Introduction
Current therapies targeting pathological ventricular remodeling1, 2 manifest significant effectiveness in reducing morbidity and mortality in patients with systolic heart failure3. However, in many instances, disease progression continues unabated. Whereas novel disease targets are continually being discovered, most innovative therapies do not demonstrate consistent efficacy in patients; indeed, many prove to be ineffective, even deleterious, before reaching phase III clinical trials. Here, we review therapeutic strategies targeting cellular pathways governing left ventricular remodeling in the two major types of heart failure, that with reduced (HFrEF) or preserved (HFpEF) systolic function. In an accompanying article, we highlight recent advances in our understanding of mechanisms underlying pathological ventricular remodeling4.
Advances in this field are conditioned by the highly heterogeneous nature of heart failure. Notably, within each of the two broad categories of HFrEF and HFpEF, a wide variety of disease etiologies dictate pathogenesis5. In other words, heart failure, a syndrome defined on clinical terms, derives from numerous different diseases, such as myocardial infarction, hypertension, cytokine or neuroendocrine dyscrasias, genetic disorders, and more5. It seems likely that the therapies which have emerged with efficacy are those targeting features which are shared among these disorders. As a corollary, it is conceivable that some of the therapies which have failed in clinical trials target relevant elements of pathogenesis but which are not common to all. As “personalized medicine” emerges in the discipline of heart failure, we envision therapies tailored to the specifics of molecular and cellular pathogenesis.
Anti-remodeling Therapies
Pharmaceutical agents
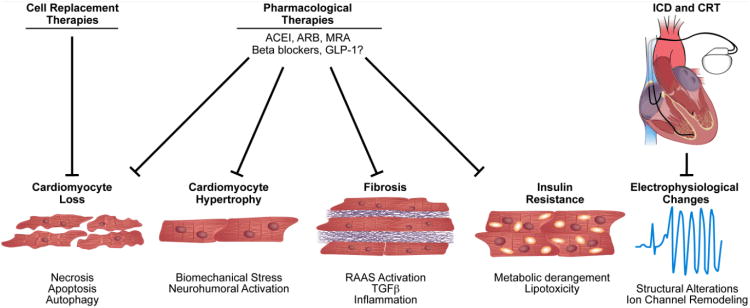
Over the last three decades, numerous randomized clinical trials have demonstrated substantial efficacy of ACE (angiotensin converting enzyme) inhibitors, ARBs (angiotensin receptor blockers), MRAs (mineralocorticoid receptor antagonists), and β-adrenergic blockers in reducing morbidity and mortality in patients with systolic heart failure. Presently, ACC/AHA guidelines for the diagnosis and treatment of heart failure in adults emphasize use of these agents in patients with HFrEF6 (Figure).
Figure.
Therapeutic interventions in pathological ventricular remodeling. Clinically proven pharmacological agents reduce morbidity and mortality, including ACE inhibitors, ARBs, β-blockers, and MRAs by reducing cell death, hypertrophy, and fibrosis. GLP-1 may prove effective in treating metabolic derangements. Mechanical support using ventricular assist device therapy unloads the failing myocardium and limits ventricular dilation. ICDs and CRTs target electrophysiological remodeling events. Finally, cell replacement therapy to replenish lost cardiomyocytes remains experimental and holds promise for the future.
Inhibitors of the renin-angiotensin-aldosterone axis
Originally, ACE inhibitors and ARBs were used to treat hypertension. However, it was subsequently found that these agents afforded substantial benefit in animal models of heart failure, including increased survival, by targeting adverse cardiac remodeling. Angiotensin receptor activation can induce cardiac remodeling independently of changes in blood pressure7, and ACE inhibitors and ARBs both act to antagonize the effects of Ang II, albeit at different points in the cascade. Numerous clinical trials have demonstrated that ACE inhibitors and ARBs reduce heart failure morbidity and mortality3. More recently, antihypertensive agents targeting renin enzymatic activity8, the rate limiting step in Ang II production, have become available and are being studied for effects on adverse cardiac remodeling. For example, the renin inhibitor, aliskiren, blunts remodeling in experimentally infarcted mouse hearts8 and has been tested for efficacy in ALOFT (Aliskiren Observation of heart Failure Treatment) and ASPITE (The Assessment of Services Promoting Independence and Recovery in Elders), however, with disparate results in efficacy (favorable and unfavorable, respectively)9, 10. Additional planned trials, such as ASTRONAUT (AliSkiren Trial ON Acute heart failure OUtcomes)11 [aliskiren vs placebo in addition to an ACEI or ARB] and ATMOSPHERE (Aliskiren Trial of Minimizing OutcomeS for Patients with HEart failuRE)12 [aliskiren vs enalapril or combination] will evaluate endpoints of death and rehospitalization due to heart failure.
Low-dose MRAs are recommended for treatment in select patients with moderately severe or severe heart failure symptoms (NYHA class III-IV), recent decompensation, or with LV dysfunction early after myocardial infarction6. The Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF) revealed that eplerenone reduces mortality and hospitalization in patients with systolic dysfunction and mild symptoms, expanding the role of MRAs to include asymptomatic patients13.
Aldosterone is a mineralocorticoid secreted by the adrenal gland in response to Ang II or cytokines; it can also signal directly within the myocardium via resident mineralocorticoid receptors and the requisite 11 beta-hydroxysteroid dehydrogenase activity14. Increases in cardiac aldosterone have been reported in experimental models of myocardial infarction15, correlating with left ventricular remodeling16. The effects of aldosterone are similar to those observed with Ang II, including inhibition of nitric oxide synthase and promotion of inflammation, fibrosis, and cardiac myocyte apoptosis17. However, the use of spironolactone is limited due to metabolic and endocrine side effects and variations in patient response18, which are largely absent with eplerenone13. In addition, patients with chronic heart failure have increased aldosterone synthase activity leading to non-mineralocorticoid receptor-mediated responses19. Blockade of mineralocorticoid receptors increases aldosterone synthase activity. Consequently, MRA therapy results in high levels of aldosterone. Aldosterone may also have non-mineralocorticoid receptor-mediated deleterious effects on cardiomyocytes20.
The aldosterone synthase inhibitor, FAD28621, reduced LV remodeling in a rat model of heart failure, and another, LCI699, demonstrated safety and tolerability in 14 patients with primary aldosteronism22. Future studies will determine whether aldosterone synthase inhibitors are more effective than MRAs in treating heart failure.
A new class of therapeutics that targets the renin-angiotensin-aldosterone axis comprises dual-acting agents for angiotensin receptor-neprilysin inhibition. Neprilysin activates the kinin and natriuretic peptide systems. One such inhibitor, LCZ696, combines a moiety of valsartan with an endopeptidase inhibitor23. An initial safety study of this inhibitor has led to the ongoing PARADIGM-HF trial (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) (NCT01035255) to compare LCZ696 with enalapril23.
Selective angiotensin receptor blockers are also currently in development. Angiotensin II can act through two different receptors: Ang II receptor type 1 (AT1) and Ang II receptor type 2 (AT2). Whereas the AT1 receptor is ubiquitously expressed in the cardiovascular system, the expression of AT2 is low in the normal adult, but elevated in heart failure. In many contexts, activation of AT1 and AT2 receptors produces opposite effects, with the AT2 receptor promoting vasodilation and blunting sof cardiac hypertrophy24. A first-in-class AT2 receptor agonist, Compound 21, has shown promising early results by decreasing infarct size in a rodent model25.
Beta-adrenergic receptor blockers
Inhibition of β-adrenergic signaling has long been used in the treatment of hypertension and cardiac arrhythmias and more recently in patients with mild to severe heart failure. β-blockers have been shown, both experimentally and clinically, to reduce adverse cardiac remodeling and improve heart failure mortality26. Specifically, cardiac myocytes express the β1-adrenergic receptor subtype, and thus manifest responses to β1-selective inhibitors. However, mechanisms underlying the full benefit of β1-adrenergic receptor blockade in reducing left ventricular remodeling remain elusive, because fibroblasts predominately express the β2-adrenergic receptor subtype27.
Agonist-promoted β-receptor desensitization occurs via G-protein Receptor Kinases (GRKs) which tag these receptors for cellular internalization and degradation. Specific to the heart, GRK2 (also known as β-ARK) has been engineered as a catalytically inactive fragment, β-ARKct, which interferes with GRK2 binding to β-adrenergic and subsequent receptor degradation, thus serving to block β-adrenergic uncoupling and desensitization28. Experimentally, β-ARKct gene therapy in infarcted rabbit or rat hearts improved function and blunted heart failure29. Although this gene therapy has not yet been tested in humans to date, it holds potential for diminishing β-receptor desensitization thereby augmenting efficacy of β-blockers in heart failure patients.
The role of β-blockers in the treatment of heart failure in patients with HPpEF remains unclear. For example, 6-month treatment with the β-blocker nebivolol did not improve 6-minute walk test performance in ELANDD (The Effect of Long-term Administration of Nebivolol on clinical symptoms, exercise capacity and left ventricular function in patients with Diastolic Dysfunction)30. Some evidence, however, suggests that its negative chronotropic effects may have contributed to this result30. There is a large ongoing trial evaluating β-blocker therapy in HFpEF (beta-PRESERVE) to test the efficacy of metoprolol succinate on mortality and hospitalization rates31.
Of note, pharmacogenetic studies of both ACEI and β-blocker therapies have uncovered specific gene mutations that impact therapeutic efficacy. For example, a deletion variant in the gene coding for angiotensin converting enzyme is associated with greater survival benefit from ACE inhibitors and β-blockers32. Ser49 (versus Gly49) in the β1 adrenergic receptor, a specific insertion in the α2C adrenergic receptor, and Gln41 (versus Leu41) in G-protein coupled receptor kinase 5 are each associated with relatively greater survival benefit from β-blocker therapy32.
Positive inotropes
Positive inotropic agents play a critical role in controlling symptoms in decompensated or end-stage HF patients6. However, chronic administration of these agents increases mortality, and long-term use of digoxin has no beneficial effect on mortality33.
Two new non-glycoside inotropic agents have entered the therapeutic arena recently. One is therapy to deliver the cDNA of the sarcoplasmic reticulum Ca2+ (SERCA2a) pump via adeno-associated virus in an effort to replenish the down-regulated SERCA2a levels typical of failing myocardium34. The second is a luso-inotropic compound, istaroxime, which inhibits the Na+/K+-ATPase, leading to accumulation of intracellular Na+, decreased activity of the Na+-Ca2+ exchanger to remove cytosolic Ca2+, and consequent activation of sarcomeric contraction (the same mechanism as digitalis glycosides). The CUPID study (Calcium Up-regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease) demonstrated safety and suggested benefit of adeno-associated virus type 1-delivered sarcoplasmic reticulum Ca2+-ATPase in advanced heart failure, supporting larger confirmatory trials34. Istaroxime manifests a dual mode of action, combining inotropic (stimulation of myocardial contractility during systole) and lusitropic (improvement of diastolic relaxation) effects. A Phase II trial to assess the hemodynamic effects of istaroxime in 120 heart failure patients (HORIZON-HF, Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure) revealed lowered capillary wedge pressure and decreased heart rate35.
Another novel and potentially exciting class of agents, cardiac myosin activators, includes omecamtiv mecarbil (CK-1827452)36. This compound binds the myosin catalytic domain, increasing the transition rate of myosin binding to actin into a more tightly bound state thereby increasing force while simultaneously inhibiting ATP turnover, together leading to more myosin heads generating force per beat. This novel mechanism increases systolic ejection time, resulting in improved systolic function without increasing myocardial oxygen demand, resulting in significant increases in cardiac efficiency37. Preliminary evidence suggests that omecamtiv mecarbil is safe in patients with ischemic cardiomyopathy and angina, increasing stroke volume and fractional shortening and decreasing ventricular volumes37.
Other promising therapeutic agents
Lipid lowering drugs
Inhibitors of HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) are well-established at lowering cholesterol, providing robust protection to patients with a variety of forms of ischemic heart disease38. Beneficial anti-remodeling effects of these “statins” may occur on top of those observed with traditional therapy (ACE inhibitors and β-blockers)39. However, in CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure), rosuvastatin did not reduce the primary endpoint or the number of all-cause deaths in older patients with systolic heart failure, although the drug did reduce cardiovascular hospitalizations40. Also in GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca - Heart Failure), rosuvastatin 10 mg daily did not alter clinical outcomes in patients with chronic heart failure of any cause41.
Vasopressin and ET1 receptor antagonists
Beyond the two major neurohormonal cascades, the sympathetic and renin-angiotensin-aldosterone axes, cascades elicited by endothelin and vasopressin are also activated during the pathogenesis of heart failure42. Stimulation of the vasopressin V1A receptor increases intracellular Ca2+ levels and promotes myocyte hypertrophy and remodeling. Stimulation of V2 receptors increases the expression and membrane incorporation of aquaporin-2 water channels into the collecting duct cells in the kidney, resulting in free water absorption. Vasopressin receptor antagonists, such as conivaptan, lixivaptan, mozavaptan, and tolvaptan, block V1A and/or V2 receptors. In patients hospitalized with heart failure in EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) trial, addition of oral tolvaptan to standard therapy improved many, though not all, heart failure signs and symptoms without serious adverse events43. However, tolvaptan initiated for acute treatment of patients hospitalized with heart failure had no effect on long-term mortality or heart failure-related morbidity44.
Endothelins, including endothelin-1 (ET-1), increase contractility and stimulate growth and myofibrillogenesis in cardiomyocytes, thereby promoting cardiac hypertrophy45. ET-1 binds to the ET-A receptor to elicit a wide range of responses, including increased expression of nuclear transcription factors, activation of protein kinases and ion channels responsible for cardiomyocyte contractility and growth45. Disappointingly, multiple trials testing a variety of endothelin antagonists have yielded negative results, including ENCOR (ENrasentan Clinical Outcomes Randomised)46, EARTH (Efficacy And safety of iRbesarTan and olmesartan in patients with Hypertension)47, and RITZ-4 (Randomised Intravenous TeZosentan 4)48.
Anti-cytokine agents
Pro-inflammatory cytokines promote hypertrophy, apoptosis, and extracellular matrix remodeling, thereby contributing to heart failure pathogenesis49. Elevated levels of pro-inflammatory cytokines are also associated with poor clinical outcomes50. However, clinical trials studying TNFα inhibitors, etanercept and infliximab, reported neutral or negative effects on all-cause mortality or hospitalization for heart failure51. Some evidence suggests that suppression of inflammatory responses globally, as opposed to inhibition of a specific pathway might hold promise52. In addition, some inflammatory responses arise secondary to other cellular events, and inhibition of the inciting signaling pathways might prove more fruitful52.
Glucagon-Like Peptide-1 (GLP1)
Metabolic derangements are significant elements of heart failure pathogenesis. Indeed, insulin resistance and lipotoxicity are recogonized as hallmarks of dilated cardiomyopathy53 and diabetic cardiomyopathy54, 55. GLP1 stimulates myocardial glucose uptake in dilated cardiomyopathy through p38 MAP kinase56, and chronic exposure to GLP1 in a rat model of heart failure sustains left ventricular systolic function and prolongs survival57. These exciting preclinical findings have led to an ongoing clinical trial, FIGHT (Functional Impact of GLP-1 for Heart Failure Treatment). (http://clinicaltrials.gov/ct2/show/NCT01800968)
Novel vasodilators
Disorders in cGMP-dependent mechanisms contribute to myocardial dysfunction and remodeling. Specific phosphodiesterases govern the amplitude, duration, and compartmentalization of cyclic nucleotide signaling. An inhibitor of phosphodiesterase-5A (PDE-5A), sildenafil, improved hemodynamics, LV diastolic function, and RV systolic function, in a small, single center trial in HFpEF58. These encouraging findings prompted the multicenter clinical trial RELAX (phosphodiesteRasE-5 inhibition to improve quality of Life And eXercise capacity in diastolic heart failure). The primary end point was change in peak oxygen consumption after 24 weeks of therapy, and secondary end points included change in 6-minute walk distance and a hierarchical composite clinical status score59. Unfortunately, sildenafil therapy did not result in significant improvement in exercise capacity or clinical status59. Another clinical trial, PITCH-HF (Phosphodiesterase Type5 inhibition with tadalafil changes outcomes in Heart Failure), is underway in patients with HFrEF (http://neriresearch.net/overview.html).
Nitric oxide production by endothelial nitric oxide synthase (eNOS) can limit cardiac hypertrophy, apoptosis, and fibrosis, thereby impacting adverse cardiac remodeling60. In light of this, a novel approach to target and increase eNOS activity has been developed. Preclinical studies of AVE9488, an oral agent targeting eNOS, suggested improvement in LV remodeling, including decreased hypertrophy, fibrosis, ventricular dilation, and enhanced contractility, yet without effects on left ventricular mass61.
Special considerations in HFpEF
HFpEF is a clinical manifestation of many different pathophysiologies5. These range from LV stiffness, impaired diastolic and/or systolic function, and metabolic dyscrasias4. In light of multiple failed clinical trials, efforts have focused on identifying biomarkers, risk factors, specific syndrome features, and appropriate study endpoints to parse these patients into defined subgroups and test therapeutic efficacy.
The most widely used HFpEF biomarkers are BNP (brain natriuretic peptide) and NT-proBNP (N-terminal pro-BNP). Outcomes in RELAX were worse relative to prior HFpEF trials when the more sensitive NT-proBNP assay was required for diagnosis59, possibly because this may indicate that the patient's shortness of breath is more likely attributed to heart failure62. Other circulating biomarkers have been used to diagnose HFpEF, including procollagen, inflammatory factors (interleukins 6 and 8 and TNFα), matrix metalloproteinase, triiodothyronine, heart-type fatty acid binding proteins, troponin T, and carbohydrate antigen-1255. However, these biomarkers remain to be validated in large cohorts.
Compared with HFrEF, HFpEF patients are classically more likely to be older, hypertensive females62. A retrospective study of new-onset HF in Framingham participants between 1981 and 2008 showed that approximately 50% were classified as HFpEF. Older age, diabetes mellitus, and a history of valvular disease predicted both HFrEF and HFpEF63. Higher body mass index, smoking, and atrial fibrillation predicted HFpEF only, whereas male sex, higher total cholesterol, higher heart rate, hypertension, cardiovascular disease, left ventricular hypertrophy, and left bundle-branch block were predictive of HFrEF63.
To date, no therapeutic agents have been shown to reduce mortality and morbidity in HEpEF; negative trials include DIG-PEF (Digitalis Investigation Group-Preserved EF), CHARM-Preserve (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity-Preserve), I-PRESERVE (Irbesartan in heart failure with PRESERVEd EF), and RELAX5. Likely, substantial heterogeneity within this patient population is a significant contributor to these negative results. Also, morbidity and mortality may not be optimal endpoints, given the age of these subjects and the presence of multiple comorbidities5.
Therapies targeting electrophysiological remodeling
Medical treatment
Agents that alter HF progression also impact disease-associated electrophysiological remodeling and may alter the risk of sudden cardiac death. For example, β-blockers reduce sudden death in both postinfarction patients and patients with HF regardless of etiology6. Aldosterone antagonists decrease sudden death and overall mortality in HF early after MI and in advanced HF6. ACE inhibitors and ARBs decrease the risk of developing atrial fibrillation in HF patients64.
Device-based therapies
HF patients have elevated risk of life-threatening ventricular arrhythmia6. Several clinical trials, including DEFINITE (Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation)65, MADIT (Multicenter Automatic Defibrillator Implantation Trial) II66 and SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial)67 demonstrated that ICDs (implantable cardioverter-defibrillators) reduced sudden cardiac death and overall mortality in HF patients. Accordingly, ICDs are class I recommendations in patients with LVEF < 35%6.
Intracardiac conduction delay in HF perturbs the coordinated mechanical contraction of the ventricle and worsens systolic performance. Therapy based on bi-ventricular pacing ameliorates the resulting contractile dyssynchrony. Several clinical trials, including MIRACLE-ICD (Multicenter InSync ICD Randomized Clinical Evaluation)68, CARE-HF (Cardiac Resynchronization in Heart Failure)69, and MADIT-CRT (The Multicenter Automatic Defibrillator Implantation Trial – Cardiac Resynchronization Therapy)70 have demonstrated that CRT (cardiac resynchronization therapy) can improve symptoms and reduce mortality. The improved outcomes derive largely from improvements in LV remodeling as assessed by enhanced systolic and diastolic function71. In animal studies, CRT can restore intracellular Ca2+ homeostasis72, renormalize the ratio of β1/β2 adrenergic receptors73, and alter the expression of genes involved in mitochondrial energetics, extracellular matrix remodeling, and myocardial stress responses74.
Analysis of all studies assessing outcomes using CRT reveals poor agreement between echocardiographic and clinical outcomes75. For example, whereas MIRACLE reported beneficial effects of CRT based on clinical indices such as NYHA symptom class and 6-minute walk test, reductions in LV volumes were found to be dependent on disease etiology, with greater reductions noted in non-ischemic patients versus ischemic patients76. On the contrary, reductions in mortality and improvements in cardiac function in CARE-HF were similar in non-ischemic and ischemic patients69. In an attempt to optimize patient selection, a multicenter trial (PROSPECT, Predictors of Response to CRT) enrolling 498 patients was conducted77, but no specific parameter emerged that conclusively improved patient selection criteria.
Mechanical support devices
Substantial evidence supports the notion that antagonism of neurohormonal stimulation improves survival in heart failure6; nevertheless, heart failure progresses in the majority of patients. In response, a variety of mechanical devices, including physical restraints, have been developed to reshape the ventricle and reverse pathological cardiac remodeling. These devices vary in terms of their design, material composition, abilities to redistribute strain across the ventricle, and technique of implantation. Overall, a critical feature is the ability to decrease ventricular wall stress, ultimately counteracting the pathological remodeling process.
Ventricular assist devices (VADs)
In the setting of end-stage heart failure, cardiac transplantation is one of the options for patients resistant to medical therapy. However, due to an enduring shortage of donors, left ventricular assist device (LVAD) therapies have emerged as an important therapeutic option, both as a bridge to transplant and more recently as “destination” therapy. Initially, pulsatile volume-displacement pumps were engineered, providing critical circulatory support to improve survival until transplantation becomes an option.
Each of the first generation VADs proved capable of improving survival, end-organ dysfunction, and quality of life78; that said, they are not without drawbacks. Adverse events such as bleeding, infection, thrombosis, and mechanical failure limit their utility to less than one year, and in the long term, they do not replace cardiac transplantation. The Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure (REMATCH) trial revealed 35% 2-year failure rate for the HeartMate XVE device with mortality in excess of 10%79. These shortcomings, coupled with chronically inadequate availability of organ donations and excessive waiting times, have prompted attempts to develop new, more reliable and smaller devices, including those with nonpulsatile dynamics.
The HeartMate 2 (Thoratec Inc.) is the most successfully used nonpulsatile device to date with over 6000 implants worldwide78. It is compact with fewer moving parts compared to first generation devices. In other second and third-generation VADs (the centrifugal pumps), devices lack bearings and are driven by a magnetically levitated impeller. Patients implanted with these second generation devices have a 65%-69% first-year survival rate with rare mechanical failure and few fatal events78. However, infection remains an important problem, even though it rarely leads to increased mortality.
Myocardial regeneration
A major mechanism of pathological cardiac remodeling involves myocyte death. And given that cardiac myocytes have only limited capacity for regeneration, there is great interest in developing progenitor cells – resident to the myocardium or otherwise – to enhance the regenerative capacity of the injured heart. Embryonic stem (ES) cells hold great promise, as they are by definition capable of differentiating into any cell type in the body. However, there are several disadvantages of using this cell type, including the possibility that they would differentiate into unwanted cell phenotypes to form teratomas. Their manipulation typically involves growth on a layer of feeder cells containing animal products, necessitating immunosuppressive therapy and predisposing to rejection. In some circles, use of ES cells has ignited ethical concerns which have led to legal restrictions. Although there are no current clinical trials underway using ES cells to treat heart disease, a first phase I application to treat spinal cord injury using human ES cells was approved by the US Federal Drug Administration (FDA) in January 2009.
One of the earliest cell types to be considered for cardiac regenerative therapy was skeletal myoblasts. These so-called satellite cells were attractive candidates, because they could be harvested from the host, expanded in vitro, followed by autologous transplantation into the heart. Furthermore, these cells are relatively resistant to ischemia. However, the MAGIC (Myoblast Autologous Grafting in Ischemic Cardiomyopathy) trial demonstrated no significant benefit from use of these cells80. Worse, patients receiving the skeletal myoblasts were at significantly increased risk of malignant ventricular arrhythmias80.
It has been demonstrated that the cardiac stem cell pool diminishes with aging81, 82. In addition, several populations of cardiac stem cells have been described; however, it is still not clear whether these cells are truly endogenous to the heart or derive from bone marrow or circulating cellular elements. Furthermore, cell surface markers used to define stem cell populations are subject to molecular regulation and may be increased or decreased depending on context. Therefore, rather than the existence of numerous different cardiac stem cell populations/infiltrating cells, it is possible that these cells derive from one population and represent a continuum of cellular phenotypes.
Stem cells being considered for myocardial regeneration derive from bone marrow, circulating pools of progenitor cells, and tissue-resident stem cells derived from adipose tissue, skeletal muscle, umbilical cord blood, myocardium, and epicardium. To date, the majority of the clinical trials using stem/progenitor cells to treat patients following an ischemic event have used adult stem cells derived from bone marrow. Recent meta-analyses of the completed clinical trials using bone marrow-derived stem cells to treat ischemic heart disease suggested that the transplantation of these cells is safe and affords benefits beyond those achieved using standard therapy83,84, 85. These studies went on to document decreases in infarct size, improvements in ejection fraction, and decreased left ventricular end-systolic volumes, suggesting improvement in overall global function. However, not all clinical trials using autologous stem cells have demonstrated efficacy, because most patients receiving autologous adult stem cells are at an advanced age and more likely to suffer from co-morbidities such as hypertension, diabetes, and ischemic disease which lead to decreased stem cell viability and function86-89. Also, whether the transplanted cells actually replace dead or dying cardiac myocytes is hotly debated. In fact, a number of other mechanisms have been proposed to underlie the benefit, including elicitation of paracrine factors which mediate endogenous repair, angiogenesis, or differentiation of native progenitors. Whether these stem cells actually differentiate into cardiac myocytes remains controversial. Further, low rates of engraftment decrease the likelihood that a sufficient number of dead myocytes can be replaced, which can approach one billion cells lost during a myocardial infarction. Therefore, strategies to reprogram cells native to the heart cells may hold significant promise.
Three recent phase I clinical trials using autologous cardiac stem cells yielded promising results in terms of myocardial repair. The Bolli and Anversa groups conducted a phase 1 trial named SCIPIO (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy) of autologous cardiac stem cells (c-kit positive and lineage negative) for the treatment of heart failure resulting from ischemic heart disease. In 14 patients receiving cell therapy, LVEF increased from 30.3% to 38.5% at 4 months after infusion. Importantly, the beneficial effects of stem cell therapy were even more pronounced at 1 year in eight patients whose LVEF increased by 12.3%. MRI measurements of infarct size demonstrated 24% and 30% decreases at 4 and 12 months, respectively90.
The recently reported CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) tested the effects of autologous stems cells cultured as cardiospheres (CDCs) as a mechanism of myocardial regeneration. These investigators reported significant reductions in scar, increases in viable heart mass, and improvements in regional contractility and regional systolic wall thickening based on MRI at six months. However, changes in end-diastolic volume, end-systolic volume, and LVEF did not differ between groups91.
The POSEIDON trial (Pilot Study of the Comparative Safety and Efficacy of Transendocardial Injection of Autologous Mesenchymal Stem Cells Versus Allogeneic Mesenchymal Stem Cells in Patients With Chronic Ischemic Left Ventricular Dysfunction Secondary to Myocardial Infarction) compared autologous and allogeneic mesenchymal stem cells (MSCs) in ischemic cardiomyopathy92. Injection of either autologous or allogeneic stem cells was associated with low occurrence of major adverse effects, and allogeneic MSCs did not stimulate significant donor-specific immune reactions. Relative to baseline, autologous, but not allogeneic, MSC therapy was associated with an improvement in the 6-minute walk test and the Minnesota Living with Heart Failure Questionnaire score; however, neither approach improved exercise VO2max. Allogeneic and autologous MSCs reduced mean infarct size and sphericity index but did not increase EF. Overall, allogeneic MSCs reduced LV end-diastolic volumes. Interestingly, in a subgroup analysis, low-dose MSCs (20 million cells) produced the greatest reductions in LV volumes and increased EF92.
Cellular reprogramming
Due to technical and ethical issues surrounding use of human ES cells for regenerative medicine, scientists have explored alternative strategies to generate cells with the capability of differentiating into cardiomyocytes. Scientists have successfully reprogrammed adult fibroblasts to de-differentiate into a embryonic stem cell-like state93, 94. These cells, termed induced pluripotent stem (iPS) cells, are viewed as a major scientific breakthrough in the field of regenerative medicine. These cells offer the advantage of being taken directly from the patient and reprogrammed into a de-differentiated state with subsequent manipulation into the desired cell type. These cells overcome obstacles associated with human ES cells in that they may escape immune rejection, and there are no ethical concerns associated with the use of human embryos. However, currently, the study of iPS cells is in its infancy, and there are several barriers to overcome before the cells can be used to regenerate the human heart. For example, iPS cells appear to be less efficient than ES cells in their capacity to differentiate into all cell types, and it is not known for each iPS cell clone whether reprogramming is complete. Even if a small number of cells are incompletely reprogrammed within the recipient tissue, the risk of teratoma formation remains present. Furthermore, to date, most iPS cells have been generated using viruses carrying transgenes, which are integrated into the host genome; reactivation could lead to tumorigenesis. However, this latter concern may be overcome by the engineering of tiny nonviral DNA vectors (rings of DNA about one-half the size of those usually used to reprogram cells)95. Other challenges include disease-related mutations and polymorphisms harbored within iPS cells derived from diseased patients and the fact that many diseases are attributable to more than one cell type.
Adult stem cells typically maintain “epigenetic memory” of their tissue or origin. As such, full cardiac myocyte differentiation may be insufficient without nuclear reprogramming. This is one reason why manipulation of cells from within the heart offers significant advantages, potentially eliminating the need for exogenous stem cell transplantation. Recently, it was demonstrated that cardiac fibroblasts could be reprogrammed into functional cardiac myocytes96-98, raising the possibility that fibroblasts within the heart might be redirected from contributing to scar formation to muscle regeneration.
Potentially important work is presently underway to discover means of reprogramming the heart using small molecule therapies99, 100. By activating resident progenitor cells, coaxing myocytes to re-enter the cell cycle, and/or transforming other cell types into a myocyte lineage, these strategies hold promise as novel ways to rebuild injured myocardium.
Tailored HF therapies
Despite the widely recognized fact that the syndrome of heart failure derives from a broad range of disease etiologies with vastly different molecular mechanisms of pathogenesis, current treatment strategies are largely uniform. Several hurdles must be overcome before etiology-specific therapy can be applied to defined patient subgroups. First, it is difficult to parse etiologic subgroups based on clinical presentation. Biomarkers may prove useful, as is emerging with NT-proBNP, procollagen, and inflammatory factors in HFpEF patients5, 59. In 2011, the UK-based National Institute for Health and Clinical Excellence issued clinical practice guidelines in heart failure based on serum NT-pro-BNP levels, echocardiography, and specialist assessment101. Second, innovative approaches may overcome deficiencies in conventional clinical trial design and/or deficient subgroup analysis. For example, Bayesian adaptive trial design uses information existing at the time of trial initiation, combined with data accumulated during the trial, to identify treatments most beneficial for specific patient subgroups102. Another approach employs multivariate prediction tools for risk stratification103. In the end, these approaches may prove superior to conventional one-variable-at-a-time subgroup analysis to minimize false positives and false negatives104.
The ultimate goal of targeted HF therapy will be individualized treatment based on each patient's clinical and genetic signatures. This, however, requires knowledge of each patient's genetic composition, meaningful interpretation of genetic variations in disease, and their interactions with therapies. For example, sexual dimorphism has been reported in the transcriptional responses to heart failure: in women, genes involved in cyclic nucleotide metabolism, glucose transport, and neurohumoral pathways are up-regulated, whereas in men, genes involved in arrhythmia, self-immunity, and cellular homeostasis are altered105. Also, pharmacogenetic studies have uncovered genetic variants contributing to variable responses to ACE inhibitor and β-blocker therapies32. Ultimately, therapeutic modalities, duration of treatment, medication doses will need to be customized for each patient.
Conclusions and perspective
In recent years, significant strides have been achieved in our understanding, and therapeutic targeting, of pathological ventricular remodeling. ACE inhibitors, ARBs, aldosterone antagonists, and MRAs, β-blockers have become standard of care; in patients with advanced heart failure, device-based therapy is important. Yet, heart failure continues to expand rapidly in incidence and prevalence, exacting enormous individual and societal tolls. Further, efforts to translate preclinical discoveries into the clinical arena have disappointed in the majority of instances. Undoubtedly, much of this failure derives from incomplete understanding of the underlying, complex biology and the currently accepted practice of aggregating all forms of heart failure together irrespective of underlying etiology. The extent and proportionate contributions of multiple events, including myocyte loss, hypertrophy, hyperplasia, extracellular matrix changes, metabolic derangements and immunological events differ by etiology and impact the response to therapy in a meaningful way.
Due to redundancy in cellular and molecular pathways involved in LV remodeling, it is unlikely that future therapeutics will target just one cell type or signaling pathway. Also, it is likely that blockade of neurohormones (e.g. catecholamines, angiotensin, aldosterone), or loading (vasodilators or diuretics), may have overlapping cellular targets (e.g. cardiac myocytes, fibroblasts, etc). Furthermore, targeting one signaling pathway or an individual molecule may be inefficient due to the existence of intricate, interlacing cellular signaling networks and regulatory loops; a multidisciplinary experimental/systems biology may be key. In the end, elucidation of the complex and fascinating biology of LV remodeling is likely to yield significant benefit to the growing number of patients with heart failure.
Acknowledgments
We thank members of the Hill lab for helpful discussions and critique.
Funding Sources: This work was supported by grants from the NIH (HL-080144, HL-0980842, HL-100401), Cancer Prevention and Research Institute of Texas (CPRIT, RP110486P3), the American Heart Association-DeHaan Foundation (0970518N), and the Fondation Leducq (11CVD04).
Footnotes
Conflicts of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. The New England journal of medicine. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN. Muscle: Fundamental biology and mechanisms of disease. Academic Press; 2012. [Google Scholar]
- 3.Goldberg LR. Heart failure. Ann Intern Med. 2010;152:ITC61–15. doi: 10.7326/0003-4819-152-11-201006010-01006. quiz ITC616. [DOI] [PubMed] [Google Scholar]
- 4.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: Mechanisms. Circulation. 2013;128:XX–XXX. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Haddad T, Dwivedi G. Heart failure with preserved ejection fraction: Current understanding and emerging concepts. Current opinion in cardiology. 2013;28:187–196. doi: 10.1097/HCO.0b013e32835c5492. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 7.Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, Ball SG. Angiotensin ii type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovascular research. 2009;81:592–600. doi: 10.1093/cvr/cvn230. [DOI] [PubMed] [Google Scholar]
- 8.Westermann D, Riad A, Lettau O, Roks A, Savvatis K, Becher PM, Escher F, Danser AHJ, Schultheiss HP, Tschope C. Renin inhibition improves cardiac function and remodeling after myocardial infarction independent of blood pressure. Hypertension. 2008;52:1068–1075. doi: 10.1161/HYPERTENSIONAHA.108.116350. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Pitt B, Latini R, Maggioni AP, Solomon SD, Keefe DL, Ford J, Verma A, Lewsey J. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circulation Heart failure. 2008;1:17–24. doi: 10.1161/CIRCHEARTFAILURE.107.740704. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SD, Shin SH, Shah A, Skali H, Desai A, Kober L, Maggioni AP, Rouleau JL, Kelly RY, Hester A, McMurray JJ, Pfeffer MA. Effect of the direct renin inhibitor aliskiren on left ventricular remodelling following myocardial infarction with systolic dysfunction. Eur Heart J. 2011;32:1227–1234. doi: 10.1093/eurheartj/ehq522. [DOI] [PubMed] [Google Scholar]
- 11.Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Bohm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled aliskiren trial on acute heart failure outcomes (astronaut) European journal of heart failure. 2011;13:100–106. doi: 10.1093/eurjhf/hfq209. [DOI] [PubMed] [Google Scholar]
- 12.Krum H, Massie B, Abraham WT, Dickstein K, Kober L, McMurray JJ, Desai A, Gimpelewicz C, Kandra A, Reimund B, Rattunde H, Armbrecht J. Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: Rationale and design of the aliskiren trial to minimize outcomes in patients with heart failure (atmosphere) study. European journal of heart failure. 2011;13:107–114. doi: 10.1093/eurjhf/hfq212. [DOI] [PubMed] [Google Scholar]
- 13.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. The New England journal of medicine. 2011;364:11–21. [Google Scholar]
- 14.Lombes M, Alfaidy N, Eugene E, Lessana A, Farman N, Bonvalet JP. Prerequisite for cardiac aldosterone action - mineralocorticoid receptor and 11-beta-hydroxysteroid dehydrogenase in the human heart. Circulation. 1995;92:175–182. doi: 10.1161/01.cir.92.2.175. [DOI] [PubMed] [Google Scholar]
- 15.Silvestre JS, Heymes C, Oubenaissa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction - effect of angiotensin ii receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694–2701. doi: 10.1161/01.cir.99.20.2694. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi M, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Tsutsui T, Matsui T, Fujii M, Matsumoto T, Yamamoto T, Horie H, Ohnishi M, Kinoshita M. Relationship between transcardiac extraction of aldosterone and left ventricular remodeling in patients with first acute myocardial infarction: Extracting aldosterone through the heart promotes ventricular remodeling after acute myocardial infarction. Journal of the American College of Cardiology. 2001;38:1375–1382. doi: 10.1016/s0735-1097(01)01539-x. [DOI] [PubMed] [Google Scholar]
- 17.Loan Le TY, Mardini M, Howell VM, Funder JW, Ashton AW, Mihailidou AS. Low-dose spironolactone prevents apoptosis repressor with caspase recruitment domain degradation during myocardial infarction. Hypertension. 2012;59:1164–1169. doi: 10.1161/HYPERTENSIONAHA.111.190488. [DOI] [PubMed] [Google Scholar]
- 18.Sica DA. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart failure reviews. 2005;10:23–29. doi: 10.1007/s10741-005-2345-1. [DOI] [PubMed] [Google Scholar]
- 19.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 20.Dooley R, Harvey BJ, Thomas W. Non-genomic actions of aldosterone: From receptors and signals to membrane targets. Mol Cell Endocrinol. 2012;350:223–234. doi: 10.1016/j.mce.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Mulder P, Mellin V, Favre J, Vercauteren M, Remy-Jouet I, Monteil C, Richard V, Renet S, Henry JP, Jeng AY, Webb RL, Thuillez C. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: A comparison with spironolactone. Eur Heart J. 2008;29:2171–2179. doi: 10.1093/eurheartj/ehn277. [DOI] [PubMed] [Google Scholar]
- 22.Amar L, Azizi M, Menard J, Peyrard S, Watson C, Plouin PF. Aldosterone synthase inhibition with lci699: A proof-of-concept study in patients with primary aldosteronism. Hypertension. 2010;56:831–838. doi: 10.1161/HYPERTENSIONAHA.110.157271. [DOI] [PubMed] [Google Scholar]
- 23.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of lcz696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (arni) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 24.Unger T, Paulis L, Sica DA. Therapeutic perspectives in hypertension: Novel means for renin-angiotensin-aldosterone system modulation and emerging device-based approaches. Eur Heart J. 2011;32:2739–2747. doi: 10.1093/eurheartj/ehr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unger T, Dahlof B. Compound 21, the first orally active, selective agonist of the angiotensin type 2 receptor (at2): Implications for at2 receptor research and therapeutic potential. J Renin Angiotensin Aldosterone Syst. 2010;11:75–77. doi: 10.1177/1470320309347792. [DOI] [PubMed] [Google Scholar]
- 26.Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of beta-adrenergic blockade in chronic heart failure - a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation. 1998;98:1184–1191. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- 27.Packer M. Do beta-blockers prolong survival in heart failure only by inhibiting the beta1-receptor? A perspective on the results of the comet trial. Journal of cardiac failure. 2003;9:429–443. doi: 10.1016/j.cardfail.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Reinkober J, Tscheschner H, Pleger ST, Most P, Katus HA, Koch WJ, Raake PW. Targeting grk2 by gene therapy for heart failure: Benefits above beta-blockade. Gene Ther. 2012 doi: 10.1038/gt.2012.9. [DOI] [PubMed] [Google Scholar]
- 29.Williams ML, Hata JA, Schroder J, Rampersaud E, Petrofski J, Jakoi A, Milano CA, Koch WJ. Targeted beta-adrenergic receptor kinase (beta ark1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109:1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 30.Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: Results of the elandd study. European journal of heart failure. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J. Rationale and design of the beta-blocker in heart failure with normal left ventricular ejection fraction (beta-preserve) study. European journal of heart failure. 2010;12:181–185. doi: 10.1093/eurjhf/hfp193. [DOI] [PubMed] [Google Scholar]
- 32.Talameh JA, McLeod HL, Adams KF, Jr, Patterson JH. Genetic tailoring of pharmacotherapy in heart failure: Optimize the old, while we wait for something new. Journal of cardiac failure. 2012;18:338–349. doi: 10.1016/j.cardfail.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 33.The effect of digoxin on mortality and morbidity in patients with heart failure. The digitalis investigation group. The New England journal of medicine. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 34.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (cupid): A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum ca2+-atpase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gheorghiade M, Blair JE, Filippatos GS, Macarie C, Ruzyllo W, Korewicki J, Bubenek-Turconi SI, Ceracchi M, Bianchetti M, Carminati P, Kremastinos D, Valentini G, Sabbah HN. Hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: A randomized controlled trial in patients hospitalized with heart failure. Journal of the American College of Cardiology. 2008;51:2276–2285. doi: 10.1016/j.jacc.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: A double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 38.Tsouli SG, Liberopoulos EN, Goudevenos JA, Mikhailidis DP, Elisaf MS. Should a statin be prescribed to every patient with heart failure? Heart failure reviews. 2008;13:211–225. doi: 10.1007/s10741-007-9041-2. [DOI] [PubMed] [Google Scholar]
- 39.Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure - a preliminary report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 40.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. The New England journal of medicine. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 41.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of rosuvastatin in patients with chronic heart failure (the gissi-hf trial): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 42.Rehsia NS, Dhalla NS. Potential of endothelin-1 and vasopressin antagonists for the treatment of congestive heart failure. Heart failure reviews. 2010;15:85–101. doi: 10.1007/s10741-009-9152-z. [DOI] [PubMed] [Google Scholar]
- 43.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The everest clinical status trials. JAMA: the journal of the American Medical Association. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 44.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The everest outcome trial. JAMA: the journal of the American Medical Association. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 45.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. Journal of molecular and cellular cardiology. 2003;35:871–886. doi: 10.1016/s0022-2828(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 46.Louis A, Cleland JG, Crabbe S, Ford S, Thackray S, Houghton T, Clark A. Clinical trials update: Capricorn, copernicus, miracle, staf, ritz-2, recover and renaissance and cachexia and cholesterol in heart failure. Highlights of the scientific sessions of the american college of cardiology, 2001. European journal of heart failure. 2001;3:381–387. doi: 10.1016/s1388-9842(01)00149-0. [DOI] [PubMed] [Google Scholar]
- 47.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Luscher TF. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the endothelina receptor antagonist trial in heart failure (earth): Randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor CM, Gattis WA, Adams KF, Jr, Hasselblad V, Chandler B, Frey A, Kobrin I, Rainisio M, Shah MR, Teerlink J, Gheorghiade M. Tezosentan in patients with acute heart failure and acute coronary syndromes: Results of the randomized intravenous tezosentan study (ritz-4) Journal of the American College of Cardiology. 2003;41:1452–1457. doi: 10.1016/s0735-1097(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 49.Epelman S, Mann DL. Communication in the heart: The role of the innate immune system in coordinating cellular responses to ischemic injury. Journal of cardiovascular translational research. 2012;5:827–836. doi: 10.1007/s12265-012-9410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fildes JE, Shaw SM, Yonan N, Williams SG. The immune system and chronic heart failure: Is the heart in control? Journal of the American College of Cardiology. 2009;53:1013–1020. doi: 10.1016/j.jacc.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 51.Cacciapaglia F, Navarini L, Menna P, Salvatorelli E, Minotti G, Afeltra A. Cardiovascular safety of anti-tnf-alpha therapies: Facts and unsettled issues. Autoimmunity reviews. 2011;10:631–635. doi: 10.1016/j.autrev.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Tamargo J, Lopez-Sendon J. Novel therapeutic targets for the treatment of heart failure. Nature reviews Drug discovery. 2011;10:536–555. doi: 10.1038/nrd3431. [DOI] [PubMed] [Google Scholar]
- 53.Fields AV, Patterson B, Karnik AA, Shannon RP. Glucagon-like peptide-1 and myocardial protection: More than glycemic control. Clinical cardiology. 2009;32:236–243. doi: 10.1002/clc.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battiprolu PK, Lopez-Crisosto C, Wang ZV, Nemchenko A, Lavandero S, Hill JA. Diabetic cardiomyopathy and metabolic remodeling of the heart. Life sciences. 2013;92:609–615. doi: 10.1016/j.lfs.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of foxo1 triggers diabetic cardiomyopathy in mice. The Journal of clinical investigation. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha map kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circulation Heart failure. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circulation Heart failure. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pde5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: Results of a 1-year, prospective, randomized, placebo-controlled study. Circulation Heart failure. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 59.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA: the journal of the American Medical Association. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prabhu SD. Nitric oxide protects against pathological ventricular remodeling -reconsideration of the role of no in the failing heart. Circ Res. 2004;94:1155–1157. doi: 10.1161/01.RES.0000129569.07667.89. [DOI] [PubMed] [Google Scholar]
- 61.Fraccarollo D, Widder JD, Galuppo P, Thum T, Tsikas D, Hoffmann M, Ruetten H, Ertl G, Bauersachs J. Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer ave9488 after experimental myocardial infarction. Circulation. 2008;118:818–827. doi: 10.1161/CIRCULATIONAHA.107.717702. [DOI] [PubMed] [Google Scholar]
- 62.Carlsen CM, Bay M, Kirk V, Gotze JP, Kober L, Nielsen OW. Prevalence and prognosis of heart failure with preserved ejection fraction and elevated n-terminal pro brain natriuretic peptide: A 10-year analysis from the copenhagen hospital heart failure study. European journal of heart failure. 2012;14:240–247. doi: 10.1093/eurjhf/hfs003. [DOI] [PubMed] [Google Scholar]
- 63.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new-onset heart failure: Differences in preserved versus reduced ejection fraction. Circulation Heart failure. 2013;6:279–286. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novo G, Guttilla D, Fazio G, Cooper D, Novo S. The role of the renin-angiotensin system in atrial fibrillation and the therapeutic effects of ace-is and arbs. British journal of clinical pharmacology. 2008;66:345–351. doi: 10.1111/j.1365-2125.2008.03234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. The New England journal of medicine. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 66.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. The New England journal of medicine. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 67.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. The New England journal of medicine. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 68.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The miracle icd trial. JAMA: the journal of the American Medical Association. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 69.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. The New England journal of medicine. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 70.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. The New England journal of medicine. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 71.Sutton MS, Keane MG. Reverse remodelling in heart failure with cardiac resynchronisation therapy. Heart. 2007;93:167–171. doi: 10.1136/hrt.2005.067967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, Abraham TP, O'Rourke B, Akar FG, Kass DA, Tomaselli GF. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119:1220–U1217. doi: 10.1161/CIRCULATIONAHA.108.794834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chakir K, Daya SK, Aiba T, Tunin RS, Dimaano VL, Abraham TP, Jacques K, Lai EW, Pacak K, Zhu WZ, Xiao RP, Tomaselli GF, Kass DA. Mechanisms of enhanced beta-adrenergic reserve from cardiac resynchronization therapy. Circulation. 2009;119:1231–U1222. doi: 10.1161/CIRCULATIONAHA.108.774752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao Z, Barth AS, DiSilvestre D, Akar FG, Tian YL, Tanskanen A, Kass DA, Winslow RL, Tomaselli GF. Key pathways associated with heart failure development revealed by gene networks correlated with cardiac remodeling. Physiological Genomics. 2008;35:222–230. doi: 10.1152/physiolgenomics.00100.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, Fyfe DA, Leon AR, Oshinski JN. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121:1985–1991. doi: 10.1161/CIRCULATIONAHA.109.910778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sutton MGS, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology - quantitative doppler echocardiographic evidence from the multicenter insync randomized clinical evaluation (miracle) Circulation. 2006;113:266–272. doi: 10.1161/CIRCULATIONAHA.104.520817. [DOI] [PubMed] [Google Scholar]
- 77.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, Sutton MS, De Sutter J, Murillo J. Results of the predictors of response to crt (prospect) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 78.Fang JC. Rise of the machines--left ventricular assist devices as permanent therapy for advanced heart failure. The New England journal of medicine. 2009;361:2282–2285. doi: 10.1056/NEJMe0910394. [DOI] [PubMed] [Google Scholar]
- 79.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P. Randomized Evaluation Mech A. Long-term use of a left ventricular assist device for end-stage heart failure. New England Journal of Medicine. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 80.Menasche P, Grp MMI. First randomized placebo-controlled myoblast autologous grafting in ischemic cardiomyopathy (magic) trial. Circulation. 2006;114:2426–2426. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 81.Torella D, Ellison GM, Mendez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: Role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3(1):S8–13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 82.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 84.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. Journal of the American College of Cardiology. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 85.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA: the journal of the American Medical Association. 2008;299:925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 86.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. Journal of the American College of Cardiology. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 87.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. Journal of the American College of Cardiology. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 88.Thijssen DH, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FC, Hopman MT, de Boer HC. Haematopoietic stem cells and endothelial progenitor cells in healthy men: Effect of aging and training. Aging Cell. 2006;5:495–503. doi: 10.1111/j.1474-9726.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 89.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired cxcr4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 90.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA: the journal of the American Medical Association. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–U311. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 94.Yu JY, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 95.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human ips cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sadek H, Hannack B, Choe E, Wang J, Latif S, Garry MG, Garry DJ, Longgood J, Frantz DE, Olson EN, Hsieh J, Schneider JW. Cardiogenic small molecules that enhance myocardial repair by stem cells. Proceedings of the National Academy of Sciences of the United States of America; 2008; pp. 6063–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Russell JL, Goetsch SC, Aguilar HR, Frantz DE, Schneider JW. Targeting native adult heart progenitors with cardiogenic small molecules. ACS chemical biology. 2012;7:1067–1076. doi: 10.1021/cb200525q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mant J, Al-Mohammad A, Swain S, Laramee P, Guideline Development G. Management of chronic heart failure in adults: Synopsis of the national institute for health and clinical excellence guideline. Ann Intern Med. 2011;155:252–259. doi: 10.7326/0003-4819-155-4-201108160-00009. [DOI] [PubMed] [Google Scholar]
- 102.Collins SP, Lindsell CJ, Pang PS, Storrow AB, Peacock WF, Levy P, Rahbar MH, Del Junco D, Gheorghiade M, Berry DA. Bayesian adaptive trial design in acute heart failure syndromes: Moving beyond the mega trial. American heart journal. 2012;164:138–145. doi: 10.1016/j.ahj.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: The need for risk stratification. JAMA: the journal of the American Medical Association. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 104.Baliga RR, Young JB. Clinical trials to “real-world” heart failure: Applying risk stratification to deliver personalized care. Heart failure clinics. 2011;7:xi–xiv. doi: 10.1016/j.hfc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 105.Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Champion HC, Breton E, Russell SD, Hall J, Kittleson MM, Baughman KL, Hare JM. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur Heart J. 2010;31:1188–1196. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]