L. Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science. 1994 Jun 17;264(5166):1762–1764. doi: 10.1126/science.8209256. [DOI] [PubMed] [Google Scholar]
- Chen D., Zeng C. Q., Wentz M. J., Gorziglia M., Estes M. K., Ramig R. F. Template-dependent, in vitro replication of rotavirus RNA. J Virol. 1994 Nov;68(11):7030–7039. doi: 10.1128/jvi.68.11.7030-7039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Protein-primed RNA synthesis in vitro by the virion-associated RNA polymerase of infectious pancreatic necrosis virus. Virology. 1995 Apr 1;208(1):19–25. doi: 10.1006/viro.1995.1125. [DOI] [PubMed] [Google Scholar]
- Enami M., Luytjes W., Krystal M., Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci U S A. 1990 May;87(10):3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M. I., Collins P. L. Intracellular amplification and expression of a synthetic analog of rotavirus genomic RNA bearing a foreign marker gene: mapping cis-acting nucleotides in the 3'-noncoding region. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5784–5788. doi: 10.1073/pnas.89.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson P. J., McKern N. M., Power B. E., Azad A. A. Genomic structure of the large RNA segment of infectious bursal disease virus. Nucleic Acids Res. 1986 Jun 25;14(12):5001–5012. doi: 10.1093/nar/14.12.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt E., Beyer J., Müller H. Identification of a novel viral protein in infectious bursal disease virus-infected cells. J Gen Virol. 1995 Feb;76(Pt 2):437–443. doi: 10.1099/0022-1317-76-2-437. [DOI] [PubMed] [Google Scholar]
- Mundt E., Müller H. Complete nucleotide sequences of 5'- and 3'-noncoding regions of both genome segments of different strains of infectious bursal disease virus. Virology. 1995 May 10;209(1):10–18. doi: 10.1006/viro.1995.1226. [DOI] [PubMed] [Google Scholar]
- Müller H., Lange H., Becht H. Formation, characterization and interfering capacity of a small plaque mutant and of incomplete virus particles of infectious bursal disease virus. Virus Res. 1986 May;4(3):297–309. doi: 10.1016/0168-1702(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Roner M. R., Sutphin L. A., Joklik W. K. Reovirus RNA is infectious. Virology. 1990 Dec;179(2):845–852. doi: 10.1016/0042-6822(90)90153-i. [DOI] [PubMed] [Google Scholar]
- Schnell M. J., Mebatsion T., Conzelmann K. K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994 Sep 15;13(18):4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg M., Silverstein S. C., Levin D. H., Acs G. Asynchronous synthesis of the complementary strands of the reovirus genome. Proc Natl Acad Sci U S A. 1971 Feb;68(2):505–508. doi: 10.1073/pnas.68.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies U., Müller H., Becht H. Properties of RNA polymerase activity associated with infectious bursal disease virus and characterization of its reaction products. Virus Res. 1987 Aug;8(2):127–140. doi: 10.1016/0168-1702(87)90024-4. [DOI] [PubMed] [Google Scholar]
- Spies U., Müller H. Demonstration of enzyme activities required for cap structure formation in infectious bursal disease virus, a member of the birnavirus group. J Gen Virol. 1990 Apr;71(Pt 4):977–981. doi: 10.1099/0022-1317-71-4-977. [DOI] [PubMed] [Google Scholar]
- Vakharia V. N., He J., Ahamed B., Snyder D. B. Molecular basis of antigenic variation in infectious bursal disease virus. Virus Res. 1994 Feb;31(2):265–273. doi: 10.1016/0168-1702(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Zou S., Brown E. G. Identification of sequence elements containing signals for replication and encapsidation of the reovirus M1 genome segment. Virology. 1992 Feb;186(2):377–388. doi: 10.1016/0042-6822(92)90003-8. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bradley J., Wimmer E., Studier F. W., Dunn J. J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]