Abstract
We have developed a bioengineered implant (BI) to evaluate strategies to promote graft survival and function in models of islet transplantation in mice. The BI, sized for implantation within a fold of intestinal mesentery, consists of a disk-shaped, polyvinyl alcohol sponge infused with a type I collagen hydrogel that contains dispersed donor islets. To promote islet vascularization, the BI incorporates a spherical alginate hydrogel for sustained release of vascular endothelial growth factor (VEGF). BIs that contained 450–500 islets from syngeneic (C57Bl/6) donors and 20 ng of VEGF reversed streptozotocin (STZ)-induced diabetes in 100% of mice (8/8), whereas BIs that contained an equivalent number of islets, but which lacked VEGF, reversed STZ-induced diabetes in only 62.5% of mice (5/8). Between these “+VEGF” and “−VEGF” groups, the time to achieve normoglycemia (8–18 days after implantation) did not differ statistically; however, transitory, postoperative hypoglycemia was markedly reduced in the +VEGF group relative to the −VEGF group. Notably, none of the mice that achieved normoglycemia in these two groups required exogenous insulin therapy once the BIs began to fully regulate levels of blood glucose. Moreover, the transplanted mice responded to glucose challenge in a near-normal manner, as compared to the responses of healthy, nondiabetic (control) mice that had not received STZ. In future studies, the BIs described here will serve as platforms to evaluate the capability of immunomodulatory compounds, delivered locally within the BI, to prevent or reverse diabetes in the setting of autoimmune (type 1) diabetes.
Keywords: Diabetes, Islet, Bioengineered implant (BI), Collagen, Vascular endothelial growth factor (VEGF), Mouse
INTRODUCTION
Type 1 diabetes (T1D) is a late-stage manifestation of an autoimmune-mediated loss of insulin-secreting pancreatic β-cells that results in the inability to maintain blood glucose homeostasis (28,47). Current options for the treatment of T1D are (1) daily insulin therapy (DIT), (2) transplantation of the pancreas, either without the kidney (pancreas transplant alone – PTA) or with the kidney (simultaneous pancreas/kidney – SPK) (39), or (3) transplantation of isolated islets (36). While PTA and SPK procedures can restore glucose homeostasis, they involve major surgery and require lifelong immunosuppression, with the problem of recurrent autoimmunity (18,23,41). Moreover, these procedures can result in significant complications that include acute rejection, infection with cytomegalovirus (43), and technical failure as a result of vascular thrombosis, bleeding, anastomotic leaks, or infection/pancreatitis (11,13). Consequently, these treatments are generally limited to severely diabetic patients with associated kidney disease, which leaves the majority of T1D patients with either DIT or islet transplantation to manage their disease.
DIT, which requires injection of short- or long-acting insulin formulations, has been made easier by the use of semiautomated pump devices (30). However, a significant problem of DIT is ineffective control of unrecognized hypoglycemic episodes, which are further amplified under more intensive glucose management protocols designed to reduce secondary complications (5,24,33). Presumably, this problem would not arise with a properly functioning islet graft, which would act like the native endocrine pancreas to maintain glucose homeostasis. Accordingly, there is a substantial effort to develop effective methods for islet transplantation (27). Notably, however, current islet transplant protocols for treatment of T1D have had limited success as a consequence of (1) poor survival of islets in therapeutically approved intrahepatic graft sites (1,9), (2) alloimmune rejection (34), and (3) recurrence of the underlying autoimmunity (23,40). These three critical barriers to success must be overcome for islet replacement therapy to be an effective treatment for T1D.
The present study describes a bioengineered implant (BI) that will be used as a test bed to evaluate a number of strategies to promote islet graft survival and function in the setting of T1D. The BI incorporates five features that make it useful in this role: (1) It is miniaturized for engraftment in mice—a species that includes specific strains that effectively model T1D. (2) The BI is disk-shaped for implantation in a fold made from gut mesentery that models the greater omentum of humans, which is considered a favorable site for islet engraftment due to its large size and rich, dynamic blood supply. (3) The supportive scaffold of the BI is comprised of polyvinyl alcohol (PVA) sponge, a material that is durable and easily shaped, elicits a minimal inflammatory response, and which supports neovascularization (17,32). (4) Islets within the BI are supported by a hydrogel comprised of native fibrillar type I collagen, a natural extracellular matrix (ECM) component that is biodegradable, elicits little or no immune response, and has been shown to improve β-cell viability and insulin secretion in vitro (10,20,25,26). (5) To improve revascularization of the islets, the BI incorporates a spherical alginate hydrogel (an “alginate macrosphere”) to provide sustained, local delivery of vascular endothelial growth factor (VEGF), a potent angiogenic cytokine.
We find that murine donor islets incorporated into BIs and transplanted into syngeneic recipients are revascularized and produce insulin. Significantly, BIs containing 450–500 donor islets reverse diabetes in streptozotocin (STZ)-treated mice. Moreover, we find that release of VEGF within the implant mitigates hypoglycemia following transplantation and improves implant performance.
MATERIALS AND METHODS
Isolation of Islets
C57Bl/6 mice 12–24 weeks of age were anesthetized by injection at 20 μl/g body weight with 2,2,2-tribromo-ethanol, prepared as a 2.5% solution in phosphate-buffered saline (PBS) from a 1:1 wt (g)/vol (ml) 2,2,2-tribromo-ethanol/tert-amyl alcohol stock. The descending aorta of each anesthetized mouse was transected and a 30-gauge needle was used to inject each pancreas, through the common bile duct, with 3 ml of 4°C “islet medium” comprised of RPMI 1640 containing 1.0 g NaHCO3, 1 mM Na-pyruvate, 100 μg/ml penicillin, and 100 U/ml streptomycin (all from Gibco/Invitrogen), and 10% fetal bovine serum (FBS) (Atlanta Biologicals, cat. #S12450H). The islet medium was supplemented with 0.8 mg/ml of collagenase P (Roche, cat. #11-249-002-001) and filtered through a 0.22-μm filter prior to injection. Subsequently, the pancreata were excised and each placed separately in a 50-ml conical centrifuge tube on ice. When two to three pancreata were obtained, 5 ml of 37°C islet medium was added to each tube, incubated at 37°C for 13 min, then decanted, and 30 ml of 4°C islet medium was added to each tube. The tubes were shaken vigorously for 1 min to disrupt the pancreata, and the tissue suspensions were filtered through a 30-mesh metal screen to remove large debris. The filtrates were centrifuged in a Beckman GS-6 at 500 rpm for 10 min at 4°C, the supernates were discarded, and the pellets were resuspended in 5 ml of 4°C islet medium. Subsequently, 5 ml of 4°C Histopaque®-1077 (Sigma-Aldrich) was injected under the medium layer and the tubes were centrifuged for 20 min at 2,000 rpm (without applied braking). The islets were collected at the Histopaque/medium interface and washed by a 500 rpm centrifugation for 10 min through 40 ml of 4°C islet medium. The washed islets were resuspended in 4 ml of islet medium, placed in 60-mm dishes, and put in a 37°C, 5% CO2 incubator. Once all pancreata were processed, the isolated islets were hand-picked into a new 60-mm dish, cultured overnight, and picked again the next day before being placed in BIs. Average yields were 100–150 islets per mouse.
In Vitro Assays of Glucose-Mediated Insulin Release From Islets
For standard in vitro assays, the islets were isolated, cultured overnight in islet medium, washed briefly in Hank’s buffered salt solution (HBSS), and then were preincubated for 2 h at 37°C in HBSS with 5.6 mM glucose (low glucose). After the 2-h interval, samples of medium were collected for insulin measurement by enzyme-linked immunosorbent assay (ELISA) (Mercodia Insulin ELISA kit, cat. #10-1247-01). Subsequently, the islets were stimulated for 3 or 30 min with 16.6 mM glucose (high glucose) and samples of medium collected for insulin measurement by ELISA. Fold changes were calculated as insulin (high glucose)/insulin (low glucose).
For experiments evaluating the effects of VEGF on insulin secretion, purified islets were cultured overnight in islet medium, washed in HBSS, and then distributed into a 24-well tissue culture plate. Each well contained 30 islets in 1 ml of HBSS/5.6 mM glucose (low glucose) with or without 10 ng/ml of human recombinant VEGF165 (Peprotech, cat. #100-20). Baseline (i.e., low glucose) samples of medium were collected after 2 h and then glucose was added to each well to a final concentration of 16.6 mM (high glucose). Samples of medium (50 μl) were then taken at 30 min and insulin concentrations were determined by ELISA as described above. Fold changes were calculated as insulin (high glucose)/insulin (low glucose).
Fabrication of the PVA Scaffolds
To fabricate the PVA scaffolds for the BIs, biopsy punches (Sklar Instruments) were used to cut 6-mm-diameter disks from 2-mm-thick sheets of PVA sponge (Type CF90, 500 μm average pore size with no surfactant treatment—a generous gift from Merocel/Medtronic, Inc.). Subsequently, each disk was through-punched with a single central hole of 1.5 mm diameter and eight peripheral holes of 1 mm diameter, using correspondingly sized biopsy punches (Acuderm, Inc.). The punched disks were washed on a rocker in 50-ml centrifuge tubes filled with 40 ml of sterile distilled water (10 min per wash, repeated five times), then air-dried on Whatman filter paper, transferred to 60-mm dishes, exposed to γ-irradiation, and stored until needed for BI assembly.
Preparation of VEGF-Alginate Macrospheres
To prepare VEGF-alginate macrospheres, a stock solution of 4% Na alginate (Sigma-Aldrich, cat. #A0682) was prepared in deionized water and filtered at 0.45 μm, using positive pressure. A stock solution of human recombinant VEGF165 was prepared at 100 ng/μl in sterile, deionized water with 0.1% normal mouse serum (NMS). For macrospheres containing VEGF, 32 μl of alginate stock was combined with 28 μl of sterile deionized water and 4 μl of VEGF stock and pipetted in 8 μl volumes (each containing 2% alginate and 50 ng of VEGF) onto a sheet of hydrophobic Parafilm™ “M” (Pechiney Plastic Packaging) that was cut into a narrow triangular shape. The Parafilm triangle was held vertically on a clamp positioned 5 cm above a 60-mm Petri dish filled with a solution of 100 mM CaCl2. The alginate/VEGF droplets were pulled to the tip of the Parafilm triangle by gravity, where they fell one at a time into the CaCl2 solution and were crosslinked by the free Ca2+ ions into spheres approximately 2 mm in diameter. The spheres were crosslinked for 15 min in the CaCl2 solution, then washed for 2 min in 10 ml of 0.15 M NaCl/25 mM HEPES/2 mM CaCl2, pH 7.0 (repeated twice), transferred to a 35-mm dish filled with serum-free Dulbecco’s modified Eagle’s medium (DMEM) (Gibco/Invitrogen) with 100 μg/ml penicillin and 100 U/ml streptomycin (P/S), and maintained in a tissue culture incubator until needed for BI assembly.
Preparation of Type I Collagen Solution
To prepare type I collagen solution for the BIs, 1 volume of a stock solution of rat tail native type I collagen in dilute acetic acid (Becton Dickinson) was combined with 1/9 volume of 10-strength NaHCO3-saturated Medium 199 (Gibco/Invitrogen) and sufficient DMEM and NMS to yield a solution containing 2.5 mg/ml collagen and 10% NMS (42). The collagen solution was prepared just prior to assembly of the BIs and maintained on ice until needed.
Assembly of the BIs
To prepare the BIs, the dry PVA sponge scaffolds were expanded for 5 min in sterile DMEM/P/S and a single, freshly prepared alginate macrosphere was gently pressed into the 1.5-mm-diameter center hole of each scaffold. The scaffolds were then blotted on sterile Whatman filter paper, transferred to 60-mm plastic tissue culture dishes lined with UV-sterilized Parafilm M, and flooded with 60 μl of type I collagen solution containing suspended islets. The PVA sponges absorbed the collagen solution, with the majority of the islets entering the 1-mm-diameter peripheral holes. Subsequently, the dishes were covered with dish tops (lined with moist filter paper) and incubated for 30 min at 37°C/5% CO2/100% humidity to gel the collagen. The completed BIs were transferred to a 24-well tissue culture plate filled with 500 μl/well of preequilibrated DMEM/10% NMS/P/S and maintained in a tissue culture incubator until implantation in subject mice.
Induction of Diabetes and Implantation of BIs in Mice
Three days prior to surgical implantation of BIs, C57Bl/6 mice were treated with a high dose (200 mg/kg) of STZ made as a stock solution of 7.5 mg/ml STZ in 100 mM citrate, pH 4.2 (prepared and filtered at 0.22 μm immediately prior to intraperitoneal injection). Over 90% of mice receiving STZ became diabetic within 72 h (and most became diabetic within 48 h). Blood glucose was measured everyday and insulin was given when needed (see following section), but insulin was not administered on the day of surgery. To implant BIs, mice were administered buprenorphine (0.05–0.1 mg/kg) prior to the surgery, which was performed under isoflurane. A 1-cm vertical, midline incision was made in the skin and peritoneum, a loop of the small intestine was extracted, and the BI placed on the intestinal mesentery. Subsequently, the intestinal loop was folded over the BI and returned to the peritoneal cavity. The incision was closed with absorbable sutures (for the peritoneum) and staples (for the skin). Removal of BIs was done in the same manner as implantation. All work with mice was done in an AAALAC-accredited facility and was approved by the Benaroya Research Institutional Animal Care and Use Committee.
Monitoring of Insulin and Glucose Levels and Intraperitoneal Glucose Tolerance Tests
The blood glucose levels (BGLs) and body weights of STZ-treated mice were measured once a day. BGLs were measured from a saphenous vein bleed (30-gauge needle) using a Wavesense Presto glucometer (AgaMatrix). For insulin treatment, sustained-release Levemir® insulin (Novo Nordisk) was diluted 1:5 to 1:20 in a solution of 20 mM NaCl/5 mM Na2HPO4/19 mM phenol/174 mM glycerol just prior to administration and was injected subcutaneously (SC). Mice were given 0.12 U of insulin for BGLs of 250–350 mg/dl, 0.23 U for BGLs of 350– 450 mg/dl, or 0.33 U for BGLs over 450 mg/dl. Mice were also given 800–1,000 μl of saline SC in the inner thigh if loss of body weight was greater than 10% from starting weight (i.e., just prior to STZ treatment). For those mice in which receipt of BIs induced normoglycemia (i.e., BGLs were <250 mg/dl), the frequency of body weight and BGL measurements was reduced.
For intraperitoneal glucose tolerance tests, mice were fasted (water, but no food) for 6 h before injection with 1 mg of glucose (in sterile PBS) per gram of body weight. BGLs in saphenous vein blood were measured at 0, 15, 30, 60, and 120 min after injection of glucose.
Histological Analyses
Implants and pancreata were fixed in neutral-buffered formalin (NBF), dehydrated, embedded in paraffin, and sectioned at 8 μm. Sections were stained with hematoxylin and eosin (H&E) for routine histological examination. For detection of von Willebrand factor (vWF), sections were subjected to epitope retrieval for 20 min at 79°C using an ethylenediaminetetraacetic acid (EDTA)-based buffer, pH 9 (Bond™ Epitope Retrieval Solution 2, Leica Microsystems, cat. #AR9640), then blocked and exposed 1 h to a 1:400 dilution of a rabbit polyclonal antibody to human vWF (Dako, cat. #A0082). Bound antibodies were visualized with a Bond™ Polymer Refine Detection Kit (Leica Microsystems, cat. #DS9800) using 3,3-diaminobenzidine as the chromogen. The vWF-labeled sections were then exposed to hematoxylin to stain cell nuclei. For detection of insulin, sections were labeled for indirect immunofluorescence with a guinea pig monoclonal antibody to human insulin (Abcam, cat. #ab7842) in conjunction with an Alexa-Fluor 488™-conjugated goat anti-guinea pig IgG secondary antibody (Molecular Probes/Invitrogen). Images were recorded with a Leica DMR brightfield/epifluorescence microscope equipped with SPOT Insight™ and RT™ digital cameras (Diagnostic Instruments).
Measurement of Release of VEGF From Alginate Macrospheres In Vitro
To measure the release of VEGF from alginate in vitro, macrospheres containing 2% alginate and 50 ng VEGF were prepared as described above. The macrospheres were placed in 96-well tissue culture plates (one sphere per well) with each of the wells filled with 200 μl of DMEM/10% FBS/P/S. The plates were placed in a tissue culture incubator maintained at 37°C/5% CO2/100% humidity. At specific time points (1, 2, 3, 6, and 14 days of incubation), a 100-μl volume of medium was removed from each well and stored at −80°C until analysis by ELISA. Following removal of the medium at each time point, the residual medium in each well was discarded, and each well was refilled with 200 μl of fresh medium. ELISA assays were performed with a DuoSet® Human VEGF ELISA Kit (R&D Systems, cat. #DY293B).
To determine the percentage of VEGF retained in alginate macrospheres during their fabrication, freshly prepared macrospheres of 8 μl volume were each dissolved in 892 μl of PBS/100 mM EDTA, followed by addition of 100 μl of FBS to the solution. VEGF in the samples was measured by ELISA, with a solution of 50 ng/ml of VEGF in PBS/100 mM EDTA/10% FBS serving as a positive control.
Statistics
Statistical p values were calculated with Prism® (GraphPad Software, Inc.) using a two-tailed t test or, for groups, a one-way ANOVA with the Bonferroni multiple comparison test. Values of p for Kaplan–Meier plots were calculated using a log-rank test.
RESULTS
BI Fabrication and Islets
The body of the BI (Fig. 1A) consisted of a disk-shaped scaffold of PVA sponge infused with a type I collagen hydrogel that held the suspended islets. An alginate macrosphere was placed in the center of the construct to provide a source of VEGF. The PVA sponge was readily infiltrated by the unpolymerized collagen solution—a consequence of the large (500 μm) pore diameter of the sponge. In contrast, penetration of the sponge by suspended islets was inhibited by the relatively narrow connections between the pores (pore throats). Consequently, we punched eight peripheral holes of 1-mm diameter into the PVA sponge in order to provide open spaces for the islets to occupy (Fig. 1A, B). The diameter of the central hole in the PVA sponge was set at 1.5 mm (Fig. 1B) so that it would stretch slightly to grip the 2 mm diameter alginate macrosphere (Fig. 1C). In fully assembled BIs, the collagen hydrogel held the suspended islets firmly in place and provided additional stabilization to the macrosphere while the construct was wrapped in a fold of small-intestinal mesentery (Fig. 1D).
Figure 1.
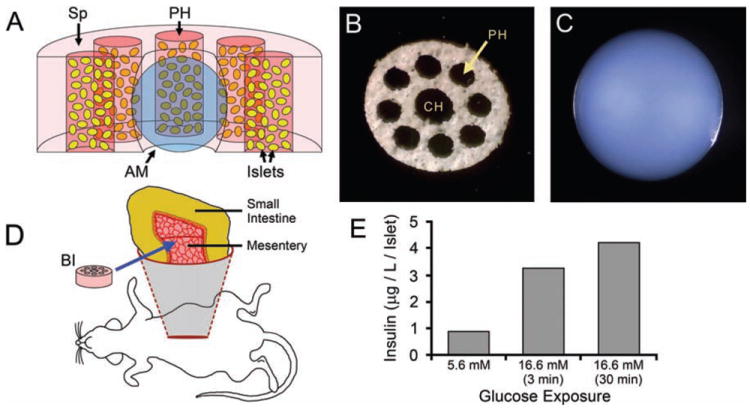
BI fabrication and implantation. (A) Cut-away diagram of the bioengineered islet (BI), with the components shown to scale. A disk-shaped polyvinyl alcohol (PVA) sponge (Sp) scaffold provides mechanical support. An alginate macrosphere (AM) (blue) occupies the central hole of the sponge. Eight peripheral holes (PH) in the sponge (5 appear in this cut-away) contain islets (yellow) suspended in a type 1 collagen hydrogel (reddish-pink). The collagen hydrogel also infuses the sponge (light pink). For clarity, the pores of the sponge are not depicted. (B) A PVA sponge scaffold oriented to show the central hole (CH) and peripheral holes (PH). The scaffold is 6 mm in diameter. (C) An alginate macrosphere of 2 mm diameter. (D) Diagram illustrating placement of the BI (pink disk) on the mesentery supporting a loop of small intestine. (E) Islets within freshly-made BIs (500 islets per BI) increased their production of insulin in vitro following 3- or 30-min exposures to elevated (16.6 mM) glucose, as compared to “resting” levels of insulin produced in the presence of low (5.6 mM) glucose.
The viability of the islets used in the BIs was confirmed by implanting 450 freshly isolated islets under the kidney capsule of STZ-treated diabetic mice. These mice became normoglycemic within 24 h of implantation (data not shown). When similar preparations of isolated islets were incorporated into freshly made BIs (500 islets per BI), the islets increased their production of insulin in vitro following 3- or 30-min exposures to elevated (16.6 mM) glucose, as compared to “resting” levels of insulin produced in the presence of low (5.6 mM) glucose (Fig. 1E). Therefore, the materials and processes used to fabricate the BIs were not immediately harmful to the islets.
Release of VEGF From Alginate Macrospheres
Solutions of alginate are rapidly crosslinked by calcium ions to form stable hydrogels. The conditions for gelation are relatively mild, which has made alginate an attractive candidate for delivery of bioactive proteins (6,12,44), including VEGF (8,21,29). We found that a simple, gravity-driven drop generator using a nonwettable (Parafilm) surface allowed us to make spheres of smaller volume than could be made using conventional, syringe-type drop generators. The method also used reagents efficiently, as we did not have to fill a syringe body with a minimum volume of alginate/VEGF solution.
As determined by ELISA assays, freshly fabricated alginate macrospheres each retained an average of 42 ± 2% (n = 5) of the 50 ng of VEGF added to the alginate before calcium crosslinking (equivalent to 21 ng of VEGF per crosslinked macrosphere). This efficiency of VEGF incorporation is comparable to results obtained by others [e.g., 31% (29)] for 2% alginate hydrogels. Based on our measurement of percent incorporation of VEGF, our macrospheres released approximately 71% of their VEGF within 6 days (Fig. 2). This value is substantially higher than the 43–47% release level reported by others (29), which might be a consequence of our inclusion of 10% FBS in the culture media, in contrast to the use of serum-free media (29).
Figure 2.
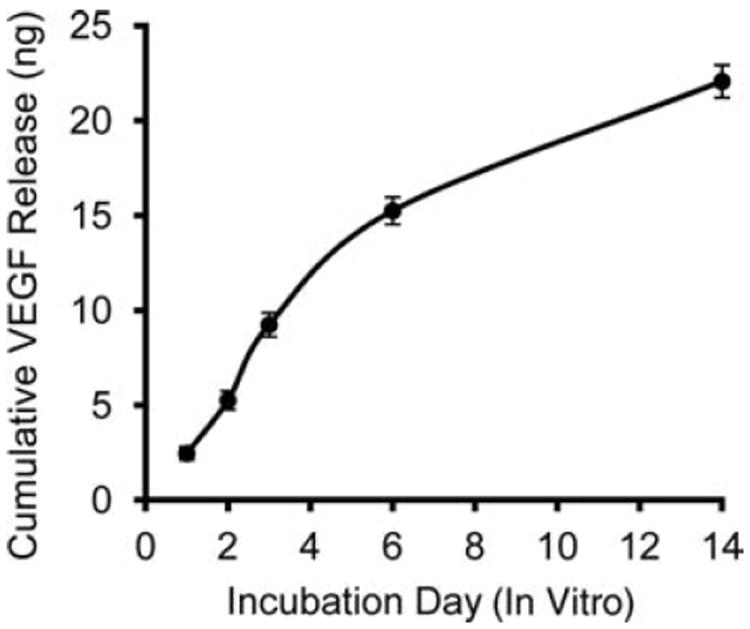
Cumulative release of vascular endothelial growth factor (VEGF) from 2% alginate macrospheres incubated for 14 days under physiological conditions in vitro (n = 5 macrospheres).
BI-Mediated Reversal of Diabetes in STZ-Treated Mice
We assessed the capacity of BIs to support islet survival and reverse acute (STZ-induced) diabetes in three groups of C57Bl/6 mice, as follows: (1) a +VEGF/+Islet group that received BIs containing 450–500 donor islets and alginate macrospheres loaded with VEGF, (2) a −VEGF/+Islet group that received BIs containing 450–500 donor islets and alginate macrospheres that lacked VEGF, and (3) a +VEGF/−Islet group that received BIs without donor islets, but with VEGF/alginate macrospheres. The BIs were surgically implanted on the intestinal mesentery under isoflurane anesthesia on day 0 in mice that were given a single high dose of STZ (200 mg/kg) 3 days earlier (day −3). The majority of recipients on the day of surgery had BGLs of at least 600 mg/dl (the maximum value on the glucometer). Basal BGLs prior to STZ-induced diabetes were 185 ± 21 mg/dl (n = 16). To prevent excessive diabetes-mediated weight loss, the STZ-treated mice were given daily insulin (Levemir) as needed beginning on day −2 for BGLs greater than 250 mg/dl, which lowered BGLs to near normal for at least 6 h after administration, but did not sustain normal BGLs after 24 h. This response profile allowed us to determine if a mouse was still diabetic under daily insulin therapy. All mice lost weight following STZ-induced diabetes but slowly gained weight after BIs were implanted, with the +VEGF/−Islet group exhibiting the greatest weight loss and the longest recovery time (Fig. 3A).
Figure 3.
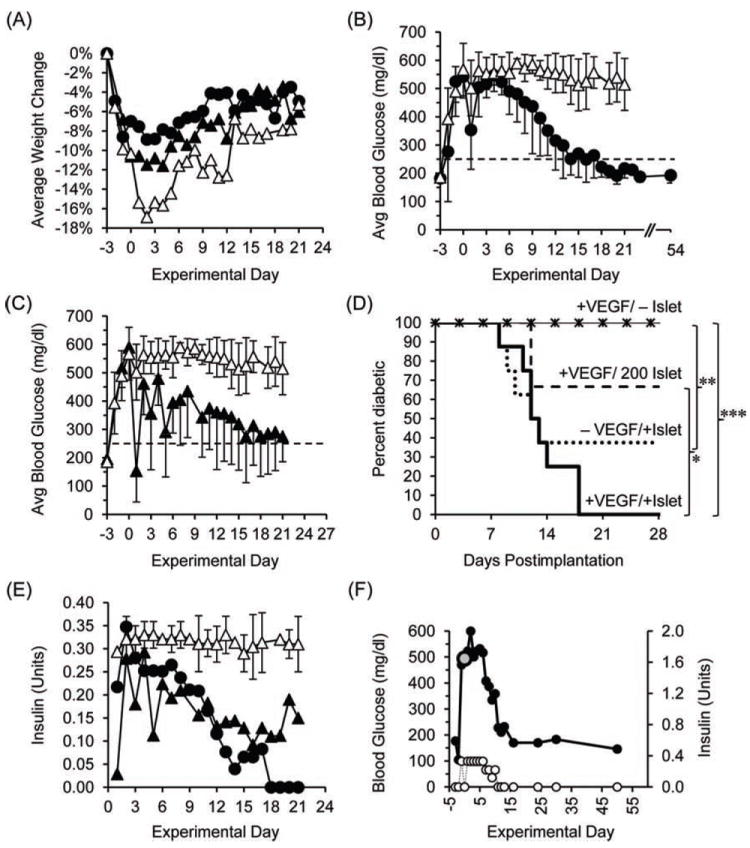
Responses of mice with streptozotocin (STZ)-induced diabetes to engrafted BIs. (A) Time course of weight change following engraftment of BIs, shown for the +VEGF/+Islet group (black circles), the −VEGF/+Islet group (black triangles), and the +VEGF/−Islet group (white triangles) (error bars are omitted for purposes of clarity). (B) In time course studies, the blood glucose levels (BGLs) of the +VEGF/+Islet group (black circles) fell to normal (dotted line) within 18 days p-i. Normoglycemic BGLs were maintained until the BIs were removed at 54 days p-i. In contrast, mice of the +VEGF/−Islet group (white triangles) remained hyperglycemic until sacrifice at 21 days p-i. (C) When averaged collectively, BGLs of the −VEGF/+Islet group (black triangles) fell to near-normal levels within 21 days p-i. BGLs of the +VEGF/−Islet group (white triangles) are included for comparison. (D) Kaplan–Meier plots of glucose regulation after BI implantation. BIs in +VEGF/+Islet (n = 8) and −VEGF/+Islet (n = 8) groups contained 450–500 islets. BIs in the +VEGF/200 Islet group (n = 3) contained 200 islets. ***p < 0.001, **p < 0.01, *p < 0.05 significant difference between the percent of animals that remained diabetic, as analyzed by a log-rank test. (E) Over time, exogenous insulin therapy could be reduced for mice of the +VEGF/+Islet group (black circles) and −VEGF/+Islet group (black triangles) that proceeded to normoglycemia. In contrast, mice of the −VEGF/−Islet group (white triangles) required a continuous, high-dose regimen of insulin. In this graph, error bars for the +VEGF/+Islet and −VEGF/+Islet groups are omitted for purposes of clarity. (F) BGLs of one mouse of the +VEGF/+Islet group, measured during the course of the experiment (black circles), are compared to the quantities of therapeutic insulin administered to the animal (white circles). The BGL measured at the time of implantation of the BI on day 0 is indicated (large gray circle). After day 10 p-i, the mouse did not require exogenous insulin.
One hundred percent (8 of 8) of diabetic mice in the +VEGF/+Islet group became normoglycemic within 18 days postimplantation (p-i) (Fig. 3B). Normoglycemia (i.e., BGLs < 250 mg/dl) was maintained until the BIs were removed for histological analysis between 40 and 54 days p-i. In contrast, 100% (10 of 10) of diabetic mice in the +VEGF/−Islet group remained hyperglycemic until sacrifice at 21 days p-i (Fig. 3B).
When averaged collectively, the mice of the −VEGF/+Islet group also achieved normoglycemia (Fig. 3C). Compared to the +VEGF/+Islet group, the percentage of mice that became normoglycemic in the −VEGF/+Islet group was lower (62.5%, 5 of 8 mice), but there was no statistical difference between the two groups (Fig. 3D), nor was there a statistical difference between the average time required for the two groups to achieve normoglycemia after transplant (13.3 ± 3.4 days and 10.4 ± 2.1 days, respectively) (Fig. 3D). In an additional group of mice that received VEGF but only 200 islets, only 33.3% (1 of 3) of the mice became normoglycemic (Fig. 3D). As expected, we were able to reduce exogenous insulin therapy for the subset of mice within the +VEGF/+Islet and −VEGF/+Islet groups that proceeded to normoglycemia (Fig. 3E). Notably, none of the mice that achieved normoglycemia in these two groups required exogenous insulin therapy once the BIs began to fully regulate levels of blood glucose by 8–18 days p-i (e.g., Fig. 3F).
As an additional assessment of BI function, intraperitoneal glucose tolerance tests were performed at 40–50 days p-i on the subset of animals from the +VEGF/+Islet group (all eight mice) and −VEGF/+Islet group (five of eight mice) that had achieved normoglycemia (Fig. 4). Compared to healthy, nondiabetic mice that had not received STZ or BIs (n = 10), the BGLs of both groups of mice carrying BIs were elevated 15–30 min after glucose administration. The rise in BGL was slightly lower for the +VEGF/+Islet group than for the −VEGF/+Islet group, but the difference was not statistically significant. Within 1–2 h of challenge, the BGLs for all three groups had returned to normal levels, with no significant difference between them.
Figure 4.
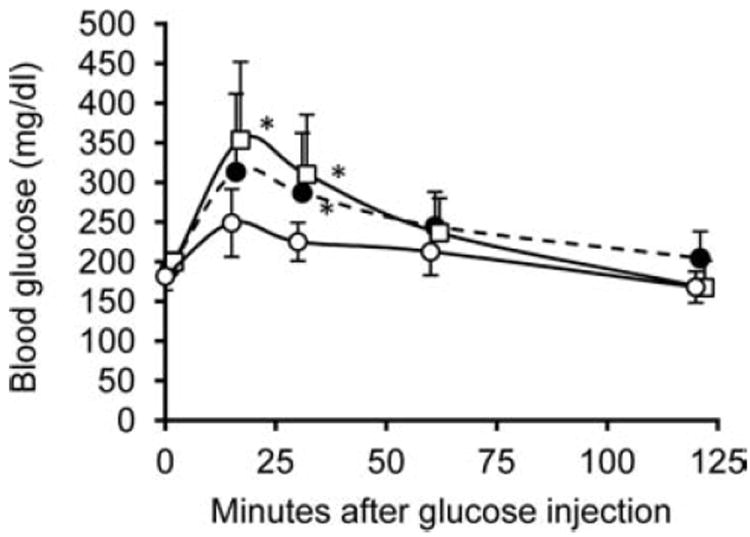
Response to glucose challenge of mice rendered normoglycemic by BIs. Intraperitoneal glucose tolerance tests were performed at 40–50 days p-i on the animals from the +VEGF/+Islet group (eight mice, black circles) and −VEGF/+Islet group (five mice, white squares) that had achieved normoglycemia. These results were compared to similar tests performed on healthy, nondiabetic mice that had not received STZ or BIs (control group) (white circles). By 15–30 min after challenge with glucose, both groups of mice implanted with BIs had elevated BGLs relative to controls. The rise in BGL was slightly lower for the +VEGF group than for the −VEGF group, but the difference was not statistically significant. Within 1–2 h of challenge, the difference in BGL between the three groups was nonsignificant. *p < 0.05 between the control group and the groups implanted with BIs.
The functionality of the BIs was further confirmed upon removal, by survival surgery, of implants from four mice of the +VEGF/+Islet group at 54 days p-i. Three of these mice became hyperglycemic within 24–48 h, while the fourth mouse remained normoglycemic (data not shown). By histological assay, all four mice had little or no islet residua in their pancreata; therefore, maintenance of normoglycemia in the one mouse may have been due to migration of islets from the BI into the animal’s peritoneal cavity. These islets would not have been removed when the BI was excised. In the four mice, we observed that removal of the BI caused devascularization of the adjacent intestine leading to focal necrosis; therefore, we did not perform survival surgeries on additional mice. Histological analyses of BIs explanted from the +VEGF/+Islet group at 54 days p-i (Fig. 5) revealed the presence of insulin-positive islets that were well-vascularized, as indicated by the presence of vWF-positive microvessels that contained luminal blood. The type I collagen hydrogel in which the islets were suspended prior to implantation was largely absent, although scattered remnants were observed.
Figure 5.
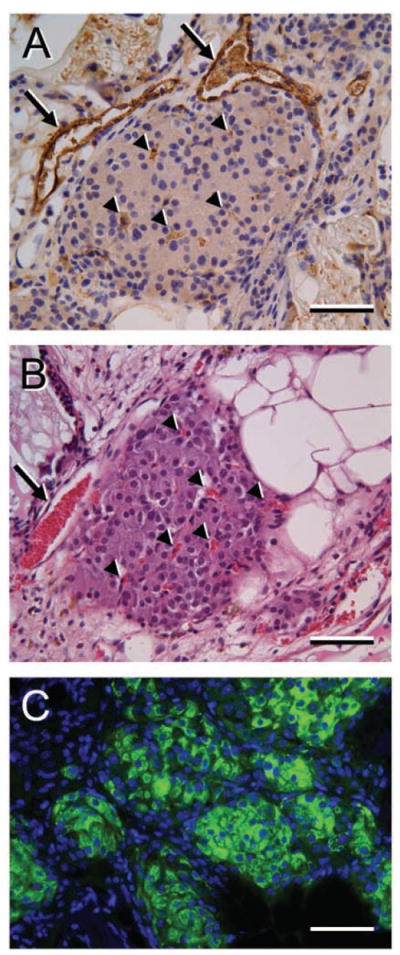
Histological evaluation of islets within BIs after 54 days in vivo. Mice of the +VEGF/+Islet group that had exhibited sustained normoglycemia for 54 days had their BIs excised and sectioned for histological analysis. (A) An islet labeled for von Willebrand Factor (vWF) (brown stain) exhibits profiles of large- and small-caliber microvessels (arrows and arrowheads, respectively) of the islet. Nuclei are counterstained with hematoxylin. (B) A slightly deeper section of the islet shown in (A), stained with H&E, indicates the presence of red blood cells (red stain) in the large- and small-caliber microvessels (arrow and arrowheads, respectively) of the islet. (C) Islet groups within a similar BI are strongly positive for insulin, as shown by indirect immunofluorescence (green). Cell nuclei in the section are stained with DAPI (blue). Scale bars: 100 μm (in A–C).
By histology, there were no obvious differences at 54–77 days p-i in the level of vascularization within BIs from the +VEGF/+Islet group compared to the normoglycemic mice of the −VEGF/+Islet group (data not shown). Interestingly, however, although the +VEGF/+Islet, −VEGF/+Islet, and +VEGF/−Islet groups all exhibited similar hyperglycemic BGLs prior to surgery on day 0 (Fig. 6A, left), by 24 h p-i, seven of eight mice of the −VEGF/+Islet group were hypoglycemic (avg. 116 ± 21 mg/dl, n = 7), whereas only one of eight mice of the +VEGF/+Islet group was hypoglycemic (group avg. 348 ± 142 mg/dl, n = 8) (Fig. 6A, center). None of the mice of the +VEGF/−Islet group were hypoglycemic (group avg. 491 ± 91 mg/dl, n = 12). Notably, by 48 h p-i, all of the mice of all three groups were hyperglycemic (Fig. 6A, right). Collectively, these results indicated that inclusion of VEGF with the implanted islets substantially mitigated the transitory hypoglycemia that occurred when islets were implanted in the absence of VEGF. To further investigate this phenomenon, we cultured isolated islets for 2 h in the presence of 10 ng/ml of VEGF and a low level (5.6 mM) of glucose, followed by a 30-min stimulus with elevated (16.6 mM) glucose to elicit insulin release. We found that the exposure to VEGF significantly reduced glucose-induced insulin release, compared to cultured islets not exposed to VEGF (Fig. 6B).
Figure 6.
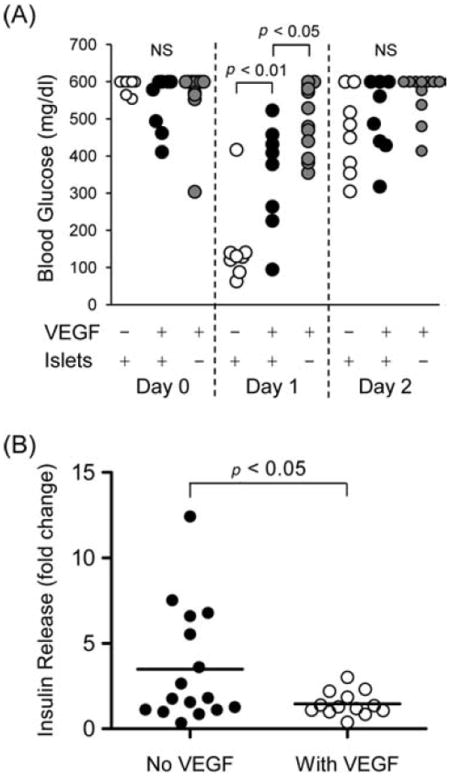
The presence of VEGF in BIs is associated with limitation of postoperative, transitory hypoglycemia. (A, left) Prior to surgery on day 0, all mice within the −VEGF/+Islet, +VEGF/+Islet, and +VEGF/−Islet groups (white, black, and gray circles, respectively) exhibited hyperglycemic BGLs. (A, center) By 24 h (day 1) p-i, seven of eight mice of the −VEGF/+Islet group were hypoglycemic, whereas only one of eight mice of the +VEGF/+Islet group was fully hypoglycemic. None of the mice of the +VEGF/−Islet group were hypoglycemic. (A, right) By 48 h (day 2) p-i, the hypoglycemic episodes had ended: all of the mice within the three groups were hyperglycemic. (B) Glucose-induced insulin release in vitro from isolated islets in the presence or absence of VEGF. Islets were preincubated for 2 h with a low level (5.6 mM) of glucose, with or without 10 ng/ml of VEGF, and then stimulated for 30 min with elevated (16.6 mM) glucose. Fold change represents insulin levels in media before and after glucose stimulus.
DISCUSSION
To achieve successful islet transplantation for treatment of T1D, the protocol must promote islet survival in the short term. Insulin secretory function in patients who receive intraportal islet transplantation averages only ~20% of that of nondiabetic persons despite the use of islets from multiple donors (35)—a result suggesting that only a small proportion of transplanted islets successfully engraft. Reasons for this loss of islet function include (1) exposure to high concentrations of cytotoxic immunosuppressive drugs via portal blood, (2) proinflammatory cytokine release by intrahepatic endothelial cells activated by islet cell contact, (3) liver ischemia, focal necrosis, and inflammation induced by islet embolism, and (4) acute inflammatory reactions that involve platelet activation and binding at the islet surface, activation of coagulation and complement systems, and leukocyte infiltration of the islet mass. To address the shortcomings of the intrahepatic environment, we developed BIs for implantation in a nonhepatic site (the intestinal mesentery). We have demonstrated that these BIs can reliably reverse drug (STZ)-induced diabetes in mice.
Injection of islet suspensions under the kidney capsule (UKC) is the most frequently used model for studies of islet engraftment in mice. With the UKC model, normoglycemia can be established in STZ-treated diabetic mice within 24–48 h using 200 islets (45). In contrast to the UKC model, we observed that engraftment of islets on the mouse mesentery in BIs required a larger number of islets (200 islets were insufficient, whereas 450–500 islets were effective) and required a longer time to achieve normoglycemia (10–13 days). Similar disparities in islet number and time to functionality between UKC and mesenteric (omental) graft sites in mice have been reported by Kim et al. (16), who found that the marginal islet mass required for the omental site was twice that of the kidney site and that time to normoglycemia averaged 14 days for omental grafts, but only 3 days for UKC grafts. Notably, the omentum performed better than liver and muscle sites, which each required threefold more islets than the omentum and comparable or longer times to achieve normoglycemia (15 and 27 days for liver and muscle, respectively). The reason why engraftment of islets is less efficient in non-UKC sites relative to the UKC site is unclear but may relate to a lower availability of vasculature, particularly for muscle (16).
Despite the efficiency of UKC transplant, the limited space within the kidney capsule cannot accommodate large numbers of injected islets or complex implants that include drug delivery devices, such as the one we describe. This limitation, among others (7,31), suggests that the UKC site may be problematic for therapeutic islet transplant in human patients. In contrast, the human omentum should be able to accommodate relatively large, multicomponent BIs. Moreover, unlike the kidney, the omentum is not a critical organ and, therefore, could be excised with few negative consequences should post-transplantation complications arise.
In contrast to most other approaches in which islets are engrafted as dispersions, our BI retains the islets in a unified structure by means of a disk-shaped scaffold. The purpose of this scaffold is fourfold: (1) to keep the islets contained within a limited volume and in close proximity to the alginate delivery device in order to maximize the effects of the released cytokine; (2) to protect the relatively soft collagen hydrogel (which supports the islets directly) from physical disruption both before and after implantation; (3) to allow the BI, with all of its components, to be rapidly assembled and implanted easily without the additional complexity of a surgically produced omental/mesenteric pouch; (4) to allow the implant to be removed easily and maintained in a compact form that makes histological analysis straightforward. For the present experimental study (which has relatively short-term endpoints), nonbiodegradable PVA sponge works well as a scaffold. For therapeutic use in human patients, the PVA could be replaced by biodegradable materials that would be resorbed after the implanted islets become fully functional.
A beneficial effect of scaffolds on islet engraftment has been observed by others. Blomeier et al. (2) reported that STZ-induced diabetic mice that received intraperitoneal implants of islets infused into cylindrical polylactide/glycolide (PLG) sponge scaffolds had higher conversions and shorter times to normoglycemia, greater weight gain, and improved response to intraperitoneal glucose tolerance tests compared to control mice that received islets not retained in scaffolds. These authors demonstrated that the islets transplanted in scaffolds remained localized at the original site of implantation, whereas the nonscaffolded islets tended to be more dispersed throughout the peritoneum. Therefore, the protective environment provided by the scaffold might have contributed to better performance of the graft. These results and our own observations that scaffolds facilitate the assembly and handling of multicomponent constructs argue for the continued development of scaffolds for islet transplantation.
In our BIs, direct physical support of the islets is accomplished by a fibrillar type I collagen hydrogel. The islets are dispersed in a single volume of monomeric collagen solution, which is infused into the protective PVA scaffold and polymerized in situ into a gel-like network of fibrils. Notably, neither the PVA scaffold, with its open structure, nor the collagen gel seem to impede the rate of vascularization from the mesentery, since dispersed islets implanted into mesenteric (omental) pouches also require the same time as our BIs to engraft and function (i.e., about 2 weeks) (16).
The endocrine cells of islets in vivo are associated with a complex peri-insular and perivascular ECM, with components of the basement membrane (BM) (e.g., laminin, type IV collagen, fibronectin) predominating (38). In this context, there is evidence that addition of specific BM components to islet graft sites improves islet graft performance. Salvay et al. (37) demonstrated that islets implanted onto the epididymal fat pads of STZ-treated mice were more effective at reversing diabetes when the islets were infused into PLG sponge scaffolds preadsorbed with type IV collagen, compared with control scaffolds pretreated with serum proteins. Adsorbed fibronectin and laminin 5 were less effective than type IV collagen but were superior to the serum-treated controls. It was proposed that the adsorbed BM proteins might be improving islet function directly and/or promoting infiltration of beneficial cell types (e.g., endothelial cells) from the host, as vascularization of the ECM-treated grafts was better than vascularization of the serum-coated grafts. Collectively, these results suggest that incorporation of individual BM components (or perhaps more complex BM mixtures) into our BIs (e.g., via direct binding to or cogelation with the collagen hydrogel) might improve the overall performance of the grafts after implantation.
Reportedly, the level of vascularization of islets transplanted into the liver or kidney is lower than that of native islets in the pancreas (22), but transfection of islets to express VEGF increases vascularization following transplantation (19,46). Transfection-based approaches are therapeutically problematic; therefore, we incorporated a device (an alginate macrosphere) within the BI to achieve a local, sustained delivery of VEGF. In preliminary experiments, we produced BIs with macrospheres that incorporated high levels (160 ng) of VEGF. The VEGF in these constructs induced a very robust response from the host within 7 days of implantation in vivo, as indicated by high levels of angiogenesis, the presence of enlarged sinusoidal neovessels, and substantial vascular permeability (i.e., extravasated blood) within the BI. Although these results were a clear indication that the VEGF was biologically active, we considered this level of response to be excessive; therefore, we reduced the level of VEGF to approximately 20 ng per macrosphere for the subsequent experiments reported here. Inclusion of 20 ng of VEGF in the BI did not decrease the average time for transplanted mice to achieve normoglycemia (approximately 2 weeks), as compared to control mice with BIs that lacked VEGF. Although VEGF increased the percentage of mice that became normoglycemic compared to the controls lacking VEGF (100% vs. 62.5%), this increase was not statistically significant for our sample size (n = 8 mice per group). These results suggest that exogenous VEGF may not have a major effect on implant performance when therapeutically “safe” (i.e., well above the minimum) numbers of islets are used.
We were particularly interested by the finding that, with the exception of one animal, the +VEGF/+Islet group did not exhibit the hypoglycemia that occurred within 24 h p-i in the −VEGF/+Islet group. Postoperative, transitory hypoglycemia has been observed in other models of islet transplantation and may be a consequence of an acute release of insulin from stressed or dying islets. Our observations suggest that the inclusion of VEGF within BIs mitigates this acute insulin release, perhaps via direct influences on the transplanted islets, as we found that exogenous VEGF suppresses glucose-stimulated release of insulin from isolated, cultured islets. This suppressive effect of exogenous VEGF on insulin release in vitro seems to support an earlier finding that islets isolated from RIP-CRE:VEGFfl/fl mice, in which production of VEGF is prevented specifically in β-cells, had higher levels of insulin mRNA and secreted more insulin after glucose stimulation in vitro than did islets from control mice that expressed VEGF (15). VEGF acts on endothelial cells to promote the maintenance and growth of vasculature, including the vasculature of islets, as illustrated by RIP-CRE:VEGFfl/fl mice, which have deficiencies in their microvessels (14). Although prosurvival, proangiogenic responses of intraislet endothelial cells to VEGF would be expected, additional responses by these cells might include the production of paracrine factors that promote the survival and function of islet endocrine cells.
In treatments of diabetic patients that involve transplantation of islets, controlling rejection is typically accomplished by systemic immunosuppressive compounds. Dosing of these compounds is a difficult balance—levels must be low enough to permit a reasonable degree of protective immunity against pathogenic organisms, but high enough to effectively suppress allo- and autoimmune activity directed against the transplant. In the case of SPK transplants, some current immunosuppression regimens are inadequate to control autoimmunity (18,41). Moreover, no matter what the dose, systemic immunosuppression can be accompanied by a variety of undesirable side effects on tissue and organ systems that are not directly associated with the transplant. In light of the problems associated with systemic treatments, an alternative approach would be to confine the delivery of immunotherapy to the implant itself. In this way, immunomodulatory compounds could be delivered at relatively high concentrations, but within the limited volume of the implant, thereby minimizing side effects on tissues and organs outside the zone of delivery. To this end, the BI described here includes a mechanically supportive scaffold and collagen hydrogel that concentrates the islets in a small volume. Localized immunotherapy might be achieved by supplementing or replacing the collagen hydrogel with ECM components that have been shown to have immunosuppressive properties, such as hyaluronan (3,4). This ECM-based approach could be augmented by sustained release of specific, immunomodulatory cytokines [e.g., interleukin-10 (IL-10) and transforming growth factor-β (TGF-β)] from biocompatible storage media placed within the implant. We are currently investigating the use of alginate-based media for this purpose.
In the present study, we have developed a BI and associated protocols for its implantation that can effectively reverse STZ-induced diabetes in syngeneic mice. Our future studies will evaluate the effectiveness of the BI in the context of strains of mice that develop autoimmune diabetes. In this way, the BI will serve as a platform to evaluate the capability of a variety of immunomodulatory compounds and formulations, delivered locally, to prevent or reverse diabetes in the setting of autoimmune dysfunction.
Acknowledgments
We would like to thank Dr. Rebecca Hull for her valuable help in optimizing the islet isolation procedures. This work was funded in part by Department of Defense Award W81XWH-10-1-049 (G.T.N.) and by an award from the Klorfine Foundation (R.B.V.).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Alejandro R, Barton FB, Hering BJ, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783–1788. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 2.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82:452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: High molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567–572. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: High molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 5.DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med. 1991;90:450–459. [PubMed] [Google Scholar]
- 6.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Gray DW, Sutton R, McShane P, Peters M, Morris PJ. Exocrine contamination impairs implantation of pancreatic islets transplanted beneath the kidney capsule. J Surg Res. 1988;45:432–442. doi: 10.1016/0022-4804(88)90193-x. [DOI] [PubMed] [Google Scholar]
- 8.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Control Release. 2004;96:463–472. doi: 10.1016/j.jconrel.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch D, Odorico J, Radke N, Hanson M, Danobeitia JS, Hullett D, Alejandro R, Ricordi C, Fernandez LA. Correction of insulin sensitivity and glucose disposal after pancreatic islet transplantation: Preliminary results. Diabetes Obes Metab. 2010;12:994–1003. doi: 10.1111/j.1463-1326.2010.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14:433–440. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 11.Hollinger EF, Powelson JA, Mangus RS, Kazimi MM, Taber TE, Goble ML, Fridell JA. Immediate retransplantation for pancreas allograft thrombosis. Am J Transplant. 2009;9:740–745. doi: 10.1111/j.1600-6143.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- 12.Hori Y, Winans AM, Irvine DJ. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 2009;5:969–982. doi: 10.1016/j.actbio.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AC, Sutherland DE. Technical failures after pancreas transplants: Why grafts fail and the risk factors—A multivariate analysis. Transplantation. 2004;78:1188–1192. doi: 10.1097/01.tp.0000137198.09182.a2. [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193–202. doi: 10.1016/s1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 15.Iwashita N, Uchida T, Choi JB, Azuma K, Ogihara T, Ferrara N, Gerber H, Kawamori R, Inoue M, Watada H. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia. 2007;50:380–389. doi: 10.1007/s00125-006-0512-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim HI, Yu JE, Park CG, Kim SJ. Comparison of four pancreatic islet implantation sites. J Korean Med Sci. 2010;25:203–210. doi: 10.3346/jkms.2010.25.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koike T, Vernon RB, Gooden MD, Sadoun E, Reed MJ. Inhibited angiogenesis in aging: A role for TIMP-2. J Gerontol A Biol Sci Med Sci. 2003;58:B798–B805. doi: 10.1093/gerona/58.9.b798. [DOI] [PubMed] [Google Scholar]
- 18.Laughlin E, Burke G, Pugliese A, Falk B, Nepom G. Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol. 2008;128:23–30. doi: 10.1016/j.clim.2008.03.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BW, Lee M, Chae HY, Lee S, Kang JG, Kim CS, Lee SJ, Yoo HJ, Ihm SH. Effect of hypoxia-inducible VEGF gene expression on revascularization and graft function in mouse islet transplantation. Transpl Int. 2011;24:307–314. doi: 10.1111/j.1432-2277.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 20.Lucas-Clerc C, Massart C, Campion JP, Launois B, Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: Morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9–20. doi: 10.1016/0303-7207(93)90046-m. [DOI] [PubMed] [Google Scholar]
- 21.Matsusaki M, Sakaguchi H, Serizawa T, Akashi M. Controlled release of vascular endothelial growth factor from alginate hydrogels nano-coated with polyelectrolyte multi-layer films. J Biomater Sci Polym Ed. 2007;18:775–783. doi: 10.1163/156856207781034160. [DOI] [PubMed] [Google Scholar]
- 22.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362–1366. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- 23.Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest. 2008;118:1806–1814. doi: 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhlhauser I, Berger M, Sonnenberg G, Koch J, Jorgens V, Schernthaner G, Scholz V, Padagogin D. Incidence and management of severe hypoglycemia in 434 adults with insulin-dependent diabetes mellitus. Diabetes Care. 1985;8:268–273. doi: 10.2337/diacare.8.3.268. [DOI] [PubMed] [Google Scholar]
- 25.Nagata N, Gu Y, Hori H, Balamurugan AN, Touma M, Kawakami Y, Wang W, Baba TT, Satake A, Nozawa M, Tabata Y, Inoue K. Evaluation of insulin secretion of isolated rat islets cultured in extracellular matrix. Cell Transplant. 2001;10:447–451. [PubMed] [Google Scholar]
- 26.Nagata NA, Inoue K, Tabata Y. Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J Biomater Sci Polym Ed. 2002;13:579–590. doi: 10.1163/15685620260178418. [DOI] [PubMed] [Google Scholar]
- 27.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58:194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 28.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes—The analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–1403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 29.Peters MC, Isenberg BC, Rowley JA, Mooney DJ. Release from alginate enhances the biological activity of vascular endothelial growth factor. J Biomater Sci Polym Ed. 1998;9:1267–1278. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- 30.Pickup J. Insulin pumps. Int J Clin Pract. 2011;170(Suppl):16–19. doi: 10.1111/j.1742-1241.2010.02574.x. [DOI] [PubMed] [Google Scholar]
- 31.Rajab A. Islet transplantation: Alternative sites. Curr Diab Rep. 2010;10:332–337. doi: 10.1007/s11892-010-0130-6. [DOI] [PubMed] [Google Scholar]
- 32.Reed MJ, Bradshaw AD, Shaw M, Sadoun E, Han N, Ferara N, Funk S, Puolakkainen P, Sage EH. Enhanced angiogenesis characteristic of SPARC-null mice disappears with age. J Cell Physiol. 2005;204:800–807. doi: 10.1002/jcp.20348. [DOI] [PubMed] [Google Scholar]
- 33.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 34.Roep BO, Stobbe I, Duinkerken G, van Rood JJ, Lernmark A, Keymeulen B, Pipeleers D, Claas FH, de Vries RR. Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes. 1999;48:484–490. doi: 10.2337/diabetes.48.3.484. [DOI] [PubMed] [Google Scholar]
- 35.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AM. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 36.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 37.Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL, Jr, Shea LD. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85:1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP, Gruessner AC, Najarian JS. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 41.Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, Diamantopoulos S, Standifer N, Geubtner K, Falk BA, Ichii H, Takahashi H, Snowhite I, Chen Z, Mendez A, Chen L, Sageshima J, Ruiz P, Ciancio G, Ricordi C, Reijonen H, Nepom GT, Burke GW, III, Pugliese A. Recurrence of type 1 diabetes after simultaneous pancreas–kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59:947–957. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernon RB, Gooden MD. New technologies in vitro for analysis of cell movement on or within collagen gels. Matrix Biol. 2002;21:661–669. doi: 10.1016/s0945-053x(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 43.Vrochides D, Paraskevas S, Papanikolaou V. Transplantation for type 1 diabetes mellitus. Whole organ or islets? Hippokratia. 2009;13:6–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 45.Yin D, Tao J, Lee DD, Shen J, Hara M, Lopez J, Kuznetsov A, Philipson LH, Chong AS. Recovery of islet beta-cell function in streptozotocin-induced diabetic mice: An indirect role for the spleen. Diabetes. 2006;55:3256–3263. doi: 10.2337/db05-1275. [DOI] [PubMed] [Google Scholar]
- 46.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, Zhang H, Song K, Meseck M, Bromberg J, Dong H. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963–970. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32:468–478. doi: 10.1016/j.immuni.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


