Abstract
Coupling the production of mature gametes and fertilized zygotes to favorable nutritional conditions improves reproductive success. In invertebrates, the proliferation of female germ line stem cells is regulated by nutritional status. But, in mammals the number of female germ line stem cells is set early in development, with oocytes progressing through meiosis later in life. Mechanisms that couple later steps of oogenesis to environmental conditions remain largely undefined. We show that in the presence of food, the DAF-2 insulin-like receptor signals through the RAS-ERK pathway to drive meiotic prophase I progression and oogenesis; in the absence of food, the resultant inactivation of insulin-like signaling leads to downregulation of RAS-ERK pathway, and oogenesis is stalled. Thus, the insulin-like signaling pathway couples nutrient sensing to meiotic I progression and oocyte production in C. elegans, ensuring that oocytes are only produced under conditions favorable for the survival of the resulting zygotes.
INTRODUCTION
To survive and propagate, organisms must respond to changes in environmental conditions by altering their physiology and behavior (Hietakangas and Cohen, 2009; Neufeld, 2003). Reproductive development is particularly well tuned to changes in environmental conditions. Reproductive needs are often coordinated with energy requirements and dictated by environmental conditions. For example, in C. elegans, larvae obtain sexual maturity rapidly in normal environmental conditions due to activation of insulin signaling, but, in harsh conditions, the larvae arrest in a sexually immature stage and enter an alternate state of development, the dauer pathway (Antebi et al., 2000; Kenyon et al., 1993). Changes in nutrient availability also impact vertebrate reproductive development and success. For example, work from cows, pigs, and sheep indicate that poor nutritional status reduce oocyte quality and fecundity (Fouladi-Nashta et al., 2007; Papadopoulos et al., 2001). And, in humans, insulin triggers the insulin growth factor receptor (IGFR1) to induce progesterone secretion, which in turn promotes the maturation of ovarian follicle cells and normal female fertility (Poretsky et al., 1999; Silva et al., 2009).
In Drosophila and C. elegans, mechanistic studies indicate that insulin signaling links nutritional conditions to the proliferation rate of germ line stem cells (Drummond-Barbosa and Spradling, 2001; Hsu and Drummond-Barbosa, 2009; Michaelson et al., 2010). For example, in flies, a protein-rich diet appears to induce the secretion of insulin-like peptides from the brain, which act systemically to activate insulin signaling in remote tissues (Colombani et al., 2003). In the ovaries, activation of the insulin signaling pathway increases the division rate of both somatic and germ line stem cells, promotes cell survival, and increases vitellogenesis, a process by which oocytes uptake yolk during ovarian follicle maturation (Drummond-Barbosa and Spradling, 2001; LaFever and Drummond-Barbosa, 2005). In worms, in response to nutrient-replete conditions activation of the DAF-2 insulin-like receptor also enhances germ line stem cell proliferation in the female germ line during larval stages and in certain tumor germ lines (Angelo and Van Gilst, 2009; Michaelson et al., 2010; Pinkston et al., 2006). To regulate stem cell proliferation in the Drosophila and C. elegans germ line, insulin-like signaling acts through its canonical pathway – PI3K (AGE-1 in C. elegans) and the AKT/ AKT-1 serine threonine kinase to phosphorylate and inactivate the FOXO/DAF-16 forkhead transcription factor (Cavaliere et al., 2005; Michaelson et al., 2010). Thus, in flies and worms insulin signaling acts as a relay system to couple external conditions to the proliferation of germ line stem cells. No link, however, has been observed between insulin signaling and meiotic progression.
During oogenesis in mammals and C. elegans, but not Drosophila, activation of ERK, the terminal kinase of the conserved RTK-RAS-ERK signaling pathway, plays a key role in meiotic maturation (Ivanovska et al., 2004; Lee et al., 2007; Miller et al., 2001; Verlhac et al., 1993). In mammalian oocytes, sustained ERK activation for ~12 hours from pro-metaphase of Meiosis I (MI) until the end of Meiosis II, turning off just minutes before fertilization, is essential for many steps of meiotic progression, such as spindle migration during meiosis I, the first meiotic division, prophase progression of meiosis II, arrest at meiosis II, and the transition from metaphase of meiosis I through metaphase of meiosis II (Brunet and Maro, 2005; Choi et al., 1996; Verlhac et al., 1996). During oogenesis in mammals, ERK is activated by Mos, a meiosis specific serinethreonine kinase, that takes the place of RAF, and activates MEK in the canonical ERK pathway (Roy et al., 1996; Verlhac et al., 1996). During meiosis, Mos activation appears to be under translational control and ERK-mediated positive feedback (Charlesworth et al., 2002; Matten et al., 1996), rather than growth factor signaling. Studies from Xenopus oocytes, however, implicate progesterone activation as an upstream signal that activates Mos (Frank-Vaillant et al., 1999), potentially placing meiotic progression in Xenopus oocytes under physiological control.
In C. elegans oocytes, sustained activation of ERK also drives meiotic progression (Lee et al., 2007). Here, ERK is activated by the conserved RAS-RAF-MEK cascade, starting in the pachytene phase of meiotic prophase I and continuing for ~18 hours into the diplotene stage of meiosis I (Lee et al., 2007). During this time, active ERK regulates many events required for meiotic progression, such as pachytene progression (into diplotene), plasma membrane organization of pachytene cells, germ cell apoptosis, and oocyte growth (Arur et al., 2009; Church et al., 1995; Gumienny et al., 1999; Lee et al., 2007). The upstream signals that trigger activation of the RAS-ERK pathway during meiotic progression in worms are unknown.
The C. elegans oogenic germ line represents a powerful model system in which to identify the upstream pathways that activate ERK during meiosis. Activation of the RAS-ERK pathway occurs in two distinct regions of the worm germ line: In the middle region, termed zone 1 in this paper, MPK-1 (ERK) activation is required, as noted, for progression of meiotic prophase I; in the proximal region of the germ line, termed zone 2 in this paper, MPK-1 activation triggers the maturation, ovulation, and ultimately fertilization of oocytes (Miller et al., 2001). This bimodal activation pattern of MPK-1 can be directly visualized by the presence of the activated, di-phosphorylated form of MPK-1 (dpMPK-1; Fig. 1A). Prior work identified that a sperm-derived signal acts through an Ephrin receptor tyrosine kinase (RTK) (Miller et al., 2001; Miller et al., 2003), to activate MPK-1 in the proximal germ line (zone 2) ensuring that oocytes ovulate only in the presence of sperm. But, neither the signal nor the receptor that activates MPK-1 in zone 1 to drive meiotic progression has been identified.
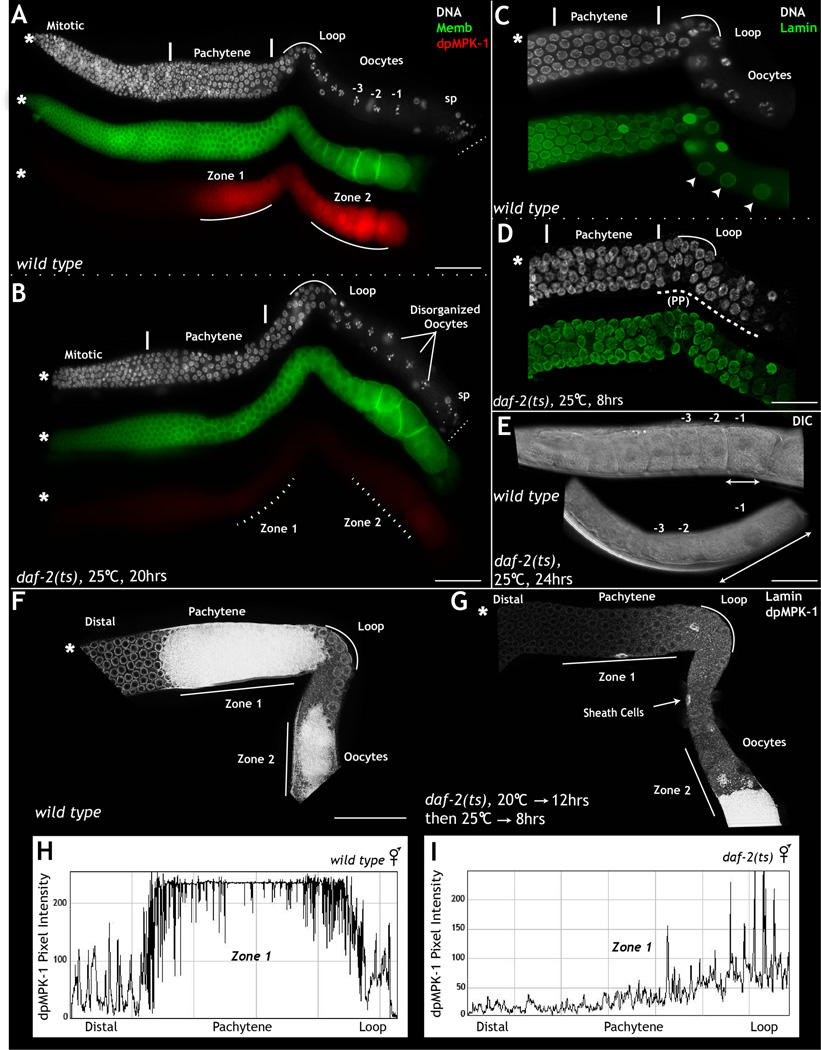
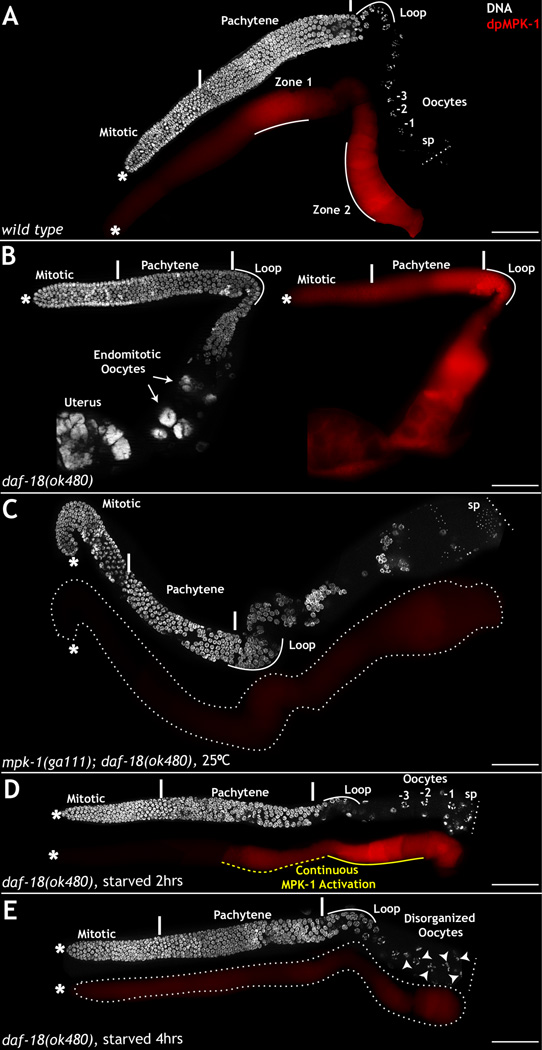
Figure 1. daf-2 regulates MPK-1 activation and function in zone 1.
Dissected C. elegans hermaphrodite germ lines, oriented from left (mitotic cells *) to right (oocytes), stained for membrane (green), dpMPK-1 (red), and DNA (DAPI, white). A: Wild type germ lines (20 hours/L4 at 25°C) exhibit dpMPK-1 in zones 1 and 2, and linear formation of oocytes (marked -1,-2,-3 per birth order). B: daf-2 mutant germ lines exhibit reduced dpMPK-1 in zones 1 and 2, and large, disorganized oocytes. C–D: Germ lines from wild type (C) and daf-2 mutant worms (D) stained for DNA (white) and lamin (green). daf-2 mutant germ lines exhibit delayed pachytene progression (PP). Wild type germ lines display normal pachytene progression and oocyte formation (arrow heads). E: DIC image of whole mount wild type and daf-2 mutant germ lines. In daf-2 loss-of-function animals, oocytes are reduced in number with a large -1 oocyte. F–G: Wild type and daf-2 mutant germ lines maintained at 25°C for 8 hours (at 20 hours/L4) stained with dpMPK-1 and lamin. daf-2 mutant germ lines display a specific loss of dpMPK-1 in zone 1. dpMPK-1 levels in daf-2 mutants are downregulated in the germ cells, as evidenced by visible somatic sheath cells (arrows). In wild type germ lines the dpMPK-1 staining in somatic sheath cell is masked by intense dpMPK-1 accumulation in germ cells. Experiments performed 5 times; 25–30 germ lines analyzed each time. H–I: Image J based pixel intensity of dpMPK-1 in zone 1 from wild type and daf-2 mutant germ lines depicted in F–G. X-axis represents position along the germ line; Y-axis represents dpMPK-1 pixel intensity. See also Figure S1 and Tables S1 and S2. Scale bar: 20µm.
Here, we show that in the C. elegans gonad the insulin-like receptor DAF-2 couples external nutritional conditions to meiotic progression by activating MPK-1 in zone 1. In the presence of food, DAF-2 activates MPK-1 in zone 1, promoting meiotic progression and oocyte production; in the absence of food, DAF-2 does not activate MPK-1 in zone 1, and meiotic progression is stalled resulting in loss of oocyte production. In this activity, DAF-2 acts through the RAS-RAF-MEK cascade rather than the canonical PI3K/AKT/FOXO pathway. Thus, the C. elegans germ line coordinates two distinct steps of meiosis with distinct external cues ensuring that mature gametes are produced in the presence of both sperm and favorable nutritional conditions.
RESULTS
DAF-2 activates MPK-1 during meiotic prophase I in the germ line
To identify the receptor that activates MPK-1 in zone 1, we first tested if the EGF receptor (LET-23) or FGF Receptor (EGL-15), canonical activators of the RAS-ERK pathway in many species are required for MPK-1 activation in zone 1. Germ lines obtained from worms homozygous mutant for null alleles of let-23 or from those in which egl-15 function was depleted specifically in the germ line, via the use of rrf-1 animals (see Supplemental Experiment Procedures), displayed wild type dpMPK-1 levels and oocyte development (Fig. S1A–C). Thus, we concluded that neither LET-23 nor EGL-15 regulate MPK-1 in the worm germ line.
The RAS-ERK pathway is typically activated by a receptor tyrosine kinase (RTK) (Sundaram, 2006). The C. elegans genome contains 29 classified and 11 unclassified RTKs (Plowman et al., 1999). Thus, to determine if a RTK activates MPK-1 in zone 1 of the germ line, we performed germ line-specific RNAi on the major family member of the 11 classes of RTKs (Table S1), scoring animals by DIC imaging for mpk-1-like loss-of-function germ line phenotypes (Lee et al., 2007). Of the 11 RTKs analyzed, only RNAi of the daf-2 type-1 insulin-like growth factor receptor elicited germ line phenotypes indicative of loss of mpk-1 function (Fig. S1D–E), suggesting that DAF-2, a type-1 insulin like receptor, activates MPK-1 in zone 1 of the germ line.
During C. elegans development, DAF-2 regulates entry into and exit from the dauer state, an alternative dormant state worms enter into at developmental stage 2 (L2) in response to stressed environmental conditions (e.g. lack of food) (Evans et al., 2008; Kenyon et al., 1993). Under normal conditions, active DAF-2 signals through AGE-1 (PI3-kinase) and AKT-1 to inhibit DAF-16 function and bypass entry into dauer. Under stressed conditions, DAF-2 signaling is inhibited and active DAF-16 induces worms to enter dauer. Re-activation of DAF-2 signaling is normally absolutely required for worms to exit dauer and continue development, although loss of daf-16 function can both trigger exit from the dauer state and suppress entry into dauer in the absence of daf-2 (or age-1 or akt-1) function (Dorman et al., 1995; Lin et al., 2001). Thus, loss of daf-2 function by itself induces worms to enter but not leave the dauer state; in these worms the germ line, which develops during young adulthood just after the L4 larval molt, never forms. Thus, the early requirement for DAF-2 to bypass or exit the dauer phase may have occluded discovery of a subsequent role for DAF-2 in the adult germ line.
To circumvent this early requirement for daf-2 function, we used three temperature-sensitive (ts) alleles of daf-2 and assayed adult germ lines for dpMPK-1 levels and phenotypes indicative of loss of mpk-1 function (Table S2). We allowed wild type and daf-2 mutant animals to develop at the permissive temperature (15°C) until young adulthood (mid-L4 stage) and then shifted the animals to the restrictive temperature of 25°C for 12–24 hours. This treatment had no effect on wild type animals: their germ lines exhibited wild type levels of dpMPK-1 and developed the characteristic linear row of 7–8 oocytes (Fig. 1A). In contrast, germ lines of daf-2 mutant animals exhibited a drastic reduction in dpMPK-1 levels in zones 1 and 2 (Fig. 1B, Fig. 1I, Fig. S1 compare S1E, G to S1L–O) and multiple phenotypes indicative of reduced mpk-1 function (Table. S2) (Lee et al., 2007): the presence of few large, disorganized oocytes (Fig. 1B and Fig. 1E), defects in meiotic prophase I progression (Fig. 1D), and increased germ cell apoptosis (Fig. S1G). More complete elimination of daf-2 function either via germ line specific daf-2 RNAi or the use of dafachronic acid to bypass dauer formation in daf-2 loss-of-function animals (Methods) led to a complete loss of detectable MPK-1 activation and germ line phenotypes essentially identical to those observed upon elimination of mpk-1 function in the germ line (Fig. S1E–G). These data suggest that DAF-2 regulates MPK-1 in the germ line, and its loss leads to germ line phenotypes that mirror those elicited upon abrogation of MPK-1 function.
The C. elegans germ line develops in an assembly-line manner, with germ cells developing into mature oocytes (zone 2) within 3–4 hours after they exit the pachytene phase of meiotic prophase I (zone 1) (Lee et al., 2007). Thus, the observed loss of MPK-1 activity in zones 1 and 2 of daf-2 mutant germ lines could arise because DAF-2 regulates MPK-1 in both zones, or because DAF-2 regulates MPK-1 specifically in zone 1, and loss of MPK-1 function in zone 1 leads to loss of MPK-1 activation in zone 2 due to subsequent defects in oocyte development. To distinguish between these models, we maintained daf-2 mutant animals at the permissive temperature of 15°C for 12 hours past L4, shifted them to the restrictive temperature for 8 hours, and then assayed the resulting effect on dpMPK-1 levels in zones 1 and 2. daf-2 mutant germ lines that underwent this treatment exhibited a specific loss of dpMPK-1 in zone 1 (pachytene) with little or no effect on dpMPK-1 levels in zone 2 (proximal oocytes) (Fig. 1F–G). Quantification of the decrease in dpMPK-1 levels in zone 1 from daf-2 mutant germ lines relative to wild type revealed a 90% reduction of dpMPK-1 levels in zone 1 but a less than 1% change in zone 2 (Fig. 1H–I and Fig. S1H–O). In addition, daf-2 mutant germ lines contained 2–3 oocytes versus 7–8 in wild type, suggesting that the reduction in daf-2 function in zone 1 resulted in halting of oocyte production (Fig. 1B, Fig. 1E and Fig. S1). Together, our results support the model that DAF-2, regulates MPK-1 activation in zone 1 of the germ line and through this regulation triggers progression of meiosis I.
DAF-2 couples the presence of nutrition to RAS-MPK-1 pathway activation independently of AGE-1/AKT-1/DAF-16 pathway
The insulin pathway is known to couple nutrient status to cell and organismal growth: in the presence of food, insulin signaling is active and promotes growth; in the absence of food, insulin signaling is inactive and growth is inhibited (Neufeld, 2003). To test whether DAF-2 couples nutritional status to activate the RAS-MPK-1 pathway and oogenesis in C. elegans, we starved fully developed wild type worms and assayed the resulting effect on dpMPK-1 in zone 1 and oocyte development. Prolonged starvation exhibits pleiotropic effects on germ cell development independent of any one signaling pathway (Angelo and Van Gilst, 2009). Thus, we starved animals for varying times to test whether loss of nutrition specifically affects dpMPK-1 prior to manifestation of any visible effects of starvation. We starved animals for 30 mins, 1 hr, 2 hr, 4 hr, 6 hr, at 24 hrs past L4 stage of development (Fig. S2, not shown) and then assayed germ lines for dpMPK-1. Starvation for as little as one hour reduced dpMPK-1 levels in zone 1 (Fig. S2B), and a two hour starvation resulted in near complete loss of dpMPK-1 levels in zone 1 but not in zone 2 (Fig. 2C, compared to Fig. 2A, Fig. S2). Starvation for 4 and 6 hours resulted in defects in pachytene progression and the formation of large, disorganized oocytes, with very reduced MPK-1 activation in zone 2 (Fig. S2C–D), similar to mpk-1 loss in the germ line (Arur et al., 2009). Quantification of the decrease in dpMPK-1 levels in two-hour starved animals relative to fed animals revealed a greater than 90% reduction in dpMPK-1 levels in zone 1, but little or no change in dpMPK-1 levels in zone 2 (Fig. 2C–D). Thus, transient starvation of adult worms yields the same germ line phenotype as transient reduction of daf-2 function: a specific reduction of MPK-1 activation during pachytene progression in zone 1 and the formation of large disorganized oocytes (Fig. 1B vs 1A, Fig. 1H vs 1I, Fig. 2C, Fig. S2B–D).
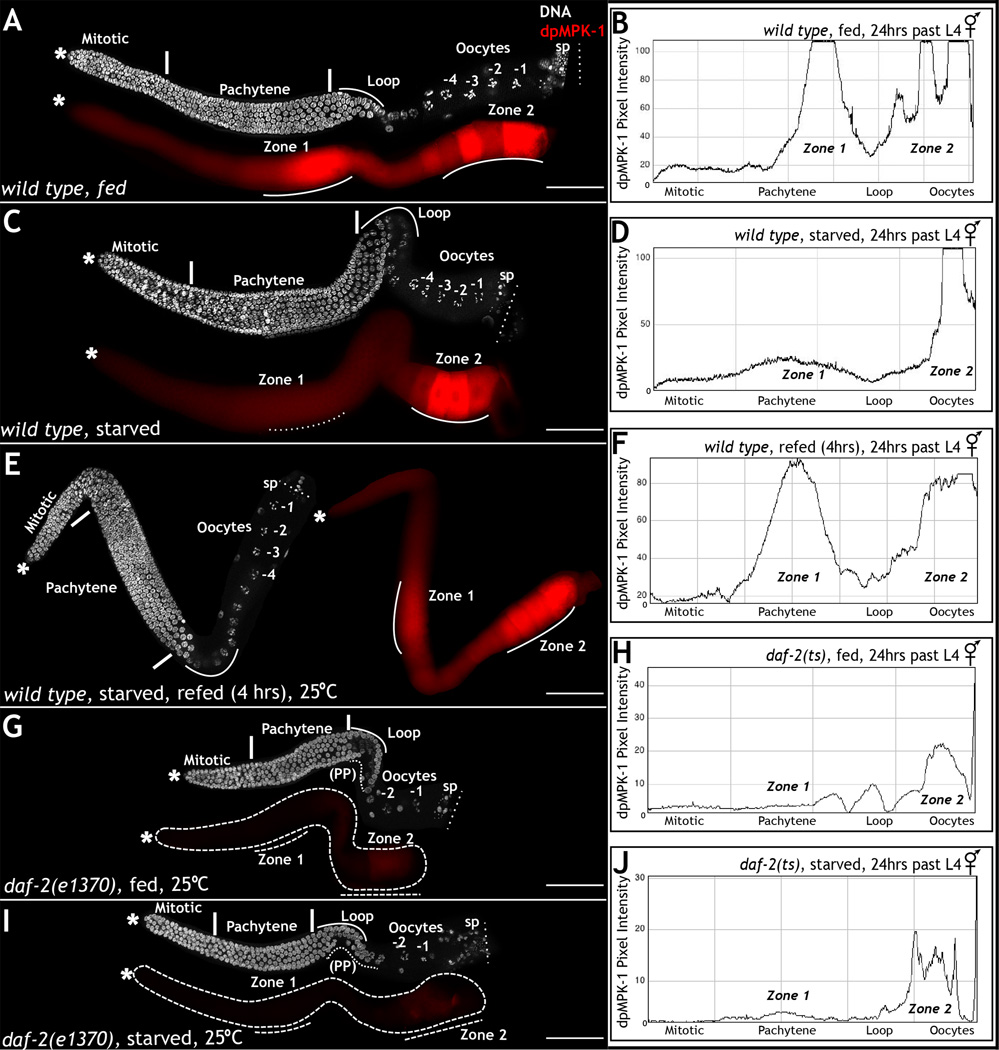
Figure 2. daf-2 couples nutritional cues to MPK-1 activation, in zone 1.
A, C, E, G, I. Dissected adult (24 hours past L4) hermaphrodite germ lines stained for DNA (DAPI, white) and dpMPK-1 (red). A: Wild type germ lines from fed conditions exhibit two zones of MPK-1 activation and 6–7 oocytes. C: Wild type germ lines from starved (2 hours) conditions exhibit reduced dpMPK-1 in zone 1, but not zone 2. E: Wild type germ lines from animals refed upon starvation reveal restoration of dpMPK-1 in zone 1. G–I: daf-2 mutant germ lines exhibit reduced dpMPK-1, pachytene progression defects, and one large oocyte both on and off food. B, D, F, H, J. Quantitative measure of dpMPK-1 levels from A, C, E, G and I taken with Image J. X-axis depicts germ cell position along the length of the germ line, and Y-axis measures the dpMPK-1 accumulation as pixel intensity. Experiment performed 4 times; 50 germ lines analyzed each time. See also Figure S2. Scale bar: 20µm.
The effect of starvation is reversible: animals starved for two hours and then refed for four hours displayed normal levels of dpMPK-1 and reinitiated oocyte production (Fig. 2E). In addition, starvation of daf-2(e1370) mutant animals for two or four hours at the restrictive temperature did not exacerbate the loss of dpMPK-1 or the oocyte phenotype (Fig. 2G–J), suggesting that nutrition and daf-2 act in the same pathway to regulate MPK-1 activation and oocyte production. Thus, DAF-2 appears to couple nutritional status to the activation of MPK-1 during meiotic prophase and thus oocyte generation.
Caloric restriction has been shown to lengthen C. elegans life span (Lakowski and Hekimi, 1998). Mutations in genes that disrupt pharyngeal function and reduce normal feeding (“eat” mutations) result in calorically restricted animals that live longer. With respect to aging, this caloric restriction pathway functions in parallel to the insulin signaling pathway (Lakowski and Hekimi, 1998). We tested whether caloric restriction had an impact on dpMPK-1 in zone 1 and oocyte production by analyzing two distinct eat-2 mutant germ lines and found that even though the animals were somatically slow growing and scrawny in appearance, loss of eat-2 function had no impact on zone 1 MPK-1 activation or oocyte production (Fig. 2E–G). Thus, as observed for aging, insulin signaling acts independently of the caloric restriction pathway to regulate meiotic progression.
DAF-2 acts through the AGE-1/AKT-1 cascade to inhibit DAF-16 function to regulate dauer formation, aging, and germ line proliferation in C. elegans (Michaelson et al., 2010). We thus asked whether DAF-2 acts through age-1 and akt-1 to regulate MPK-1 activation in zone 1. Since AGE-1 and AKT-1, like DAF-2 regulate entry into the dauer state in C. elegans, we performed RNAi analysis of age-1 and akt-1 in wild type animals and in rrf-1 animals. As reported earlier (Dorman et al., 1995), RNAi of age-1 and akt-1 in wild type worms triggered entry into dauer state (not shown). When depleted in the rrf-1 mutant background, germ lines with loss of akt-1 or age-1 exhibit normal dpMPK-1 in zone 1 and oocyte development (Fig. S3A–D). Thus, DAF-2 does not appear to function through the AGE-1 or AKT-1 to regulate dpMPK-1 in zone 1.
Next, we asked whether daf-2 acts through daf-16 to activate MPK-1 in zone 1. In the canonical Insulin signaling pathway, daf-2 activates insulin signaling by repressing daf-16, and loss of daf-16 results in active insulin signaling regardless of whether daf-2 function is present (Apfeld and Kenyon, 1998). Thus, if daf-2 acts through daf-16 to activate MPK-1 in zone 1 of the germ line, loss of daf-16 should reverse the effects of both starvation and loss of daf-2 function on dpMPK-1 in the germ line. But we found that daf-16 null mutant worms starved for 2 hours behaved identically to wild-type worms: they downregulated dpMPK-1 levels in the germ line (Fig. 3B to 3A). Thus, loss of daf-16 function fails to reverse the effects of starvation, consistent with daf-2 acting independently of daf-16 to activate MPK-1 in zone 1 of the germ line.
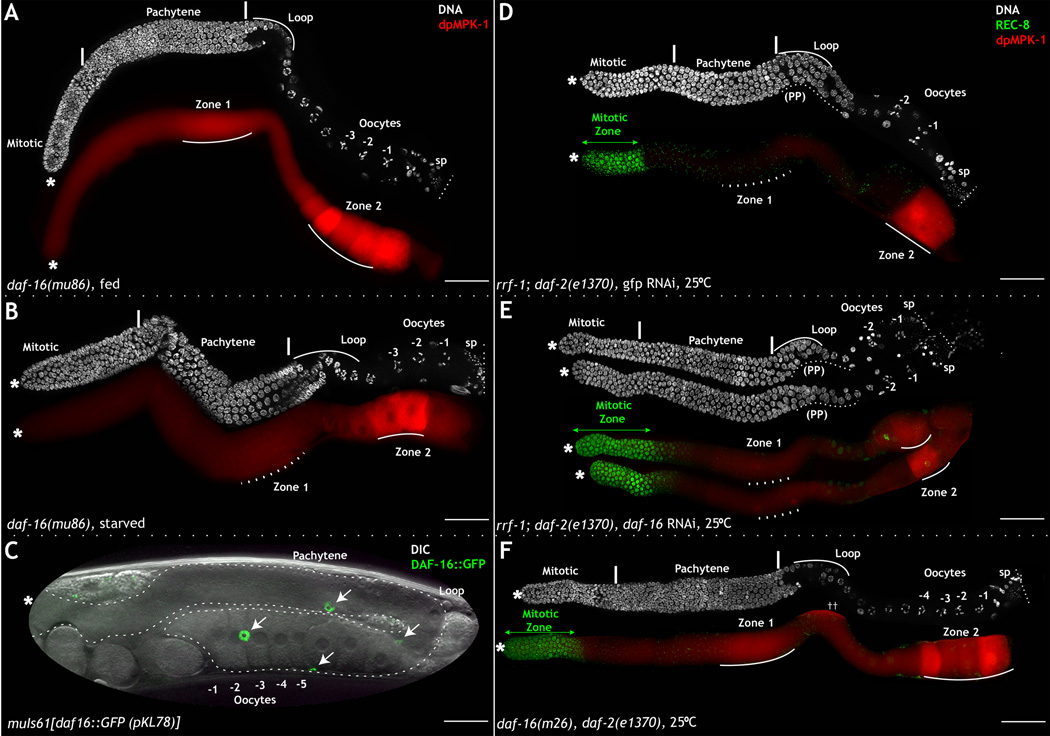
Figure 3. daf-16 does not function downstream to daf-2 / nutritional cues to regulate zone 1 MPK-1 activation and oocyte development.
Dissected adult hermaphrodite germ lines stained for DNA (DAPI, white), dpMPK-1 (red), and REC-8 (green). A–B: daf-16(mu86) germ lines on either fed (A) or starved (B) conditions. daf-16 germ lines from fed animals reveal normal dpMPK-1 in zone 1 and oocyte development (A), but reduced dpMPK-1 in zone 1 and decreased oocyte production in starved condition (B). RNAi analysis was performed in triplicate and 50 germ lines analyzed for each genotype. Starvation experiment was performed five times, and 20–25 germ lines analyzed each time. C: Whole mount DIC and GFP analysis of muIs61, DAF-16∷GFP animals. Arrows indicate nuclear staining of DAF-16∷GFP in somatic gonadal sheath cells. D–E: Germ lines from rrf-1;daf-2 animals at the restrictive temperature with gfp (D) or daf-16 (E) RNAi treatment. E: Reduction of daf-16 in daf-2 mutant animals results in restoration of the mitotic proliferative germ cells (green arrow), but does not rescue pachytene progression defects (PP), stalled oocyte development, or MPK-1 activation in zone 1. F: Germ lines from daf-16;daf-2 double mutant animals have normal mitotic zone development and dpMPK-1. ++ marks non-specific signal from the intestine. See also Figure S3. Scale bar: 20µm.
Next, we assayed a daf-16∷GFP transgene, muIs61, that fully rescues the daf-16(mu86) null mutant background to follow DAF-16 expression and localization (Lin et al., 2001). In the germ line itself, DAF-16∷GFP is barely detectable, indicating daf-16 is expressed at low levels in this tissue. DAF-16∷GFP, however, is expressed at high levels in the somatic gonadal sheath cells, localizing to the nuclei of these cells under normal fed conditions (Fig. 3C). As DAF-2 signaling leads to the phosphorylation of DAF-16 and its subsequent nuclear exclusion, our observation suggests DAF-16 acts independently of DAF-2 in the somatic gonad, a tissue that exerts profound non-autonomous control over many aspects of germ line development, including mitosis, meiotic progression, and ovulation (McCarter et al., 1997).
Due to DAF-16 expression in the somatic gonad, we assessed the effect of removing daf-16 function from daf-2 mutant worms via germ line-specific depletion of daf-16 function (daf-16 RNAi in the rrf-1 background) and systemic depletion of daf-16 function via RNAi or the use of daf-16 null alleles. Depletion of daf-16 function in rrf-1;daf-2(e1370) mutant worms suppressed the daf-2 proliferation phenotype observed in the mitotic zone of the germ line (as previously reported by (Michaelson et al., 2010); compare Fig. 3D to 3E), but had no effect on the loss of dpMPK-1 in zone 1 or decreased oocyte production observed in the proximal germ line of daf-2 mutant worms (compare Fig. 3D to 3E). This result indicates that within the germ line daf-2 does not signal through daf-16 to activate MPK-1. In contrast, systemic loss of daf-16 function in a daf-2 mutant background, either via the use of RNAi or three daf-16 null alleles (see methods), rescued both the germ line proliferation phenotype and the loss of dpMPK-1 in zone 1 (Fig. 3F). This result indicates that systemic loss of daf-16 impacts germ line development and MPK-1 activation within it, in an indirect manner via a function in the somatic gonadal sheath cells. Integrated together, we believe the simplest interpretation for all of our results is that within the germ line daf-2 acts independently of age-1, akt-1, and daf-16 to activate MPK-1 and meiotic progression, and that a previously unappreciated and daf-2-independent role for DAF-16 in somatic gonadal sheath cells accounts for the restoration of dpMPK-1 levels in zone 1 in daf-16, daf-2 double mutant worms.
DAF-2 signals through the RAS-MPK-1 cascade to regulate meiotic progression and oocyte development
Our data indicate that DAF-2 activates MPK-1 during meiotic prophase I (zone 1). Prior work indicates that the RAS (LET-60), RAF (LIN-45), and MEK (MEK-2) module activates MPK-1 during meiotic prophase I in the C. elegans germ line (Lee et al., 2007). Thus, we asked if DAF-2 signals via this cascade to activate MPK-1 and regulate germ line development. To assay if DAF-2 acts through MEK-2 and MPK-1, we generated a GFP∷DAF-2 transgene (Fig. 4A) wherein GFP∷DAF-2 expression was placed under the control of the germ line specific pie-1 promoter (Supplemental Experimental Procedures). We first tested whether the presence of the transgene rescues the daf-2(e1370) mutant phenotype at the restrictive temperature and found that it restored normal oocyte development and zone 1 MPK-1 activation (Fig. 4B). Analysis of the localization of GFP∷DAF-2 in the germ line reveals that GFP∷DAF-2 localizes to the cell membrane, the cytoplasm, and cytoplasmic vesicles (Fig. S4B, inset).
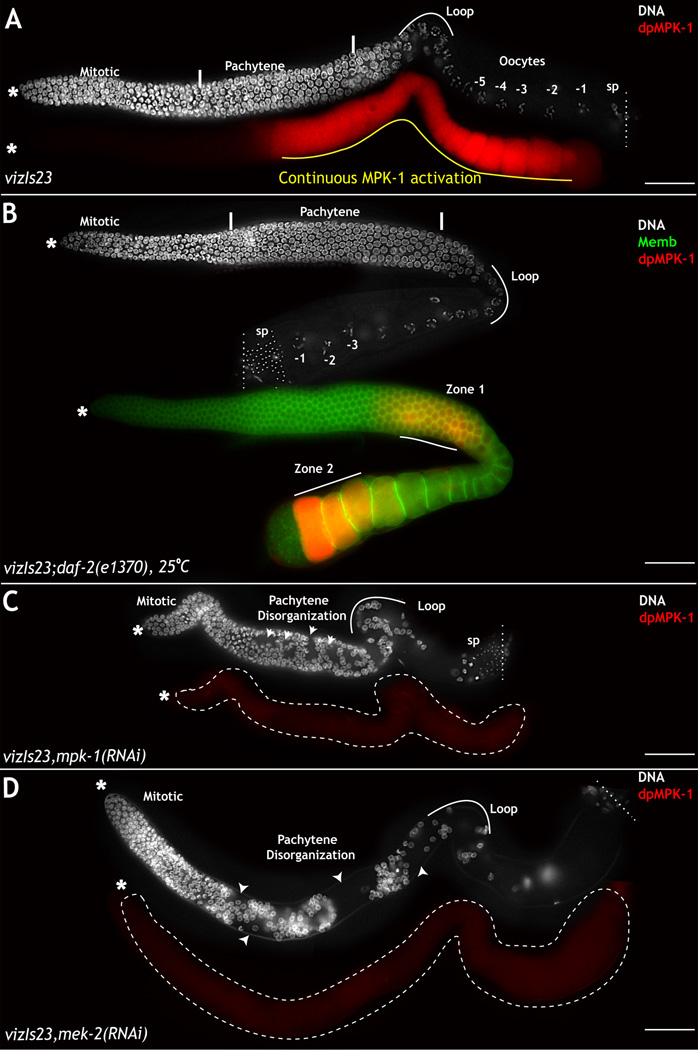
Figure 4. MPK-1 and MEK-2 are epistatic to DAF-2 over-expression.
Dissected adult hermaphrodite germ lines stained with dpMPK-1 (red) and DNA (DAPI, white). A: Germ lines obtained from GFP∷DAF-2 transgenic animals exhibit continuous dpMPK-1 through zone 1 (yellow line). B: Germ lines from vizIs23 (GFP∷DAF-2);daf-2(e1370) animals at 25°C exhibit normal dpMPK-1 levels in zone 1, and oocyte development. C–D: Germ lines obtained from DAF-2 overexpression animals upon RNAi treatment with mpk-1 or mek-2 reveal mpk-1 loss-of-function phenotypes. RNAi experiment performed 3 times; 30–35 germ lines assayed each time. See also Figure S4. Scale bar: 20µm
In the wild type background, the presence of the transgene elicited in heightened accumulation of dpMPK-1 in zone 1 and the loop region (Fig. 4A), consistent with DAF-2 activating MPK-1 in zone 1. Depletion of either mek-2 or mpk-1 function in this background resulted in complete loss of dpMPK-1 levels in meiotic prophase I and the loop region and also produced phenotypes similar to mek-2 and mpk-1 mutants: pachytene arrest of cells and clumping and disorganization of pachytene cells, as evidenced by the ‘holes’ in zone 1 (Fig. 4C–D). Thus, daf-2 requires mek-2 and mpk-1 for its function in zone 1, suggesting that DAF-2 acts upstream of MEK-2 and MPK-1 to regulate oogenesis in the germ line.
In support of daf-2 acting through the RAS-MPK-1 pathway in zone 1 of the germ line, we found that mutations in mpk-1 are also epistatic to mutations in daf-18. DAF-18, the worm PTEN homolog, negatively regulates DAF-2 signaling downstream of receptor activation (Ogg and Ruvkun, 1998), and daf-18 mutant worms display heightened dpMPK-1 levels in zone 1 and an increased ovulation rate (Fig. 5B vs 5A), presumably due to both increased insulin signaling as well as a reported role for DAF-18 downstream to VAB-1 Eph receptor signaling to negatively regulate oocyte ovulation (Brisbin et al., 2009). To test whether mpk-1 functions downstream to daf-18 during zone 1 activation and meiotic progression, we generated daf-18;mpk-1 double mutants, and conducted RNAi analysis of mpk-1 in rrf-1;daf-18 animals. Worms of both genetic backgrounds exhibited germ line phenotypes indistinguishable from mpk-1 mutant worms: pachytene progression defects, increased germ cell death and loss of oocyte production (Fig. 5C and not shown). These data suggest that DAF-18 attenuates DAF-2 mediated activation of the RAS-MPK-1 pathway in zone 1 of the germ line.
Figure 5. daf-18/PTEN functions downstream to nutritional cues and upstream to mpk-1.
Dissected adult hermaphrodite germ lines stained for DNA (DAPI, white) and active MPK-1 (red). A–B: Germ lines from wild type (A) or daf-18(ok480) (B) animals. Loss of daf-18 results in continuous dpMPK-1 through zone 1 (yellow line) and endomitotic oocytes in the germ line (arrows) and the uterus. C: daf-18;mpk-1 double mutants exhibit pachytene arrest and no oocyte production (B). D–E: daf-18 loss-of-function animals after 2 hours (D) and 4 hours (E) of starvation exhibit downregulation of dpMPK-1 in zone 1 and suppression of the hyper-ovulation phenotype (D). Starvation for 4 hours (E) results in further reduction in dpMPK-1 and oocyte disorganization phenotypes (arrow heads). Experiments performed 3 times; 50–60 germ lines analyzed each time. Scale bar: 20 µm
Consistent with loss of daf-18 function leading to increased daf-2 signaling in zone 1, the germ lines of daf-18 mutant are partially resistant to starvation. Germ lines of daf-18(ok480) worms subjected to a two-hour starvation retained elevated dpMPK-1 levels in zone 1 and continued to produce oocytes, albeit at a decreased rate relative to fed daf-18 mutant worms (Fig. 5D). Germ lines of daf-18(ok480) animals subjected to a four-hour starvation, however, exhibited a starvation phenotype: they downregulated dpMPK-1 levels in zone 1, ceased ovulation, and contained only one to two large oocytes as in wild type animals (Fig. 5E). Thus, daf-18 mutant animals are resistant to short, but not extended, stretches of starvation, consistent with the ability of DAF-18 to oppose insulin signaling downstream of DAF-2 receptor activation.
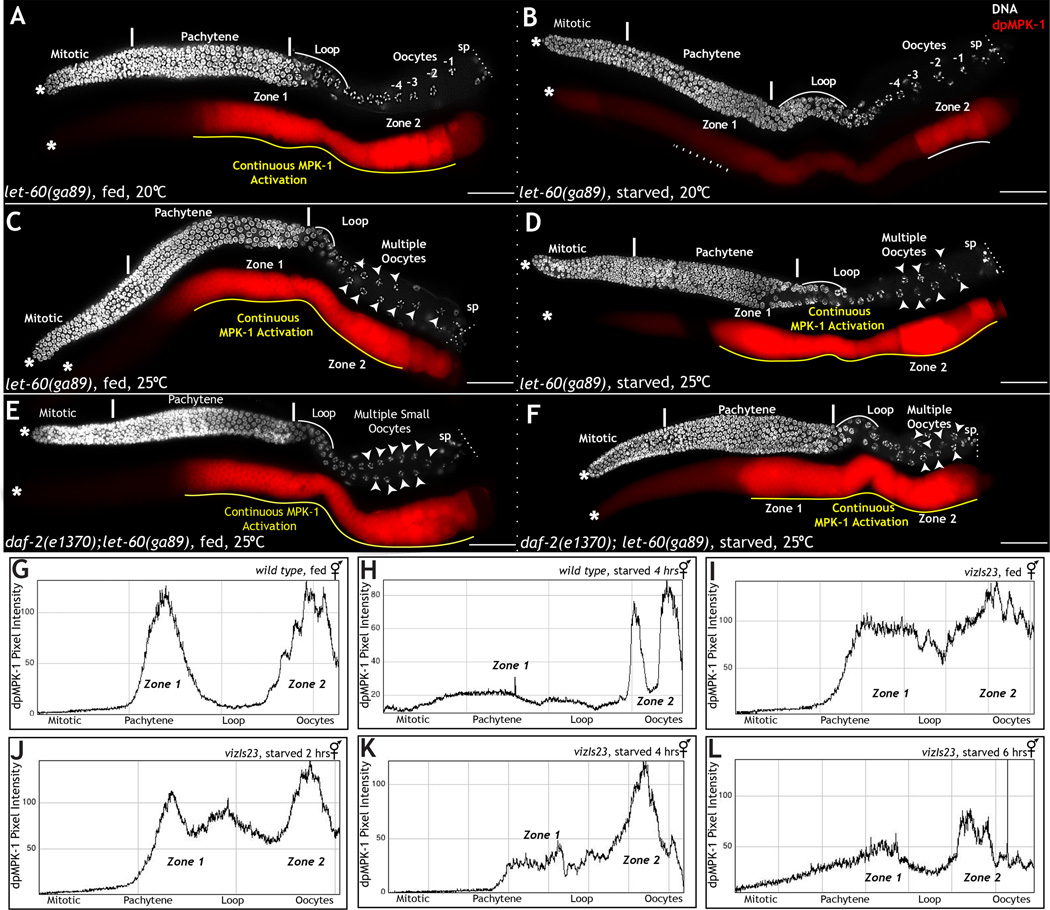
If nutrition and daf-2 act through the RAS-MPK-1 pathway to regulate oogenesis, then constitutive activation of let-60 (ras) should reverse the effect of loss of daf-2 function and starvation with respect to oocyte development and production. To test this model, we used the gain-of-function, temperature sensitive let-60(ga89gf) mutant. At permissive temperature (20°C), let-60(ga89gf) animals exhibit normal germ line development, but heightened dpMPK-1 levels in zone 1 and the loop region (Fig. 6A). At restrictive temperature (25°C), the germ lines of these animals exhibit hyperactivation of dpMPK-1 and strong gain-of-function phenotypes, including the production of multiple, small oocytes (Fig. 6C) (Lee et al., 2007). At permissive temperature, we found that the germ lines of let-60(ga89gf) animals were partially resistant to starvation: dpMPK-1 was downregulated after 4 hours of starvation, but not after two hours of starvation (Fig. 6B). At restrictive temperature, however, the germ lines of daf-2(e1370); let-60(ga89gf) (Fig. 6F) or let-60(ga89gf) animals starved for two (Fig. 6D) or four hours (not shown) looked identical to those of fed let-60(ga89gf) worms (Fig. 6C): the germ lines retained elevated dpMPK-1 levels in zone 1 and continued to produce small oocytes. This was true even though after the four-hour starvation let-60 animals were overtly thinner (and thus starved of nutrients). Thus, the let-60 gain-of-function phenotype is epistatic to daf-2 and starvation, supporting the idea that nutrition signals via DAF-2 and the RAS-MPK-1 pathway to activate MPK-1 in zone 1 and promote progression through meiosis I.
Figure 6. LET-60 RAS functions downstream to DAF-2 and nutritional cues to regulate MPK-1 activation.
Dissected adult hermaphrodite germ lines stained for DNA (DAPI, white) and dpMPK-1 (red). A–F: let-60(ga89gf) (A–D) and daf-2;let-60(ga89gf) (E–F) animals fed or starved for 2 hours. A–D: At 25°C upon starvation (D) let-60(ga89gf) worms displays continuous dpMPK-1 through zone 1 (yellow line) 1 and produce double rows of small oocytes (arrow heads). At 20°C let-60(ga89gf) worms downregulate dpMPK-1 in zone 1 (B). E–F: daf-2;let-60(ga89gf) worms display continuous dpMPK-1 and multiple oocytes both on and off food at 25°C. Experiments performed 3 times; each time 40-germ lines analyzed. G–L: Quantitative measure of dpMPK-1 levels in zones 1 and 2 from germ lines of fed or starved DAF-2∷GFP (vizIs23) worms, taken with Image J. X-axis depicts germ cell position along the length of the germ line; Y-axis measures the dpMPK-1 levels as pixel intensity. Scale bar: 20µm
To assay whether over expression of the DAF-2 receptor was also epistatic to starvation, we starved wild type worms that harbored the GFP∷DAF-2 transgene for two, four, and six hours. These worms displayed partial resistance to starvation effects after two and four hours (Fig. 6D–L), but not six hours (Fig. 6L), suggesting that the receptor may be downregulated or turned over in the absence of signal over time.
DAF-2 acts in a homeostatic regulatory mechanism that couples oogenesis to environmental conditions
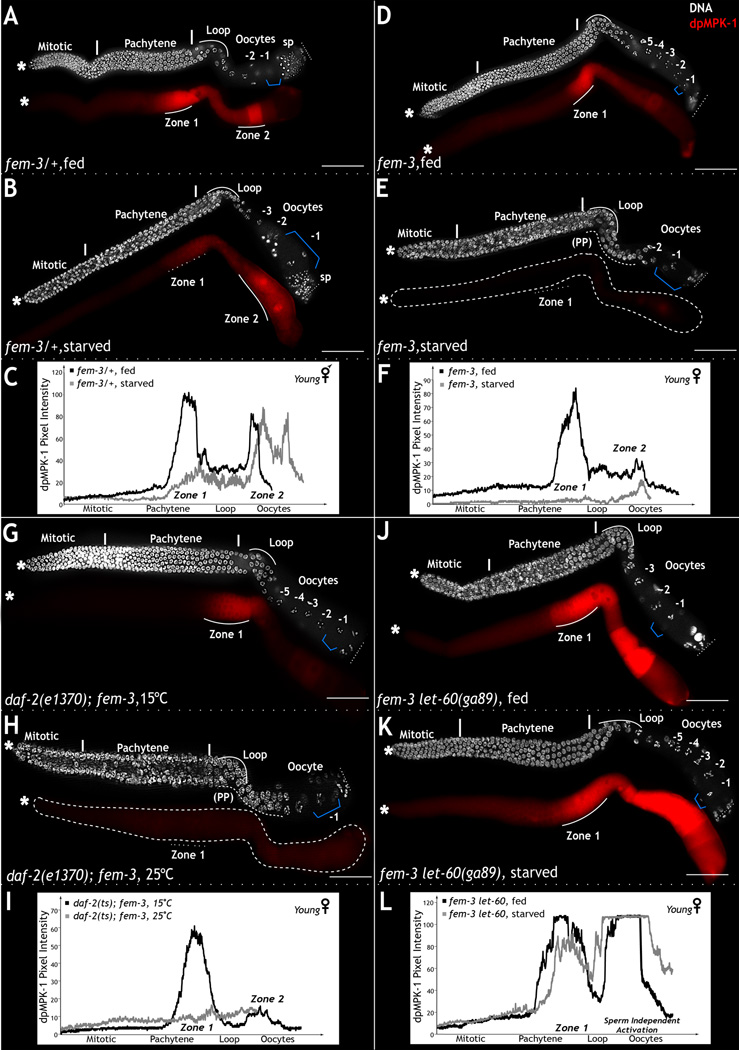
In wild type hermaphrodites, two independent signals activate MPK-1 in the germ line: daf-2 and nutrition in zone 1 (this study); the sperm signal in zone 2 (Miller et al., 2001). To isolate the effect of DAF-2 and nutrition-mediated activation on MPK-1 in zone 1 and germ line development from sperm-mediated activation of MPK-1 in zone 2, we used fem-3 (feminized mutant) animals. The germ lines of fem-3 animals are essentially identical to wild type hermaphrodites (Fig. 7A and Fig. 7C), except that they lack sperm and thus sperm-mediated activation of MPK-1 in zone 2 (Fig. 7D and Fig. 7F). Young females (6 hours after L4 molt) possess relatively normal levels of dpMPK-1 in zone 1 compared to age matched hermaphrodites (Fig. 7A, Fig. 7C vs 7D and Fig. 7F). Interestingly in the absence of sperm dependent high zone 2 MPK-1 activation, a dpMPK-1 signal in growing oocytes is visible, which we term as zone 2, sperm independent signal (Fig. 7). Much like the hermaphrodites, fem-3 germ lines generate 5–6 oocytes, but in contrast to the hermaphrodites, these oocytes arrest because in the absence of sperm there is no dpMPK-1 in zone 2 (Fig. 7A vs 7D and Fig. 7C vs 7F). Upon introduction of sperm, these arrested oocytes activate MPK-1 in zone 2 and undergo maturation, ovulation, and fertilization (Miller et al., 2001).
Figure 7. daf-2 and nutritional cues regulate dpMPK-1 in zone 1 for oocyte production.
A–B, D–E, G–H and J–K. Dissected hermaphrodite (A, B) and female (D–E, G–H, J–K) germ lines stained for DNA (DAPI, white) and dpMPK-1 (red). A–B: Young (6 hours/L4) fem-3 heterozygous germ lines on food (A) or starved (B) for two hours. Germ lines from fed worms exhibit dpMPK-1 in zone 1 and 2 and 3–4 oocytes. Starved germ lines display reduced dpMPK-1 in zone 1, defects in pachytene progression, and large oocytes (blue bracket). C: Quantitative measure of dpMPK-1 pixel intensity in zones 1 and 2 from A and B, collected with Image J. X-axis depicts germ cell position along the length of the germ line, and Y-axis measures the dpMPK-1 accumulation as pixel intensity. D–E: Young female germ lines from fed or starved (2 hours) conditions. Fed females display normal dpMPK-1 in zone 1. Starved (E) females display reduced dpMPK-1 in zone 1, pachytene progression defects, oocyte loss, and larger -1 oocyte (blue bracket). F: Quantitative analysis of dpMPK-1 levels from D and E, acquired using Image J, displayed as per C. G–H: Young daf-2 loss-of-function females at 15°C (G) or 25°C (H). Young daf-2 females at 25°C display defects in pachytene progression exhibit a reduction in oocyte number and contain large oocytes (blue bracket). I: Quantitative analysis of dpMPK-1 levels in germ lines analyzed in panels G and H, displayed as per C. J–K: Young let-60(ga89gf) females either fed (J) or starved (K). Fed let-60(ga89gf) females reveal continuous dpMPK-1 in zone 1 and sperm independent oocyte activation (L). Starved let-60(ga89gf) females reveal continuous dpMPK-1 through zone 1 and sperm independent region in the oocytes. L: Quantitative analysis of dpMPK-1 from J and K, displayed as per C. Experiments performed 5 times; each time 30–35 germ lines analyzed. See also Figure S5. Scale bar: 20µm.
To investigate the effect of loss of daf-2 function or starvation specifically on zone 1 MPK-1 activation, we analyzed the germ lines of young daf-2; fem-3 double mutants or of fem-3 animals starved for two hours. In both cases, we observed the same effect: the germ lines displayed a dramatic loss of dpMPK-1 in zone 1 and mpk-1 like loss-of-function phenotypes, such as loss of pachytene cell membranes, pachytene progression defects, and the formation of large, disorganized oocytes (Compare Fig. 7H to 7E, and Fig. 7I to 7F) – phenotypes essentially identical to those observed in daf-2 hermaphrodites or starved wild type animals (compare Fig. 1B–E to Fig. 2C and Fig. S1). Thus, as observed in hermaphrodites, DAF-2 acts through MPK-1 to drive the formation of oocytes in young female worms. Also as observed in hermaphrodites, constitutive activation of the RAS-MPK-1 pathway, through the use of the let-60 (ras) gain-of-function allele, is sufficient to drive oogenesis in young females even when starved. Young, fed or starved, fem-3(0);let-60(ga89gf) animals exhibited the same phenotype: high levels of dpMPK-1 in zone 1, and sperm independent zone 2 dpMPK-1, and the continual production of many small oocytes – phenotypes indistinguishable from those observed in animals singly-mutant for let-60(ga89gf) (Fig. 7J–L vs Fig. 6C–D). Thus, constitutive activation of the RAS-MPK-1 pathway in zone 1 is sufficient to drive oogenesis in the absence of nutrition and sperm signal in young females.
In contrast to young females, old females (24 hours after L4 larval molt) possess low levels of dpMPK-1 in zone 1 and a stockpile of 14–16 arrested oocytes (Fig. S5C). In these females, starvation did not appreciably reduce dpMPK-1 levels or impact germ line morphology beyond that typically observed in old females that were fed (Fig. S5C–D). But, when allowed to mate with males, within two-hours old females exhibited high levels of dpMPK-1 in zones 1 and 2 of their germ lines and their oocytes underwent maturation, ovulation and subsequent fertilization (Miller et al., 2001; not shown). It is formally possible that introduction of sperm, and activation of the sperm signal, directly activates MPK-1 in zones 2 and 1, but we favor a different model. We propose that in the absence of sperm, the stockpiling of arrested oocytes in old females eventually triggers a feedback mechanism that blocks MPK-1 activation and oocyte production in zone 1. Release of the block would require introduction of sperm and the subsequent resumption of oocyte maturation and fertilization. Regardless of the exact cause of this observation, we note that extended loss of MPK-1 activation in either zone 1 (lack of food) or in zone 2 (lack of sperm) ultimately results in loss of MPK-1 activation in both zones 1 and 2 and cessation of oocyte production
DISCUSSION
“It is not the strongest of the species that survives, nor the most intelligent that survives. It is the one that is the most adaptable to change”- Charles Darwin (Darwin, 1859).
Our work suggests the presence in C. elegans of a physiological relay system that couples nutrient availability to meiotic progression during oogenesis through the action of the DAF-2 insulin-like receptor and the RAS-MPK-1 pathway. Below we discuss the role of insulin signaling in coupling animal physiology and development to environmental conditions, the apparent PI3K-independent function of PTEN during meiotic progression in the C. elegans germ line, and the C. elegans germ line as an organ that coordinates oocyte (and progeny) production to two independent external cues.
DAF-2 couples nutrient availability to meiotic progression by activating the RAS-MPK-1 signaling pathway
The insulin-like signaling pathway has been shown to link metabolic inputs to developmental and cellular outcomes in multiple different model systems and in humans (Colombani et al., 2003; Hietakangas and Cohen, 2009; Michaelson et al., 2010). In Drosophila, the cellular and molecular pathways through which the insulin-like signaling pathway couples nutritional status to cell division, cell growth, and tissue development has been particularly well delineated. Here, the presence of nutrition in the form of amino acids has been shown to elicit the fat body, the fly adipose tissue, to secrete a diffusible signal that triggers the secretion of insulin-like peptides (ILPs) from a small set of neurons in the brain (Colombani et al., 2003). The ILPs then diffuse systemically and activate the insulin-like signaling pathway in diverse tissues. In the ovary, the insulin-signaling pathway acts through the canonical PI3K/AKT cascade to regulate stem cell proliferation and the uptake of yolk proteins by maturing oocytes (LaFever and Drummond-Barbosa, 2005).
We suspect a similar physiological relay system occurs in C. elegans. In the meiotic germ line, we find that the DAF-2 insulin-like signaling pathway responds to the presence of food by driving progression of meiosis I and oocyte production (Fig. 1B–E). Lack of either DAF-2 function or nutrition stalls oocyte production (Fig. 1, Fig. 2 Fig. S1). DAF-2 couples nutritional status to meiotic progression via sustained activation of MPK-1 for ~18 hours in each germ cell, with MPK-1 activity then orchestrating a suite of biological events that drives meiotic progression and oogenesis (Arur et al., 2009; Lee et al., 2007).
DAF-2 activity couples external cues to meiotic progression, but what ligands activate DAF-2 in zone 1 of the germ line and what is their cellular source? Several studies suggest that sensory neurons secrete ILP’s in response to nutrient availability during larval development (Apfeld and Kenyon, 1999; Bargmann and Horvitz, 1991; Michaelson et al., 2010). By analogy we speculate that in adult animals, the presence of food triggers neurons to secrete ILPs that then act remotely to trigger DAF-2 receptor activation in zone 1 of the germ line. The signals that trigger ILP secretion in this system, however, remain unknown. Interestingly and unlike in the Drosophila ovary, in the relay system uncovered in this study, DAF-2 Insulin-like signaling pathway does not utilize the canonical AGE-1/AKT-1/DAF-16 module, and instead appears to integrate directly with the RAS-RAF-MEK-ERK cascade to mediate meiotic prophase progression and oocyte production. Future work is required to delineate all of the players – both molecules and tissues – in this relay system in C. elegans and to reveal the similarities and differences between the processes in C. elegans and mammals that couple nutrient availability to ERK activation and meiotic progression.
Our data also uncovered an unappreciated and likely DAF-2 independent function of DAF-16 in somatic gonadal sheath cells to regulate germ line development. DAF-16 is expressed at low levels in the germ line ((Michaelson et al., 2010); this paper), but at high levels in somatic gonadal sheath cells (Fig. 3C). Removal of daf-16 function specifically in the germ line of daf-2 mutant worms had no effect on dpMPK-1 levels in zone 1. But, complete, systemic loss of daf-16 function in daf-2 mutant worms reversed the loss of dpMPK-1 staining in zone 1. As daf-16 is expressed in the somatic gonad and the somatic gonad exerts significant influence over germ line development (McCarter et al., 1997), we speculate that daf-16 acts in an autonomous manner to regulate the function of somatic gonadal sheath cells and through them acts in a non-autonomous manner to influence germ line development. In this function, daf-16 likely acts independently of daf-2, as under normal fed conditions in which daf-2 signaling is active, DAF-16 localizes to the nucleus of somatic gonadal sheath cells and is thus presumably active (Fig. 3C). Recent evidence reveals that DAF-16 also acts independently of daf-2 via miRNA-mediated regulation of akt-1 in the somatic gonad to promote longevity in C. elegans (Shen et al., 2012). Thus, in the future, it will be important to dissect the previously undefined function of daf-16 in the somatic gonad and to investigate how it impacts germ line development in a daf-2 and insulin signaling independent manner.
DAF-18/PTEN acts independently of PI3 Kinase to control meiotic progression downstream to DAF-2
During meiotic progression in zone 1, we find that DAF-2 acts independently of the canonical AGE-1, AKT-1, and DAF-16 pathway (Fig. 3 and Fig. S3), and instead functions via the RAS-RAF-MEK-ERK cascade (Fig. 2 and Fig. 6). Insulin and insulin-like signaling have been shown to function independently of the PI3K pathway in multiple systems, usually regulating the ERK or the JNK pathway; thus, engagement of the ERK signaling cascade by DAF-2 is not by itself surprising. In fact, studies in humans indicate that circulating insulin triggers ovarian follicle maturation by activating the insulin growth factor receptor (IGFR1) in granulosa-luteal cells and inducing progesterone secretion (reviewed in Silva., et al., 2009). Here, IGFR also appears to act independently of the PI3K and AKT pathway, with speculation that it might instead act either through the ERK or the JNK pathway (Poretsky et al., 2001).
What was surprising, however, was that DAF-2 signaling was still regulated by PTEN, which typically inhibits insulin signaling by opposing the activity of PI3K (AGE-1), and converting PIP3 back into PIP2 via its phosphatase activity (Das et al., 2003). A recent study also found that PTEN acts independently of PI3K in a distinct region of the C. elegans germ line. Brisbin et al., 2009 showed that DAF-18/PTEN acts downstream of the VAB-1 Eph RTK/MSP sperm signal to negatively regulate ovulation independently of both PI3K and FOXO. Here, DAF-18 also acts to negatively regulate RAS-MPK-1 signaling, in this instance, via the sperm receptor. But, how might DAF-18 work in an AGE-1 independent pathway to regulate RAS signaling downstream to DAF-2 in zone 1 of the germ line? One possibility is that an as yet unidentified PI3K exists in the C. elegans genome that links the DAF-2 signal to DAF-18 and then relays the signal to the RAS-MPK-1 pathway; another is that DAF-18 function may itself be modified by phosphorylation as suggested by Brisbin et al; A third is that DAF-18 regulates RAS-MPK-1 activity downstream to DAF-2 independent of its phosphatase activity. Consistent with the last idea, PTEN has been found to inhibit the function of other proteins that promote growth via protein-protein interactions rather than through its phosphatase action (Okumura et al., 2005; Song et al., 2011). . Clarifying the molecular basis, through which DAF-18 regulates DAF-2 signaling in the C. elegans germ line independently of PI3K, thus represents a key area of future study.
The C. elegans germ line couples oocyte production and maturation to two distinct external cues
Our work together with that of Miller et al (2001) indicate that the C. elegans germ line couples two distinct external cues to meiotic progression and oocyte maturation and ovulation, in both cases through the activation of the ERK signaling pathway. Here, we showed that in the presence of food, DAF-2 functions in zone 1 to activate the RAS-MPK-1 pathway and drive meiotic progression. Previously, Miller et al (2001) showed that the presence of sperm activates MPK-1 in zone 2 and drives oocyte maturation, ovulation, and fertilization. Integration of these mechanisms generates a seemingly adaptive system that ensures organismal resources are shepherded towards procreation only under conditions in which fertilization can occur and that favor survival of the progeny (Fig. 8). Thus, in the presence of favorable nutrient conditions and sperm, the C. elegans germ line continually produces oocytes, which are then fertilized, and the resulting progeny born under hospitable conditions. In the absence of either signal, oogenesis is inhibited in mechanistically distinct ways: In the absence of food and daf-2 signaling, MPK-1 activation does not occur in zone 1 and meiotic progression is stalled (over time, dpMPK-1 is also lost in zone 2); in the absence of sperm results, MPK-1 activation in zone 2 does not occur (over time, dpMPK-1 is also lost in zone 1, but not before a stockpile of oocytes has been produced). In either scenario, the germ line is poised to respond to a change in environment – the appearance of food or sperm - via rapid reactivation of MPK-1 in zone 1 (food) or zone 2 (sperm) and oocyte production, maturation, and fertilization. Thus, C. elegans oogenesis appears to provide an elegant example of how evolution has sculpted an adaptive organ system that helps ensure the survival of its (fittest) progeny.
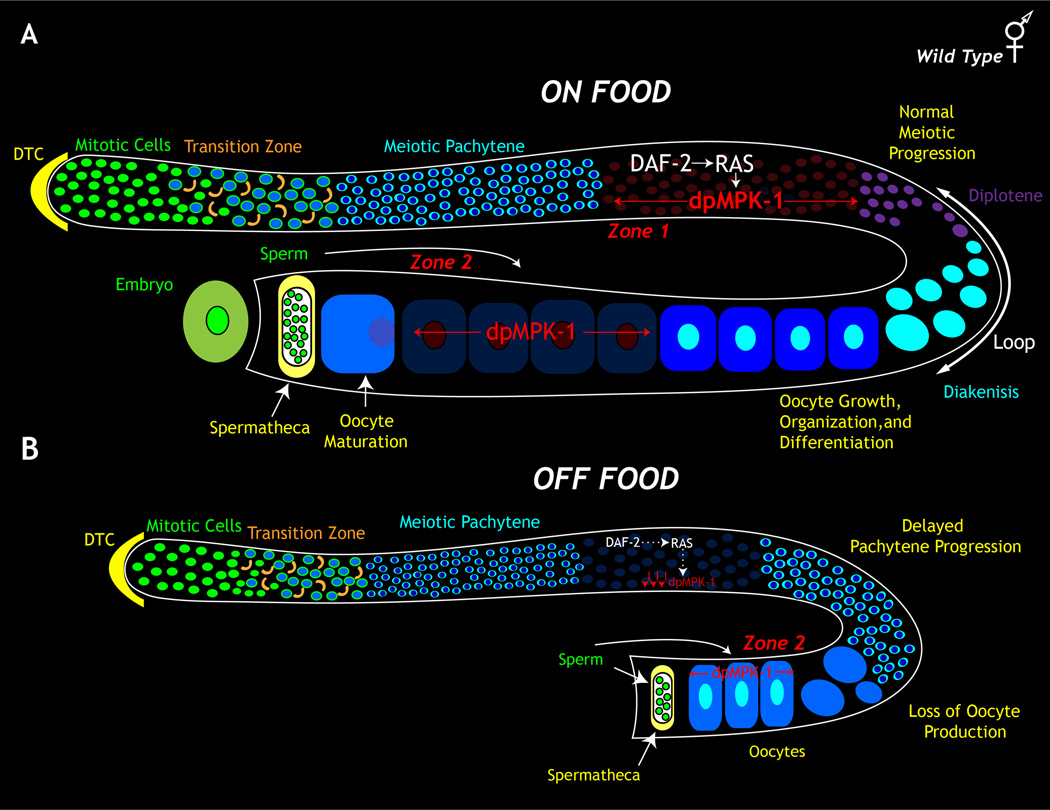
Figure 8. Nutrition cues and DAF-2 / LET-60 RAS / MPK-1 ERK signaling pathway regulate zone 1 MPK-1 activation and oocyte production in C. elegans germ line.
A. Nutritional cues signal via the DAF-2 insulin-like receptor and result in activation of the RAS-MPK-1 in zone 1. MPK-1 activation in zone 1 drives progression of meiotic prophase and oocyte production. The sperm signal activates MPK-1 in zone 2, which ensures oocyte maturation and ovulation. B. In the absence of nutrition the germ line turns off the MPK-1 signal in zone 1, stalling meiotic prophase progression and oocyte production.
EXPERIMENTAL PROCEDURES
Starvation assay
Indicated genotypes were grown on NGM plates with E. coli OP50 bacteria to indicated developmental stage and then transferred to an unseeded NGM plate (minus peptone) as described earlier and starved for indicated time points (Angelo and Van Gilst, 2009).
Dafachronic Acid experiments
NGM plates minus cholesterol were supplemented with 1mM of Dafachronic Acid (DA) (Sharma et al., 2009) and seeded with E. coli OP50 for use.
Dissections and staining
Dissections were performed as described earlier (Arur et al., 2009). All dissections were performed under 5 minutes (immediately after adding levamisole) to achieve optimal dpMPK-1 staining. The dissected germ lines were fixed in 3% paraformaldehyde for ten minutes, followed by a post-fix in 100% methanol at −20°C. The fixed germ lines were then processed for immunoflourescence staining as described (Arur et al., 2009).
Supplementary Material
HIGHLIGHTS.
DAF-2 and ERK couple nutrient availability to oocyte production
DAF-/Insulin-like signaling activates RAS-ERK during oogenesis
DAF-2 activates RAS-ERK pathway independent of PI3K and FOXO
ACKNOWLEDGEMENTS
Worm strains were obtained from C. elegans Genetics stock center at University of Minnesota funded by NIH (P40 OD010440). We thank Jim Skeath, Awdhesh Kalia and Jill Schumacher for critical review of the manuscript and valuable discussions, and David Mangelsdorf for providing Dafachronic acid and the idea to test daf-2 mutant worms on DA to assay adult germ line phenotypes. CPRIT (RP101502) to JC, NIH (GM98200) and MD Anderson CCSG grant from NCI to SA supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS:
ALL, JC and SA conceived and designed the study. ALL, JC, HJJ, MND, MS, CK and SA performed experiments and analyzed data. SA wrote the paper.
REFERENCES AND NOTES
- Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Arur S, Ohmachi M, Nayak S, Hayes M, Miranda A, Hay A, Golden A, Schedl T. Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc Natl Acad Sci U S A. 2009;106:4776–4781. doi: 10.1073/pnas.0812285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction. 2005;130:801–811. doi: 10.1530/rep.1.00364. [DOI] [PubMed] [Google Scholar]
- Cavaliere V, Donati A, Hsouna A, Hsu T, Gargiulo G. dAkt kinase controls follicle cell size during Drosophila oogenesis. Dev Dyn. 2005;232:845–854. doi: 10.1002/dvdy.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth A, Ridge JA, King LA, MacNicol MC, MacNicol AM. A novel regulatory element determines the timing of Mos mRNA translation during Xenopus oocyte maturation. Embo J. 2002;21:2798–2806. doi: 10.1093/emboj/21.11.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7032–7035. doi: 10.1073/pnas.93.14.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. 1 edn. Vol 1. London: W Clowes and Sons; 1859. [PMC free article] [PubMed] [Google Scholar]
- Das S, Dixon JE, Cho W. Membrane-binding and activation mechanism of PTEN. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7491–7496. doi: 10.1073/pnas.0932835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman JB, Albinder B, Shroyer T, Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141:1399–1406. doi: 10.1093/genetics/141.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Evans EA, Chen WC, Tan MW. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell. 2008;7:879–893. doi: 10.1111/j.1474-9726.2008.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladi-Nashta AA, Gutierrez CG, Gong JG, Garnsworthy PC, Webb R. Impact of dietary fatty acids on oocyte quality and development in lactating dairy cows. Biol Reprod. 2007;77:9–17. doi: 10.1095/biolreprod.106.058578. [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M, Jessus C, Ozon R, Maller JL, Haccard O. Two distinct mechanisms control the accumulation of cyclin B1 and Mos in Xenopus oocytes in response to progesterone. Mol Biol Cell. 1999;10:3279–3288. doi: 10.1091/mbc.10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Hietakangas V, Cohen SM. Regulation of tissue growth through nutrient sensing. Annu Rev Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska I, Lee E, Kwan KM, Fenger DD, Orr-Weaver TL. The Drosophila MOS ortholog is not essential for meiosis. Curr Biol. 2004;14:75–80. doi: 10.1016/j.cub.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics. 2007;177:2039–2062. doi: 10.1534/genetics.107.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Matten WT, Copeland TD, Ahn NG, Vande Woude GF. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137:671–680. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld TP. Shrinkage control: regulation of insulin-mediated growth by FOXO transcription factors. J Biol. 2003;2:18. doi: 10.1186/1475-4924-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- Okumura K, Zhao M, DePinho RA, Furnari FB, Cavenee WK. PTEN: a novel anti-oncogenic function independent of phosphatase activity. Cell Cycle. 2005;4:540–542. doi: 10.4161/cc.4.4.1614. [DOI] [PubMed] [Google Scholar]
- Papadopoulos S, Lonergan P, Gath V, Quinn KM, Evans AC, O'Callaghan D, Bolan MP. Effect of diet quantity and urea supplementation on oocyte and embryo quality in sheep. Theriogenology. 2001;55:1059–1069. doi: 10.1016/s0093-691x(01)00466-6. [DOI] [PubMed] [Google Scholar]
- Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science. 2006;313:971–975. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- Plowman GD, Sudarsanam S, Bingham J, Whyte D, Hunter T. The protein kinases of Caenorhabditis elegans: a model for signal transduction in multicellular organisms. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13603–13610. doi: 10.1073/pnas.96.24.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Seto-Young D, Shrestha A, Dhillon S, Mirjany M, Liu HC, Yih MC, Rosenwaks Z. Phosphatidyl-inositol-3 kinase-independent insulin action pathway(s) in the human ovary. J Clin Endocrinol Metab. 2001;86:3115–3119. doi: 10.1210/jcem.86.7.7617. [DOI] [PubMed] [Google Scholar]
- Roy LM, Haccard O, Izumi T, Lattes BG, Lewellyn AL, Maller JL. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- Sharma KK, Wang Z, Motola DL, Cummins CL, Mangelsdorf DJ, Auchus RJ. Synthesis and activity of dafachronic acid ligands for the C. elegans DAF-12 nuclear hormone receptor. Mol Endocrinol. 2009;23:640–648. doi: 10.1210/me.2008-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Wollam J, Magner D, Karalay O, Antebi A. A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science. 2012;338:1472–1476. doi: 10.1126/science.1228967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JR, Figueiredo JR, van den Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology. 2009;71:1193–1208. doi: 10.1016/j.theriogenology.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–199. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram MV. RTK/Ras/MAPK signaling. WormBook. 2006:1–19. doi: 10.1895/wormbook.1.80.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, de Pennart H, Maro B, Cobb MH, Clarke HJ. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol. 1993;158:330–340. doi: 10.1006/dbio.1993.1192. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development. 1996;122:815–822. doi: 10.1242/dev.122.3.815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.