Abstract
Inflammation plays an important role in the pathogenesis of ischemic stroke and other forms of ischemic brain injury. Increasing evidence suggests that inflammatory response is a double-edged sword, as it not only exacerbates secondary brain injury in the acute stage of stroke but also beneficially contributes to brain recovery after stroke. In this article, we provide an overview on the role of inflammation and its mediators in acute ischemic stroke. We discuss various pro-inflammatory and anti-inflammatory responses in different phases after ischemic stroke and the possible reasons for their failures in clinical trials. Undoubtedly, there is still much to be done in order to translate promising pre-clinical findings into clinical practice. A better understanding of the dynamic balance between pro- and anti-inflammatory responses and identifying the discrepancies between pre-clinical studies and clinical trials may serve as a basis for designing effective therapies.
Keywords: Inflammation, leukocytes, inflammatory mediators, brain ischemia
Introduction
Stroke is the leading cause of death and permanent disability worldwide [1]. Inflammation acts importantly in the progression of ischemic stroke, although the underlying mechanisms are largely unclear [2, 3, 4]. Cerebral ischemia could break the dynamic balance between the pro-inflammatory and anti-inflammatory responses. Pre-clinical stroke studies indicate that inhibition of inflammatory responses could decrease brain injury and improve neurological outcome [5]. Clinical studies indicate that systemic inflammation could influence the susceptibility of the patients to stroke and the subsequent prognosis [6, 7]. However, inhibition of inflammatory responses could worsen brain repair and long-term functional recovery after ischemic stoke. A comprehensive understanding of the dynamic balance between pro-inflammatory and anti-inflammatory responses is a prerequisite for developing effective therapies to treat ischemic stroke. In current review, we provide an overview on the role of inflammation and its mediators in acute ischemic stroke. We discuss the role of blood-borne and brain resident inflammatory cells, pro- and anti-inflammatory mediators, and related pathways in ischemic brain injury.
1. Blood-borne inflammatory cells in ischemic stroke
Of the various types of leukocytes, neutrophils are among the first to infiltrate ischemic brain (30 min to a few hours of focal cerebral ischemia), peak earlier (Days 1–3), and then disappear or decrease rapidly with time [8]. Infiltrating neutrophils contribute to brain inflammation and injury by releasing a number of pro-inflammatory mediators, such as inducible nitric oxide synthase (iNOS) and matrix metalloproteinases (MMPs) [9], which are stored in granules and vesicles of neutrophils. Neutrophils may act as a deleterious role in the stroke [10], because immuno-depletion of neutrophils or antibody inhibition of neutrophil infiltration could significantly decrease ischemic brain injury and improve neurological outcome [11, 12, 13, 14, 15].
Monocytes and macrophages play dual functions after stroke due to their expressions of anti- and pro-inflammatory mediators. Blood monocytes are divided into at least two subtypes, namely “anti-inflammatory” (Ly-6Chigh/CCR2+) and “pro-inflammatory” (Ly-6Clow/CCR2−) subpopulations [9]. The Ly-6Clow/CCR2− monocytes exhibit anti-inflammatory property by the expression of anti-inflammatory cytokines, such as interleukin 10 (IL-10). In contrast, the Ly-6Chigh/CCR2+ monocytes exhibit pro-inflammatory property by the expression of pro-inflammatory cytokines, such as IL-1β and tumor necrosis factor (TNF-α) [9, 16, 17]. During cerebral ischemia, peripheral blood monocytes migrate into the ischemic brain tissues where they mature into different types of microglia- and macrophage-like cells or dendritic cells [18, 19]. However, the exact roles of infiltrating monocytes in acute ischemic brain injury and stroke recovery remain to be elucidated. Macrophages are also divided into two subtypes: inflammatory M1 and anti-inflammatory M2 macrophages. M1 macrophages exbibite inflammatory property by producing inflammatory mediators, such as IL-1β, TNF-α and chemokines (e.g. MCP-1, MIP-1α), whereas M2 macrophages exhibit anti-inflammatory property by producing anti-inflammatory cytokines, such as IL-10 and transforming growth factor beta (TGF-β) [20].
Lymphocytes play complex roles in pathogenesis of ischemic stroke. There are many subtypes of lymphocytes, and several subtypes of T cells have been implicated in the pathogenesis of ischemic stroke [21, 22]. In recent years, increasing research efforts have been devoted to the roles of specific T cell subtypes in ischemic stroke. However, the time course of the recruitment of different subtypes of T cells into the ischemic brain remains largely undetermined. Recent studies suggest the importance of CD4+, and CD8+ T cells and γδT cells in the pathogenesis of ischemic stroke [23]. These subtypes of T cells act deleterious roles in stroke by producing pro-inflammatory cytokines (e.g. IFN-γ and IL-17), whereas Treg cells (CD4+CD25+Foxp3+ Treg cells) seem to act beneficial role by producing anti-inflammatory cytokines (e.g. IL-10).
2. Brain resident cells in ischemic stroke
Microglia are the main resident immunological macrophage-like cells in the central nervous system (CNS) [24], and served as scavenger cells in the event of inflammation, ischemia, and neurodegeneration [25, 26]. Microglia could be activated rapidly (within minutes) in response to cerebral ischemia [27, 28]. Its activation and expansion peaked at 2–3 days after ischemic stroke and lasted for weeks after initial injury [25, 26]. The exact roles of microglia in ischemic stroke are largely unclear. It seems that microglia play dual functions in ischemia stroke. On activated, microglia can produce inflammatory mediators leading to cell damage and death. Meanwhile, microglia can also produce TGF-β1, which acts as a neuroprotective role [27]. These dual functions may be related to the time of microgial activation since data suggested that early activation is detrimental and later activation is beneficial [25]. Furthermore, different subsets of microglia act different roles in cerebral ischemia and could increase or decrease the brain injury [28].
Astrocytes play important roles in the function of normal CNS and also in stroke pathology [29]. They may proliferate and differentiate (astrogliosis) following ischemic stroke with increased expression of glial fibrillary acidic protein (GFAP). Most of the astroglial response starts within 4 h in the core area of trauma and last more than 28 days after the photo-thrombosis stroke onset [30]. However, other data showed that the response could be activated only after 24 h and with a peak expansion at 4 days after the insult [31]. Astrocytes may produce a number of inflammatory mediators [32, 33] and develop neuroinflammation by secreting major histocompatibility complex and costimulatory molecules, which can activate anti-inflammatory responses (e.g. Th2) [34]. Like microglia, astrocytes also act dual functions, some beneficial and some detrimental. For example, inhibition of astrocyte proliferation improve functional recovery [35], however, administration of TGF-α, a mitogen of astrocytes [36], decreases infarct size and increases functional recovery after focal cerebral ischemia [37].
3. Inflammatory mediators in ischemic stroke
After an ischemic insult, inflammatory mediators in the ischemic brain are upregulated from resident brain cells and infiltrating immune cells, which play a complex role in the pathophysiology of cerebral ischemia [Figure 1]. A number of major pro- and anti-inflammatory mediators are summarized in Table 1.
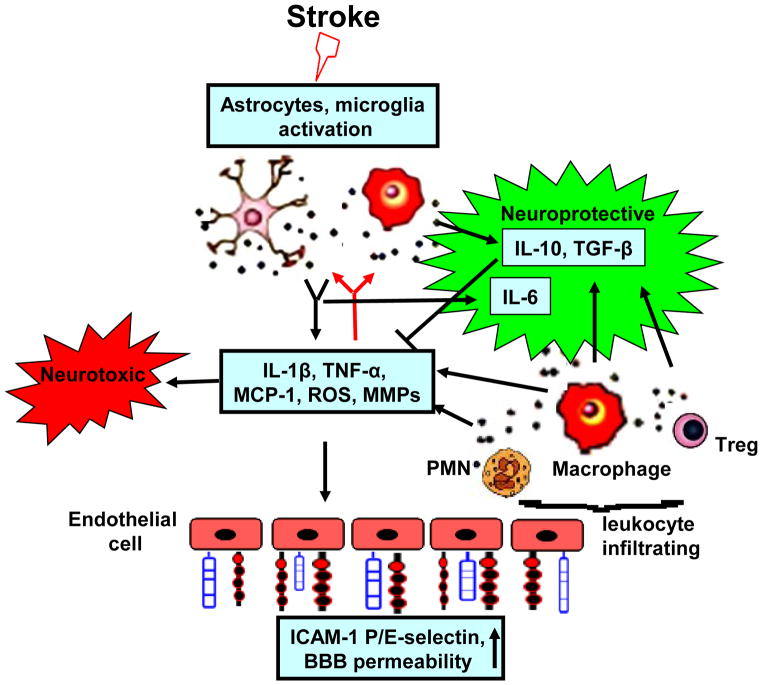
Figure 1. Postischemic inflammatory response.
Stroke induces activation of microglia and astrocytes which react by secreting cytokines, chemokines, ROS and matrix metalloproteases (MMPs). These mediators increase the expression of adhesion molecules on cerebral endothelial cells, which promotes adhesion and infiltration of the blood-derived leukocytes (neutrophils, macrophages and lymphocytes) to ischemic brain. Infiltrating leukocytes further amplify brain inflammatory response by secreting a variety of proinflammatory mediators. Meanwhile, activated microglia/macrophage and infiltrated Treg cells also secrete some neuroprotective factors (e.g. IL-10, TGF-β) that could suppress postischemic inflammation.
Table 1.
Summary of pro- and anti-inflammatory mediators involved in brain ischemia injury.
| Name | Main sources | Time course | Pathway | Effects | Ref. | |
|---|---|---|---|---|---|---|
| Pro-inflammatory | ||||||
| Cytokines | TNF-α | Microglia/macrophage, astrocyte, neuron, and endothelial cell | increased 0.5–6h, peak at 12–24h and remains elevated for days | TNF-α/TNF-R p75 pathway | Neurotoxic | 43, 44 |
| TNF-α/TNF-R p55 pathway | Neuroprotective | 45, 46 | ||||
| IL-1β | Microglia/macrophage, astrocyte, neuron and endothelial cell | increased 2–6h, peak at 12–24h | IL-1β/NF-kB and MAPK pathway | Neurotoxic | 57, 58 | |
| IL-6 | Microglia/macrophage, astrocyte, neuron and endothelual cell. | increased 10h, peak at 18h | IL-6/gp130/JAK/STAT pathway | Neuroprotective | 64 | |
| Chemokines | MCP-1 | Neuron, astrocyte and microglia | increased 3–6h, peak at 12h to 2day | MCP-1/PI3K, MAPK, and PKC pathway | Neurotoxic | 97 |
| MIP-1α | Neuron and microglia | increased 2h, peak at 4h | MIP-1α/PKC and MAPK pathway | Neurotoxic | 96 | |
| Anti-inflammatory | ||||||
| IL-10 | Microglia/macrophage, Th1, Th2, B cells and astrocyte | increased 12h, peak at 72h | IL-10/JAK/STAT, PI3K and MAPK pathway | Neuroprotective | 111, 112 | |
| TGF-β | Microglia/macrophage Treg, | increased 3h, peak at 24h | TGF-β/Smad pathway | Neuroprotective | 118, 119 | |
| TIPE-2 | Microglia/macrophages, | increased 3h, peak at 48h | Negatively regulate TLR and TCR pathway | Neuroprotective | 123 | |
| IGF-1 | Microglia, astrocytes and endothelual cell | increased 12h, peak at 24h | IGF-1/PI3K pathway | Neuroprotective | 125, 126 | |
3.1. Pro-inflammatory mediators
Cytokines
TNF-α
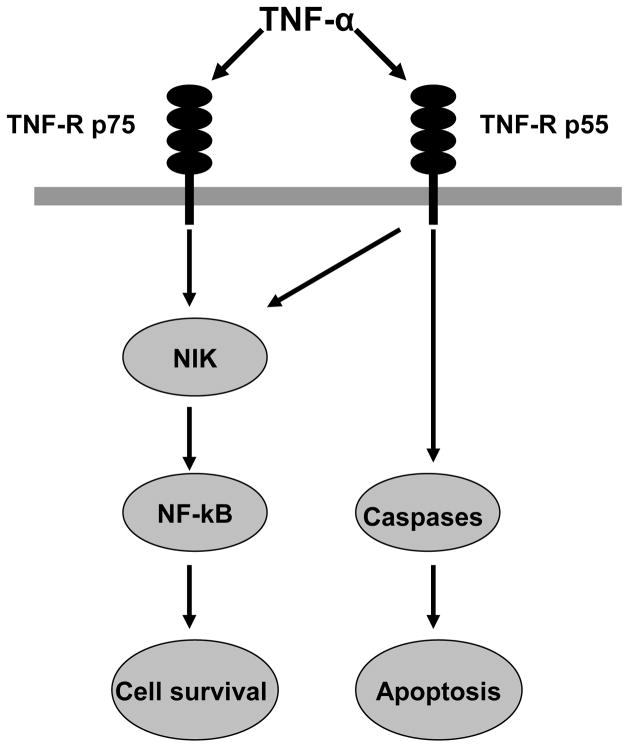
TNF-α, a potent pro-inflammatory cytokine, is upregulated in the brain after both permanent [38] and transient MCAO [39]. Its expression is initially increased at 1– 3 h after the ischemic onset and then has a second peak at 24–36 h [40, 41]. TNF-α orchestrates pleiotropic functions in ischemic brain injury [42]. Increasing brain TNF-α before stroke worsens brain damage while blocking TNF-α reduces ischemic brain injury [43, 44]. However, TNF-α is also implicated in neuroprotective mechanisms of ischemic brain injury [45, 46]. Pre-exposure of cultured neurons to TNF-α caused a protection against hypoxic injury, and inhibition of TNF-α in hypoxia-preconditioned cell abolished the tolerant state [47]. The dual functions of TNF-α may be related to different TNF-α receptors: tumor necrosis factor receptor (TNF-R) p55 and TNF-R p75. TNF-R p55 seems to mediate TNF-α-induced apoptosis by activating sequential caspases [48], whereas TNF-R p75 appears to involve in cell survival via mediating the transcription factor nuclear factor-kappaB (NF-κB) [49] [Figure 2].
Figure 2. TNF-α pathway.
The TNF-α signaling is mediated via TNF-R p55 and TNF-R p75. TNF-R p55 seems to lead to TNF-α-induced apoptosis, whereas TNF-R p75 appears to involve in cell survival.
IL-1α and IL-1β
IL-1α and IL-1β, two pro-inflammatory isoforms of IL-1 family, have been implicated in the pathogenesis of many human diseases, including stroke [50]. Both IL-1α and IL–1β are precursor proteins with a small molecular mass of approximately 33 kDa. The precursor form of IL-1α is biologically active [51], whereas the precursor form of IL-1β is minimal biological activity and needs cleavage to mature IL-1β by proteases such as IL- 1β converting enzyme, leukocyte elastase, granzyme A and MMPs [52, 53, 54]. IL-1α and IL-1β orchestrate similar functions, although these may vary among different cell types and organ systems [55, 56]. They are key contributors to ischemic brain injury. IL-1α/β double knockout mice have markedly reduced brain damage induced by middle cerebral occlusion (MCAO) [57] and increased brain damage also occurred when IL-1β was administered to rats [58]. IL-1α and IL-1β act mainly via two receptors (IL-1R1 and IL-1R2) [59]. IL-1R1, which binds to both IL-1α and IL-1β, can be detected in a variety of cell types, whereas the IL-1R2 is found on the cell surface of type B lymphocytes, neutrophils and macrophage, and binds with IL-1β with higher affinity [60]. Inactivating or knocking out the IL-1R1 decreased the extent of brain damage caused by a hypoxic–ischemic (H/I) insult and preserved neurological functions [61].
IL-6
IL-6 is a pro-inflammatory cytokine with several potentially important functions in the pathogenesis of stroke. Previous studies suggested that IL-6 is increased during the ischemic stroke [62] and seems to be a robust early marker for outcome in acute ischemic stroke [63]. Some data show that IL-6 leads to an excessive inflammatory response, which might increase injury due to stroke. However, paradoxical pre-clinical data suggested that IL-6 might be as a beneficial role. Administration of recombinant human IL-6 significantly reduces ischemic injury in rat model of stroke [64]. In fact, IL-6 also acts as an anti-inflammatory cytokine [65], although it is largely thought of a pro-inflammatory cytokine.
Selectins
Selectins are a family of three types of cell-surface proteins specialized in vascular endothelium (E-selectin and P-selectin), leukocytes (L-selectin) and platelets (P-selectin) [66, 67, 68]. E-selectin and P-selectin are mainly implicated in leukocyte rolling and recruitment during the early stage of activation, whereas L-selectin acts key roles in unstimulated leukocytes [69]. Although with distinct functions, all selectins are closely related to injury due to stroke and their effects have been documented by both pre-clinical and clinical studies. In animal studies, blocking or knocking out the P-selectin or E-selectin decreased brain injury and improved neurological function [70], whereas upregulation of P-selectin or E-selectin promoted ischemic inflammation and brain injury [71, 72, 73]. Interestingly, blocking P-selectin also reduced survival [74]. The reasons for these paradoxical outcomes may be implicated to differences between focal and global ischemic models. In clinical studies, P-selectin level is positively correlated with NIHSS scores [75]. E-selectin is associated with increased risk for the development of ischemic stroke [76]. The association between L-selectin and stroke is less clear. Early studies suggested that it does not significantly influence stroke outcome. Blocking L-selecin in MCAO rabbits did not reduce brain injury. However, recent study showed attenuation of ischemia/reperfusion (I/R) injury may be related to the nearly complete removal of L-selectin from the neutrophil surface [77]. These paradoxical functions might be related to different models.
Immunoglobulin superfamily
The immunoglobulin superfamily contains five members, namely intercellular adhesion molecule-1 (ICAM-1), ICAM-2, vascular adhesion molecule- 1 (VCAM-1), platelet–endothelial cell adhesion molecule-1 (PECAM-1), and the mucosal vascular addressing cell adhesion molecule 1 (MAdCAM-1). ICAM-1 is found on the surface of endothelial cells, leukocytes and epithelial cells, and can be upregulated by various pro-inflammatory cytokines [78, 79]. ICAM-2 is expressed on endothelial cells, leukocytes, and platelets [80] and not upregulated after stimulation, whereas VCAM-1 can be increased by IL-1β and TNF-α. PECAM-1 is implicated in leukocyte adhesion to endothelial cells and transmigration across the endothelium. MAdCAM-1, a ligand of L-selectin and α4β7 integrin, is involved in the selective homing of lymphocytes.
Among of the five members, ICAM-1 and VCAM-1 are the main pro-inflammatory mediators and have been mostly investigated in stroke. In animal studies, increased ICAM-1 expression was involved in the pathogenesis of focal ischemia [81] and blocking or knocking out the ICAM-1 decreased brain damage and improved outcome of experimental stroke [82, 83, 84, 85]. In clinical studies, ICAM-1 was also closely related to stroke. Soluble ICAM-1 was upregulated and reached a peak within 24 h onset of acute ischemic stroke [86]. Furthermore, ICAM-1 is significantly upregulated in the brain tissue after cerebral ischemia [87]. Taken together, it seems that the ICAM-1 might be a therapeutic target in acute stroke. But, unfortunately, a phase III clinical trial of anti-ICAM therapy failed to show a beneficial effect on ischemic stroke. Oppositely, this kind of treatment significantly worsened clinical outcome [88]. The interpretation of the adverse effects may be related that the anti-ICAM-1 antibody activates neutrophilic granulocytes in a complement-dependent manner [89]. The functions of VCAM-1 in stroke are controversial. One study suggested that blocking VCAM-1 improved neurological deficits and decreased neuronal death [90]. However, other data showed blocking VCAM-1 with anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice [91].
Chemokines
Chemokines are a class of small cytokines with important functions in cell communication and inflammatory cell recruitment, such as recruiting neutrophils to the site of infection and controlling the migration of leukocytes. According to the positions of cysteine residues, chemokines are usually classified into four groups: C, CC, CXC, and CX3C, with which chemokines act by both specific and shared receptors belonging to the superfamily of G-protein-coupled receptors [92].
Monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and fractalkine (CX3CL1) are three well known pro-inflammation chemokines and can be up-regulated in animal models of cerebral ischemia [93]. Upregulation of chemokines after focal ischemia is thought to act as deleterious role [94]. Studies showed that MCP-1, MIP-1α and CX3CL1 can contribute to stroke pathology. Their inhibition or deficiency can decrease ischemic brain injury [95, 96]. In addition to their chemotactic properties, studies also suggested that chemokines can directly affect the permeability of blood–brain barrier (BBB). MCP-1 can enhance 17-fold BBB’s permeability and cause alterations of BBB tight junction proteins, suggesting that MCP-1 may play an important role in regulating the BBB [97]. Furthermore, chemokines may also play an important role in homing stem cells to injured regions and be implicated in migration of bone marrow derived stromal cells into ischemic brain [98, 99].
MMPs
MMPs, a family of zinc-dependent proteolytic enzymes, are normally as a pro- or inactivated forms and can be activated by the removal of amino-terminal propeptides. Their constitutive expression is low, but can be upregulated by many pathogenic events, including stroke. MMPs play important roles in brain injury after stroke. Previous studies showed that MMPs were implicated in neurogenic migration since a broad spectrum MMP inhibitor can significantly decrease the migration of doublecortin-positive cells in transient focal cerebral ischemia in mice [100]. Inhibiting the activation of MMPs may decrease ischemic injury. However, MMPs may be beneficial in the later stage of cerebral ischemia. Treatment with the MMP inhibitor 7 d post MCAO inhibited neurovascular remodeling and increased brain injury after stroke [101].
Among various MMPs, MMP-9 is most closely implicated in cerebral ischemia [102]. MMP-9 was upregulated in brain tissue and also in serum of patients with acute ischemic stroke [103]. It was associated with BBB disruption, edema development, and hemorrhagic transformation of ischemic stroke [104, 105]. At the early stage, MMP-9 gene knock-out decreased the brain injury [106], whereas inhibiting the activation of MMP-9 released by neutrophils may be as a viable therapeutic method to decrease brain injury. At the later stage, MMP-9 is beneficial due to its association with several growth factors [e.g. vascular endothelial growth factor (VEGF)] which are involved in angiogenesis after stroke.
3.2. Anti-inflammatory mediators
IL-10
IL-10, an anti-inflammatory cytokine, is produced mainly by Th2-lymphocytes and also by other cells such as monocytes/macrophages. It can inhibit IL-1 and TNF-α and decrease both cytokine receptor expression and receptor activation. IL-10 is increased in brain tissue after stroke [107, 108, 109] and acts importantly in pathogenesis of stroke. Pre-clinical data suggested that IL-10 deficient mice have a larger lesion size after MCAO [110], and administration [111] and gene transfer of IL- 10 [112] in animal models appear to decrease brain injury after stroke [113]. Clinical data showed that low IL-10 levels predict an increased risk of stroke [114]. Taken data together, IL-10 may be a predictable therapeutic target for the treatment of ischemic stroke in future.
TGF-β
TGF-β is highly conserved and contains three isoforms that bind to the same receptors, namely TGF-β1, TGF-β2 and TGF-β3. It can be upregulated by focal cerebral ischemia [115] and TGF-β mRNA was increased in 1–6 h and remained up to 15 days after stroke [116, 117]. TGF-β plays both neuroprotective and anti-inflammatory roles in stroke and may be an effective therapeutic agent for stroke. When overexpressed, TGF-β can reduce the ischemic injury and decrease the accompanying inflammation [118]. TGF-β blockade exacerbated ischemic brain damage [119]. However, other data have reported its insignificant role when TGF-β is administered after stroke. This may be related to the TGF-β administration area, as TGF-β was shown to have a neuroprotective role when it was injected into the penumbra area, but no beneficial effect when injected into the core area [120].
TIPE-2
TIPE2 (TNF-α-inducible protein 8-like 2, or TNFAIP8L2), a recently identified anti-inflammatory protein, is important in maintaining immune homeostasis [121, 122]. Deleting TIPE2 in mice can induce multiorgan inflammation and splenomegaly. TIPE2 regulates immune homeostasis mainly through negatively regulating signaling by T cell receptors and Toll-like receptors (TLRs). Recently data suggested TIPE2 was highly expressed in microglia/macrophages in the ischemic brain and contributed to pathogenesis of stroke [123]. Blocking TIPE2 can increase the infarct size, neurological dysfunction, inflammatory cytokine expression and infiltration of inflammatory cells after MCAO in mice [123]. Targeting TIPE2 may be a new therapeutic strategy in the future.
IGF-1
The insulin-like growth factor 1 (IGF-1) is a multifunctional hormone similar in molecular structure to insulin. Most circulating IGF-1 is in a complex consisting insulin-like binding protein 3, IGF-1, and an acid-labile subunit, which transports IGF-1 in the circulation and prolongs its half-life [124]. IGF-1, among its many functions, is also closely related to neuronal maintenance. In animal models, IGF-1 has neuroprotective effect after stroke and can reduce the infarct volume, increase cell survival, and improve functional outcome [125, 126, 127]. IGF-1 is available for clinical use for treatment of growth disorders. However, there is still few of clinical trails in its neuroprotective roles in stroke patients. A small study in elderly stroke patients showed an inverse relation between circulating IGF-1 levels and outcome [128]. Another study suggested that high serum IGF-I levels just after ischemic stroke onset are associated with neurological recovery and a better functional outcome [129]. More clinical trails are needed and enhancing IGF-I levels may be an interesting therapeutic target for stroke in future.
4. Activation of inflammation-related signaling pathways in ischemic stroke
4.1. TLRs pathway
TLRs are transmembrane proteins expressed by a variety of immune cells and brain resident cells, such as B cells, dendritic cells, microglia, cerebral endothelium, astrocytes, oligodendrocytes, and neurons [130, 131, 132, 133]. They are divided into 14 types according to their different roles in recognizing pathogen-associated molecular patterns, such as those found in the bacterial cell wall components peptidoglycan (TLR2), lipopolysac-charide (LPS) receptor (TLR4) and nonmethylated cytosine-guanine (CpG) DNA (TLR9) [134]. TLRs are critical components in the innate immune system. Activation of TLRs signaling can induce inflammatory responses by regulating cytokine and chemokine production.
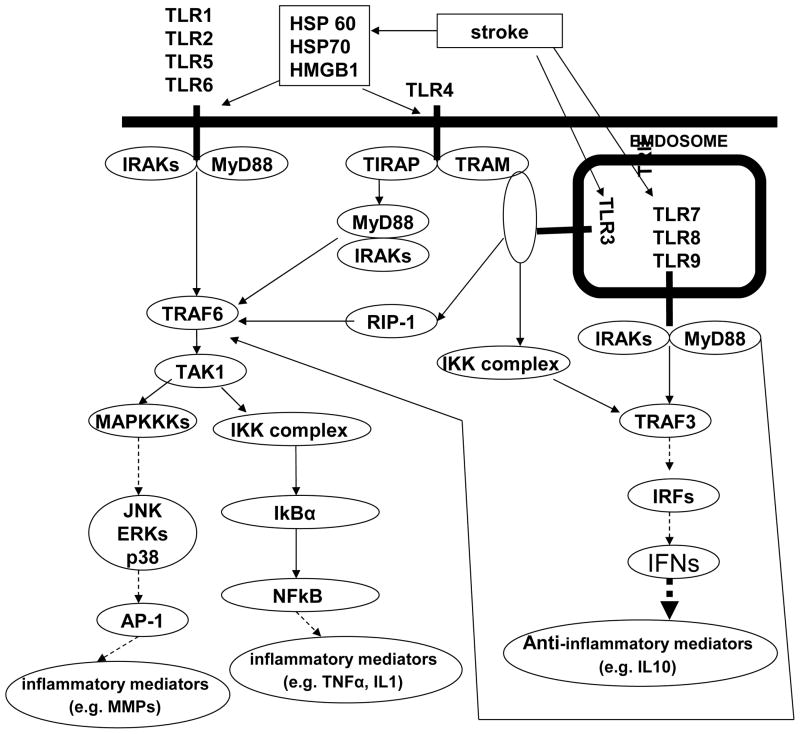
TLRs signaling include two manners, named a MyD88-dependent and a MyD88-independent manner [135, 136]. All TLRs, except TLR3, initiate the signaling in a MyD88- dependent manner. When enlisted to plasma membrane-associated TLRs, either directly (e.g. TLR2) or by the Toll/IL-1 receptor (TIR) domain-containing adaptor protein (TIRAP) (e.g. TLR4), MyD88 members of the IL-1 receptor-associated kinases (IRAKs) family (e.g. IRAK1 and IRAK4) begin a process of auto- and cross-phosphorylation. After phosphorylated, IRAKs disconnect from MyD88 and bind to TNF receptor associated factor 6 (TRAF6). TRAF6 in turn activates activated kinase 1 (TAK1) which can activate the IκB kinase (IKK) complex and mitogen-activated protein kinase kinase kinase (MAPKKKs). Activation of IKK complex leads to the degradation of IkBα and the subsequent nuclear translocation of the transcription factor NF-kB and finally induces inflammatory mediators (e.g. TNF-α, IL-1β). Activation of MAPKKs increases the transcription factor activator protein 1 (AP-1) and induces other inflammatory mediators (e.g. MMPs). When enlisted to endosomal TLRs, MyD88 is recruited almost as it does in the plasma membrane-associated TLRs. Due to the endosomal location of the complex, phosphorylated IRAKs can also bind TRAF3 in addition to TRAF6. Activation of TRAF3 induces phosphorylation, dimerization, and nuclear localization of the transcription factors [e.g. Interferon regulatory factor 3 (IRF3), IRF7 and interferon] [Figure 3].
Figure 3. TLRs pathway following ischemic stroke.
TLRs pathway could be activated by stroke and induce a serious of pro-inflammatory and anti-inflammatory responses.
TLR3 is unique among the TLRs, because its signal is in a MyD88-independent manner, which signals via recruitment of the TIR domain-containing adaptor inducing interferon (TRIF). TRIF enlists the IKKs complex and Receptor interacting protein-1 (RIP-1), which activates TRAF3 and TRAF6. Then activation of TRAF3 and TRAF6 induce the activation of MAPK, IKKs and IRFs [figure 2]. Of all the TLRs, only TLR4 can recruit in both a MyD88-dependent (via TIRAP) and a MyD88-independent manner (via TRAM) [Figure 3].
TLRs and cerebral ischemia
TLRs are closely implicated in cerebral ischemia. Mice lacking either functional TLR2 or TLR4 were less susceptible to brain damage due to stroke and also with smaller infarcts than wild type controls [137, 138, 139]. Furthermore, TLR4−/− mice could decrease the damage due to global cerebral ischemia and permanent focal ischemia [140, 141]. TLR endogenous ligands (e.g. HSP 60, HSP70 and HMGB1) were detected in injury brain [142, 143]. These molecules could activate TLRs (e.g. TLR2 and TLR4) in brain and induce inflammatory mediators (e.g. TNF-α, IL-1 and IL-6) which contribute to stroke pathology.
In contrast to the detrimental role of TLRs, stimulation of TLRs prior to brain ischemia could be neuroprotective. Pretreatment with TLR4 ligands (e.g. LPS) leads cells to switch their transcriptional response to TLR4 stimulation by enhancing IFN expression and suppressing the NF-kB-induced TNF-α expression. Inhibition of NF-kB would protect the brain since mice lacking the p50 subunit of NF-kB decrease brain damage compared to the wild type mice [144]. Increasing of IRF signaling would also protect the brain, as IFN could downregulate the IRF3 induction and act as an acute neuroprotectant [145, 146]. This pretreatment can induce a finely controlled shift in the balance of pro-inflammatory and anti-inflammatory cytokines.
4.2. Mitogen-activated protein kinases (MAPKs) pathway
MAPKs can transduce cellular stress and mediate various intracellular activities via a three-tiered kinase cascade (MAPKKK, MAPKK, MAPKs) [147, 148, 149]. The MAPK family consists of four major members, namely extracellular signal–regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinases (JNK), p38 MAPK, and extracellular signal-regulated kinase 5 (ERK5) [150].
ERK1/2 regulates a board range of activity from metabolism, motility, inflammatory responses to cell death and survival. ERK1/2 could be stimulated by both mitogens (e.g. growth factors) and various stressors (e.g. cytokines, microtubule disorganization and osmotic stress) [151]. On stress, the three-tiered Raf/MEK/ERK cascade could be upregulated by cell surface receptors (e.g. Ras and receptor tyrosine kinases) [152, 153]. Details are as follows: Ras activates Raf, that can phosphorylate MEK1/MEK2, which in turn activate ERK1/2. The phospho-ERK1/2 could activate transcription factors Elk-1, that could transcription of some immediately genes such as c-fos, which could exaggerate inflammation by upregulating inflammatory mediators (e.g. IL-1β) [154] [Figure 4].
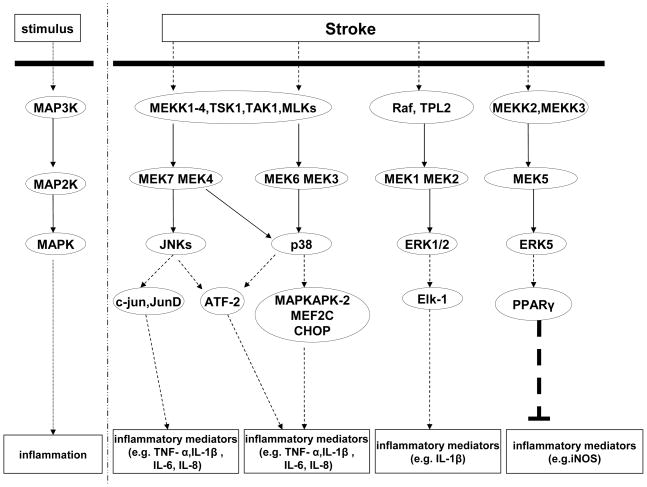
Figure 4. MAPK pathways following ischemic stroke.
MAPK pathway could be activated by stroke and induce a serious of pro-inflammatory and anti-inflammatory responses.
p38 MAPK acts as a nexus for signal transduction and is closely implicated in numerous biological processes. The p38 can be activated by various stressors and mitogens as well. But p38’s three-tiered MEKK1-4/MEK/JNK cascade is different from ERK1/2. Details are as follows: stressors or mitogens activate MEKK1-4 which can phosphorylate MEK3, MEK4 and MEK6, and in turn activate p38. Then, p38 activate meditators (e.g. MAPKAPK-2, CHOP, MEF2C and ATF2) which regulates expression of inflammatory meditators, such as TNF- α, IL-1β, IL-6, and IL-8 [155] [Figure 4].
JNK is activated in a manner almost like p38. The difference is that MEK4 and MEK7 are involved but not MEK3 and MEK6 during the three-tiered cascade [Figure 4].
ERK5, a most recently identified member of MAPK family, consists with a relatively large carboxy-terminal of unique structure that makes it different. The ERK5 signaling is related to inflammation. On stress, the three-tiered MEKK2/3/MEK/ERK cascade could be upregulated. Activation of MEKK2/3 activates MEK5, which in turn activates ERK5. Activation of ERK5 can increase peroxisome proliferator-activated receptor δ (PPARγ) transcriptional activity and then inhibit TNF-α-mediated NF-κB activation and the pro-inflammatory meditators (e.g. iNOS) [156] [Figure 4].
MAPK and cerebral ischemia
All the four MAPK pathways are activated in cerebral ischemia, but their roles are complicated and not yet adequately understood. Activation of JNK and p38 seem to be detrimental since injury due to stroke could be decreased after using their inhibitors [157, 158, 159]. ERK5 activation appears to be beneficial while ERK1/2 activation could be both beneficial and detrimental [160].
JNK pathway can lead to the production and activation of pro-inflammatory meditators (e.g. cytokines) in several inflammatory cells [161, 162]. Inhibition of JNK pathway with JNK inhibitor could decrease ischemic injury via reducing neuroinflammation [163]. p38 pathway is almost similarly with JNK pathway. It is linked to production and activation of pro-inflammatory meditators as well. Administration of SB 239063, a p38 pathway inhibitor, could reduce p38 activity following stroke and also downregulate the stroke-induced cytokines (e.g. TNF- α and IL-1β) which contribute to stroke-induced brain injury [164]. Activation of ERK1/2 in cerebral ischemic is associated with ischemic brain injury. Inhibition of ERK1/2 with a specific MEK1/2 inhibitor produced a neuroprotection by suppression of IL-1β expression [165]. Administration of inhibitors of the MEK/ERK1/2 pathway could reduce ischemic brain injury and improve neurological outcome [166, 167, 168]. On the other hand, Activation of ERK1/2 might also block apoptosis by upregulating expression of the anti-apoptotic protein Bcl-2 or by downregulating of the pro-apoptotic protein Bad [169]. Furthermore, ERK1/2 could reduce cerebral hypoxic-ischemic injury and survive neurons by activation of neurotrophins (e.g. brain derived neurotrophic factor) [170]. ERK5 pathway was a recently identified member of MAPK family. Its effects and mechanism on inflammation in stroke are still largely unknown. But data showed that ERK5 activation may act in neuroprotection of ischemic preconditioning [171].
4.3. Nuclear factor-kappa B (NF-κB) pathway
The mammalian NF-κB family comprises five members: p65 (RelA), RelB, c-Rel, p50/p105 (NFκB1), and p52/p100 (NF-κB2), which can form homodimers and heterodimers [172]. The activation of NF-κB is required for the transcriptional induction of many proinflammatory genes, such as cellular adhesion molecules, cytokines, MMPs, and growth factors [172]. NF-κB dimers are normally retained in the cytosol bound to a family of inhibitory proteins known as the inhibitor of κB (IκB). Activation of NF-κB signaling is initiated by extracellular stimuli. These stimuli are recognized by receptors and transmitted into the cell which leads to the activation of the IKK (IκB kinase). Phosphorylation of IκBs results in their proteasomal degradation and the release of NF-κB for nuclear translocation and activation of gene transcription [173].
NF-κB and cerebral ischemia
NF-κB, a key regulator of a variety of genes involved in cell survival and inflammation, is activated after cerebral ischemia in neurons, astrocytes, microglia, and infiltrating inflammatory cells [172]. Among the 5 NF-κB subunits, p65/RelA and p50 are known to be responsible for a detrimental effect in cerebral ischemia [172]. Previous studies showed that expression of p65 and p50, and DNA binding activity were increased in the brain after cerebral ischemia. Increased DNA binding reflects activation of NF-κB. NF-κB subunit p50 knockout mice have a smaller infarct in both transient and permanent stroke models [174]. Similar observations were made by inhibiting activation of NF-κB with the treatment of S-nitrosoglutathione [175]. However, NF-κB activation is also implicated in neuroprotective mechanisms of ischemic brain injury. For example, one study showed that rats treated with diethyldithiocarbamate, a NF-κB inhibitor, had enhanced neuronal DNA fragmentation and larger infarct sizes compared to controls, suggesting a beneficial role [176].
5. Systemic inflammation, atherosclerosis, and acute ischemic stroke
There is growing evidence that inflammatory events outside the brain have an important impact on stroke susceptibility and outcome. Recent clinical and pre-clinical studies suggest that the systemic inflammatory status prior to and at the time of stroke is a key determinant of acute outcome and long-term prognosis. Several clinical studies have reported more severe neurological deficits in stroke patients presenting with preceding infection [177, 178]. A number of studies using experimental models of systemic inflammation in rodents, in combination with cerebral ischemia have reported findings similar to those in stroke patients. Systemic challenge with the bacterial endotoxin, lipopolysaccharide (LPS), which mimics aspects of gram-negative bacterial infection, markedly exacerbates the extent of ischemic brain damage and the severity of neurological deficit after focal cerebral ischemia in mice [179]. The majority of stroke patients present with co-morbid disease, such as atherosclerosis, obesity, diabetes, hypertension and peripheral infection, all of which are risk factors for stroke [7]. A common theme among these conditions is their association with an elevated systemic inflammatory profile and increasing evidence implicates inflammation as a causative factor in the development and/or progression of these diseases [180, 181] Indeed, poorer outcomes have been reported in diabetic mice and spontaneously hypertensive rats after experimental stroke [182, 183]. Here, we discuss the link between atherosclerosis and ischemic stroke as an example of chronic inflammatory conditions that modulate stroke pathology.
Thromboembolism resulting from the rupture of atherosclerotic plaques is the most common etiological factor in stroke [184]. Atherosclerosis is now considered an inflammatory disorder of the vessel wall in which molecular and cellular inflammatory mediators initiate and drive the progression of plaques through various stages from a relatively benign to highly unstable state prone to rupture which triggers thrombotic and/or embolic events [181]. Endothelial dysfunction and activation are critical events for the initiation of lesion development and result in the expression of adhesion molecules, such as VCAM-1 and ICAM-1 that promote recruitment of immune cells, in particular monocyte-derived macrophages and T lymphocytes. Activated macrophages and T cells, which are abundant in the shoulder region of the plaques, secrete proteolytic enzymes, such as matrix metalloproteinases (MMPs), which destabilize the plaque and ultimately lead to its rupture, a common cause of thromboembolic occlusion of cerebral vessels in ischemic stroke.
Another important mechanism by which systemic inflammation contributes to the pathogenesis of ischemic stroke may be involved in cerebral microvascular dysfunction. It is well known that the structure and function of cerebral microvasculature can be profoundly altered after ischemic stroke [185]. The diverse responses of the microvasculature to stroke include enhanced oxidative stress, activation of ischemic brain endothelial cells, platelet-leukocyte-endothelial cell interaction in the cerebral microvasculature, and thrombus formation in cerebral blood vessels, leading to disruption of the blood-brain barrier and hemorrhagic transformation. These cerebral microvascular responses could be worsened due to pre-existing infection or systemic inflammation. It has been shown that systemic inflammation caused an alteration in the kinetics of the BBB disruption through conversion of a transient to a sustained disruption of the tight junction protein, claudin-5, and also markedly exacerbated disruption of the cerebrovascular basal lamina protein, collagen-IV after experimental stroke in mice [179].
6. Anti-inflammatory therapeutic approaches for ischemic stroke
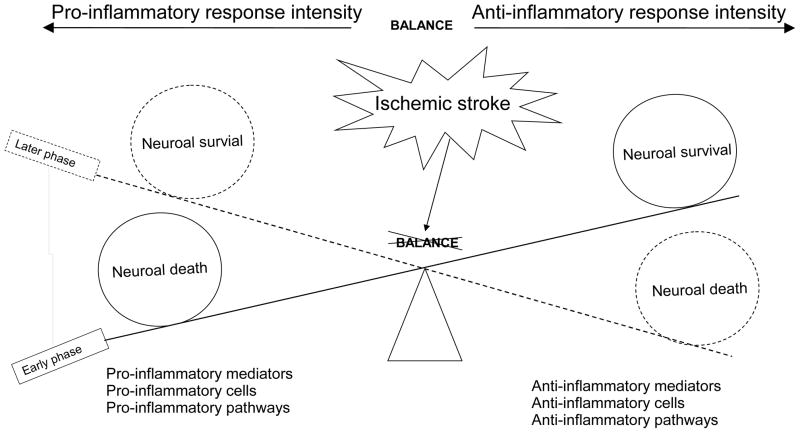
The pathologic processes after ischemic stroke can be separated into acute (minutes to hours), subacute (hours to days), and chronic (days to months) phases. In the acute phase, ROS and proinflammatory cytokines and chemokines are produced rapidly from injured brain tissue and released into extracellular compartment [8]. These mediators activate brain endothelial cells and induce the expression of adhesion molecules on cerebral endothelium, which promote the adhesion and transendothelial migration of circulating leukocytes across the BBB into brain parenchyma [8] [Figure. 1]. In the subacute phase, infiltrating leukocytes release a variety of proinflammatory mediators including cytokines and chemokines, ROS, MMPs and other proteases, which further amplify the brain inflammatory responses and cause more extensive activation of brain resident cells and infiltration of blood leukocytes, eventually exaggerating BBB disruption, brain edema, neuronal death, and hemorrhagic transformation [186, 187]. However, post-ischemic inflammation is also thought to promote tissue repair and functional recovery in the chronic phase after stroke [186]. Therefore, it is important to understand the dynamic balance between the neurotoxic and neuroprotective effects of post-ischemic inflammation in different stages of ischemic stroke [Figure 5].
Figure 5. the roles of pro-inflammatory and anti-inflammatory responses in ischemic stroke.
The balance between pro-inflammatory and anti-inflammatory responses acts key roles in injury following ischemic stroke.
Pre-clinical studies have demonstrated that inhibition of brain leukocyte infiltration using agents directly blocking adhesion molecules (e.g., CD11b/CD18, ICAM-1, P-selectin) and neutrophil inhibitory factor inhibition [Table 2] reduces infarct size, edema, and neurological deficits in transient MCAO models, even administrated up to 12–24h after ischemia, but the benefits do not extend to permanent stroke models [188,189,190]. Additionally, development of an anti-inflammatory milieu, and generation of pro-survival factors fostering tissue reconstruction and repair may be another useful therapeutic strategy. Recently, animal studies have demonstrated that TGF-β and IL-10 are key cerebroprotective immunomodulators in acute experimental stroke [109, 191]. TGF-β, which is upregulated after brain ischemia, has neuroprotective properties by inhibiting Th1 and Th2 responses and promoting regulatory T cells (Treg cells) development [9, 192]. Treg cells are key cerebroprotective modulators in acute cerebral ischemia by secreting IL-10 and suppressing the neurotoxic function of TNF-α and IFN-γ [193]. Considering that the benefits of anti-inflammatory therapy are often associated with reperfusion, anti-inflammation approach would be a fitting complement to reperfusion therapy using thrombolytics for stroke patients.
Table 2.
Selected pre-clinical studies targeting inflammatory mediators in acute ischemic stroke.
| Agent | Administration | Ischemic model | Outcome | Ref. |
|---|---|---|---|---|
| rhIL-1Ra | i.c.v. injection of rhIL-1Ra at 30 min before surgery and 40 min after reperfusion | Mouse tMCAO | Reduced infarct volume | 57 |
| rhIL-6 | i.c.v. injection of rhIL-6 at 30 min before surgery and 15 min after pMCAO | Rat pMCAO | Reduced infarct volume | 64 |
| Nonspecific selectin antagonist | i.c.v. injection of antagonist at 10 min before reperfusion and 1 h after reperfusion | Rat tMCAO | Reduced infarct volume | 188 |
| Anti-ICAM-1 antibodies | i.v. injection of anti-ICAM-1 antibodies at 1 and 22h after reperfusion i.v. injection of anti-ICAM-1 antibodies at 2 h after pMCAO |
Rat tMCAO Rat pMCAO |
Reduced infarct volume No significant effects |
189 |
| Anti-MAC-1 antibodies | i.v. injection of anti-MAC-1 antibodies at 1 and 22h after reperfusion | Rat tMCAO | Reduced infarct volume | 190 |
| IL-10 | i.c.v. injection of IL-10 at 30 min and 3 h following pMCAO./Iv at 30 min after pMCAO and continued for 3 h | Rat pMCAO | Reduced infarct volume | 111 |
| TGF-1 | Intranasal delivery of TGF-1 at 2 and 24 h after reperfusion. | Mouse tMCAO | Reduced infarct volume | 191 |
| IGF-1 | Intranasal delivery of IGF-1, the first dose given at 2, 4 or 6h respectively; the second and third dose given at 24 and 48h after reperfusion. | Rat tMCAO | First dose given at 2 and 4h, but not at 6h reduced infarct volume | 125 |
Clinically, several drugs targeting leukocyte recruitment have been investigated for the treatement of ischemic stroke [194, 195, 196], such as antibody to ICAM-1 (Enlimomab, R6.5), antibody to the CD11b/CD18 (Hu23F2G or LeukArrest), and recombinant neutrophil inhibitory factor [Table 3]. However, all of these clinical trials have failed, which raise the question of why anti-inflammatory therapy succeeded in animal models but not in clinical application. There are several possible explanations for of clinical trial failure: i) The unhumanized antibodies (e.g. mouse anti-human ICAM-1) used in clinical trials. ii) The heterogeneity and complexity of human stroke compared with animal models. Human stroke is a heterogeneous condition made up of three pathological types: ischemic stroke, cerebral haemorrhage and subarachnoid haemorrhage. Ischemic stroke is then further divided into several subtypes, such as intracranial small vessel disease, large-vessel atherosclerotic disease, and embolism from the heart. These types and subtypes differ in terms of cause, outcome and treatment. Different types of ischemic stroke also have distinct inflammatory features. In addition, the composition of emboli and the location (arterial or venular) of occlusion may alter stroke pathophysiology [199]. Thus, different therapeutic strategies should be considered for different types of stoke in patients. iii) Limitation of animal stroke models. Heterogeneous nature of human stroke is not well reproduced in animal models [200]. At present, young and healthy animals were mostly used in pre-clinical stroke research. In order to model the human stroke more closely, animals in both sex, aging, and with stroke-related comorbidities, such as diabetes mellitus, atherosclerosis, hyperlipidemia, hypertension or obesity should be used in pre-clinical studies [201]. iv) Limitation of outcome measures. At present, most of experimental stroke studies only report short-term outcome measures. However, the most important outcome parameters of any intervention in human stroke are long-term (3-month) survival and functional recovery [201]. For translation into clinical application, long-term survival and behavioral and functional analysis should be performed in experimental stroke studies. v) Post-ischemic inflammation may act through multiple redundant pathways, this may be another possible reason why blocking a single cytokine or leukocyte adhesion molecule failed in clinical trials. In addition, even the same molecule produced by different cells (e.g., microglia- and leukocyte-derived TNF-α) may play different roles in stroke pathology [202, 203]. Thus, identifying and blocking a common molecular signal shared by different inflammatory cells and mediators would be a more effective approach to stroke treatment.
Table 3.
Selected clinical studies targeting inflammatory mediators in acute ischemic stroke.
| Intervention | Mode of action | Results | Ref. |
|---|---|---|---|
| rhIL-1Ra | Interleukin-1 receptor antagonist | unsuccessful | 194 |
| Enlimomab | Anti-ICAM-1 monoclonal antibody | unsuccessful | 88 |
| UK-279,276 | Neutrophil inhibitory factor | unsuccessful | 195 |
| Hu23F2G (LeukArrest) | Anti-CD11/CD18 monoclonal antibody | unsuccessful | 196 |
| Minocycline | Broad-spectrum tetracycline antibiotic | Phase I, II (completed) NCT00630396 | 197 |
| ONO-2506 | Glial modulator | Phase II, III(completed) NCT00229177 | 198 |
Conclusions
Inflammation plays an important role in the pathogenesis of ischemic stroke and other forms of ischemic brain injury. Although several approaches for anti-inflammatory treatment have proven effective for treating acute stroke in animal models, none of these treatments has proven effective in clinical trials. Increasing evidence suggests that inflammatory response is a double-edged sword, as it not only exacerbates secondary brain injury in the acute stage of stroke but also beneficially contributes to brain recovery after stroke [Figure 5]. Undoubtedly, there is still much to be done in order to translate promising pre-clinical findings into clinical practice. For example, how to clearly define the early or later phases of experimental ischemic stroke under clinically relevant conditions; how to clearly define the dynamic balance between pro- and anti-inflammatory pathways activated in different stages of ischemic stroke; when and how to activate or inhibit the pro-inflammatory or anti-inflammatory pathways as therapies; and which pro-inflammatory or anti-inflammatory mediators to be targeted. Future studies should consider the pro- and anti-inflammatory responses as a whole, but not to evaluate them separately. A better understanding of the dynamic balance between pro- and anti-inflammatory responses and identifying the discrepancies between pre-clinical studies and clinical trials may serve as a basis for designing effective therapies.
Acknowledgments
The work was supported by the National Institutes of Health Grant HL087990 (Dr. Li) and by American Heart Association grant 0530166N (Dr. Li).
Footnotes
Conflict of Interest
None
References
- 1.Wang X. Investigational anti-inflammatory agents for the treatment of ischemic brain injury. Expert Opin Investig Drugs. 2005;14:393–409. doi: 10.1517/13543784.14.4.393. [DOI] [PubMed] [Google Scholar]
- 2.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samson Y, Lapergue B, Hosseini H. Inflammation and ischaemic stroke: current status and future perspectives. Rev Neurol (Paris) 2005;161:1177–1182. doi: 10.1016/s0035-3787(05)85190-2. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 7.McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neurosci. 2009;158:1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–89. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl. 1996;66:27–31. doi: 10.1007/978-3-7091-9465-2_5. [DOI] [PubMed] [Google Scholar]
- 11.Chopp M, Li Y, Jiang N, Zhang RL, Prostak J. Antibodies against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J Cereb Blood Flow Metab. 1996;16:578–584. doi: 10.1097/00004647-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, Naka Y, Solomon RA, Pinsky DJ. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81:304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- 13.Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, Mennini T, Ghezzi P, Villa P. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–131. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, Whitaker S, Steinberg GK. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153:223–233. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 16.Barone FC, Arvin B, White RF, Miller A, Webb CL, Lysko PG, Feuerstein GZ. Tumor necrosis factor-α: a mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest. 1997;100:2648–2652. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felger JC, Abe T, Kaunzner UW, Gottfried-Blackmore A, Gal-Toth J, McEwen BS, Iadecola C, Bulloch K. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24:724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neurosci. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 20.Konsman JP, Drukarch B, Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond) 2007;112:1–25. doi: 10.1042/CS20060043. [DOI] [PubMed] [Google Scholar]
- 21.Shigematsu T, Wolf RE, Granger DN. T-lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia-reperfusion. Microcirculation. 2002;9:99–109. doi: 10.1038/sj/mn/7800126. [DOI] [PubMed] [Google Scholar]
- 22.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 24.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 25.Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res. 1992;17:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- 26.Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol. 2006;84:49–59. doi: 10.1139/Y05-143. [DOI] [PubMed] [Google Scholar]
- 28.Denes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppi-nen RA, Allan SM. Proliferating resident microglia after fo-cal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- 29.George B, Robin E, White YO, Lijun X, Rona G Giffard. Astrocytes: Targets for Neuroprotection in Stroke. Cent Nerv Syst Agents Med Chem. 2011;11:164–173. doi: 10.2174/187152411796011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowicka D, Rogozinska K, Aleksy M, Witte OW, Skangiel-Kramska J. Spatiotemporal dynamics of astroglial and microglial responses after photothrombotic stroke in the rat brain. Acta Neurobiol Exp (Wars) 2008;68:155–168. doi: 10.55782/ane-2008-1685. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Roth-Eichhorn S, Braun N, Culmsee C, Rami A, Krieglstein J. The expression of transforming growth factor-beta1 (TGF-beta1) in hippocampal neurons: a temporary upregulated protein level after transient forebrain ischemia in the rat. Brain Res. 2000;866:286–298. doi: 10.1016/s0006-8993(00)02240-x. [DOI] [PubMed] [Google Scholar]
- 32.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 33.Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902:171–177. doi: 10.1016/s0006-8993(01)02328-9. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Redecker C, Yu ZY, Xie MJ, Tian DS, Zhang L, Bu BT, Witte OW. Rat focal cerebral ischemia induced astrocyte proliferation and delayed neuronal death are attenuated by cyclin-dependent kinase inhibition. J Clin Neurosci. 2008;15:278–285. doi: 10.1016/j.jocn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Sharif A, Legendre P, Prevot V, Allet C, Romao L, Studler JM, Chneiweiss H, Junier MP. Transforming growth factor alpha promotes sequential conversion of mature astrocytes into neural progenitors and stem cells. Oncogene. 2007;26:2695–2706. doi: 10.1038/sj.onc.1210071. [DOI] [PubMed] [Google Scholar]
- 37.Justicia C, Perez-Asensio FJ, Burguete MC, Salom JB, Planas AM. Administration of transforming growth factor-alpha reduces infarct volume after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2001;21:1097–1104. doi: 10.1097/00004647-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC. Tumor necrosis factor-α expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF- α and IL-1 α mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- 40.Murakami Y, Saito K, Hara A, Zhu Y, Sudo K, Niwa M, Fujii H, Wada H, Ishiguro H, Mori H, Seishima M. Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J Neurochem. 2005;93:1616–1622. doi: 10.1111/j.1471-4159.2005.03163.x. [DOI] [PubMed] [Google Scholar]
- 41.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 42.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 43.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 44.Meistrell ME, 3rd, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, Vishnubhakat JM, Ghezzi P, Tracey KJ. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–348. [PubMed] [Google Scholar]
- 45.Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1996;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22:142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Alikhani M, Alikhani Z, Raptis M, Graves DTJ. TNF-alpha In vivo stimulates apoptosis in fibroblasts through caspase-8 activation and modulates the expression of pro-apoptotic genes. Cell Physiol. 2004;201:341–348. doi: 10.1002/jcp.20067. [DOI] [PubMed] [Google Scholar]
- 49.Plumpe J, Malek NK, Bock CT, Rakemann T, Manns MP, Trautwein C. NF-kappaB determines between apoptosis and proliferation in hepatocytes during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2000;278:173–183. doi: 10.1152/ajpgi.2000.278.1.G173. [DOI] [PubMed] [Google Scholar]
- 50.Zeng L, Liu J, Wang Y, Wang L, Weng S, Chen S, Yang GY. Cocktail Blood Biomarkers: Prediction of Clinical Outcomes in Patients with Acute Ischemic Stroke. Eur Neurol. 2012;14, 69(2):68–75. doi: 10.1159/000342896. [DOI] [PubMed] [Google Scholar]
- 51.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 52.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;15:2095–2147. [PubMed] [Google Scholar]
- 53.Black RA, Kronheim SR, Sleath PR. Activation of interleukin-1β by a co-induced protease. FEBS Lett. 1989;247:386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- 54.Schonbeck V, Mach F, Libby P. Generation of biologically active IL-1β by matrix metalloproteinase a novel caspase-1 independent pathway of IL-1β processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- 55.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 56.Bankers-Fulbright JL, Kalli KR, McLean DJ. IL-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 57.Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 59.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 60.Koga S, Ogawa S, Kuwabara K, Brett J, Leavy JA, Ryan J. Synthesis and release of interleukin 1 by reoxygenated human mononuclear phagocytes. J Clin Invest. 1992;90:1007–1015. doi: 10.1172/JCI115913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, Levison SW. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- 62.Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- 63.Waje-Andreassen U, Kråkenes J, Ulvestad E, Thomassen L, Myhr KM, Aarseth J, Vedeler CA. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 64.Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 66.Connolly ESJ, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–16. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N acetyllactosamine. J Biol Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- 68.Stenberg PE, Shuman MA, Levine SP, Bainton DF. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984;98:748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P-selectin deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 71.Huang J, Kim LJ, Mealey R, Marsh HC, Zhang Y, Tenner AJ, Connolly ES, Pinsky DJ. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 72.Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31:3047–3053. [PubMed] [Google Scholar]
- 73.Mocco J, Choudhri T, Huang J, Harfeldt E, Efros L, Klingbeil C, Vexler V, Hall W, Zhang Y, Mack W, Popilskis S, Pinsky DJ, Connolly ES. HuEP5C7 as a humanized monoclonal anti-E/P-selectin neurovascular protective strategy in a blinded placebo-controlled trial of nonhuman primate stroke. Circ Res. 2002;91:907–914. doi: 10.1161/01.res.0000042063.15901.20. [DOI] [PubMed] [Google Scholar]
- 74.Lehmberg J, Beck J, Baethmann A, Uhl E. Effect of P-selectin inhibition on leukocyte endothelium interaction and survival after global cerebral ischemia. J Neurol. 2006;253:357–363. doi: 10.1007/s00415-005-0996-4. [DOI] [PubMed] [Google Scholar]
- 75.Cha JK, Jeong MH, Kim EK, Lim YJ, Ha BR, Kim SH. Surface expression of P-selectin on platelets is related with clinical worsening in acute ischemic stroke. J Korean med Sci. 2002;17:811–816. doi: 10.3346/jkms.2002.17.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao DX, Feng J, Cong SY, Zhang W. Association of E-selectin gene polymorphisms with ischemic stroke in a Chinese Han population. J Neurosci Res. 2012;90:1782–1787. doi: 10.1002/jnr.23075. [DOI] [PubMed] [Google Scholar]
- 77.Kaba NK, Schultz J, Law FY, Lefort CT, Martel-Gallegos G, Kim M, Waugh RE, Arreola J, Knauf PA. Inhibition of Na+/H+ exchanger enhances low pH-induced L-selectin shedding and beta2-integrin surface expression in human neutrophils. Am J Physiol Cell Physiol. 2008;295:C1454–1463. doi: 10.1152/ajpcell.00535.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 79.Gahmberg CGM, Tolvanen P, Kotovuori Leukocyte adhesion: structure and function of human leukocyte β2-integrins and their cellular ligands. Eur J Biochem. 1997;245:215–232. doi: 10.1111/j.1432-1033.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 80.Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J Clin Invest. 1994;94:1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang RL, Chopp M, Chen H, Garcia JH. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci. 1994;125:3–10. doi: 10.1016/0022-510x(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 82.Kanemoto Y, Nakase H, Akita N, Sakaki T. Effects of anti-intercellular adhesion molecule-1 antibody on reperfusion injury induced by late reperfusion in the rat middle cerebral artery occlusion model. Neurosurgery. 2002;51:1034–1041. doi: 10.1097/00006123-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 83.Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M, Yanagihara T. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18:1336–1345. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Khan M, Jatana M, Elango C, Singh PA, Singh AK, Singh I. Cerebrovascular protection by various nitric oxide donors in rats after experimental stroke. Nitric Oxide. 2006;15:114–124. doi: 10.1016/j.niox.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Vemuganti R, Dempsey RJ, Bowen KK. Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke. 2004;35:179–184. doi: 10.1161/01.STR.0000106479.53235.3E. [DOI] [PubMed] [Google Scholar]
- 86.Shyu KG, Chang H, Lin CC. Serum levels of intercellular adhesion molecule-1 and E-selectin in patients with acute ischaemic stroke. J Neurol. 1997;244:90–93. doi: 10.1007/s004150050055. [DOI] [PubMed] [Google Scholar]
- 87.Lindsberg PJ, Carpen O, Paetau A, Karjalainen-Lindsberg ML, Kaste M. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. 1996;94:939–945. doi: 10.1161/01.cir.94.5.939. [DOI] [PubMed] [Google Scholar]
- 88.Enlimomab AST. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 89.Vuorte J, Lindsberg PJ, Kaste M, Meri S, Jansson SE, Rothlein R, Repo H. Anti-ICAM-1 monoclonal antibody R6.5 (Enlimomab) promotes activation of neutrophils in whole blood. J Immunol. 1999;162:2353–2357. [PubMed] [Google Scholar]
- 90.Zhang LH, Wei EQ. Neuroprotective effect of ONO-1078, a leukotriene receptor antagonist, on transient global cerebral ischemia in rats. Acta Pharmacol Sin. 2003;24:1241–1247. [PubMed] [Google Scholar]
- 91.Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, Chamorro A, Planas AM. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab. 2006;26:421–432. doi: 10.1038/sj.jcbfm.9600198. [DOI] [PubMed] [Google Scholar]
- 92.Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 94.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garau A, Bertini R, Colotta F, Casilli F, Bigini P, Cagnotto A, Mennini T, Ghezzi P, Villa P. Neuroprotection with the CXCL8 inhibitor repertaxin in transient brain ischemia. Cytokine. 2005;30:125–131. doi: 10.1016/j.cyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 96.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D, Pan Y. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 97.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van RN, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 98.Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 99.Wang L, Li Y, Chen J, Gautam SC, Zhang Z, Lu M, Chopp M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- 100.Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 102.Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 104.Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 105.Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32:1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 106.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jensen C, Ekholm S, Tarkowski A. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997;110:492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pelidou SH, Kostulas N, Matusevicius D, Kivisakk P, Kostulas V, Link H. High levels of IL-10 secreting cells are present in blood in cerebrovascular diseases. Eur J Neurol. 1999;6:437–442. doi: 10.1046/j.1468-1331.1999.640437.x. [DOI] [PubMed] [Google Scholar]
- 109.Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 110.Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E. Interleukin-10 modulates neuronal threshold of vulner-ability to ischaemic damage. Eur J Neurosci. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- 111.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 112.Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- 113.Kim JS, Yoon SS, Kim YH, Ryu JS. Serial measurement of inter-leukin-6, transforming growth factor-beta, and S 100 protein in patients with acute stroke. Stroke. 1996;27:1553–1557. doi: 10.1161/01.str.27.9.1553. [DOI] [PubMed] [Google Scholar]
- 114.van Exel E, Gussekloo J, de Craen AJ, Bootsma-van DWA, Frolich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33:1135–1138. doi: 10.1161/01.str.0000014206.05597.9e. [DOI] [PubMed] [Google Scholar]
- 115.Buckwalter M, Wyss-Coray T. Modelling neuroinflammatory phenotypes in vivo. J Neuroinflammation. 2004;1:10. doi: 10.1186/1742-2094-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klempt ND, Sirimanne E, Gunn AJ, Klempt M, Singh K, Williams C. Hypoxia-ischemia induces transforming growth factor beta 1 mRNA in the infant rat brain. Mol Brain Res. 1992;13:93–101. doi: 10.1016/0169-328x(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 117.Wienner C, Gehrmann J, Lindholm D, Topper R, Kreutzberg GW, Hossmann KA. Expression of transforming growth factor-beta1 and interleukin-1 beta mRNA in rat brain following transient fore-brain ischemia. Acta Neuropathol. 1993;86:439–46. doi: 10.1007/BF00228578. [DOI] [PubMed] [Google Scholar]
- 118.Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- 119.Ruocco A, Nicole O, Docagne F, Ali C, Chazalviel L, Komesli S, Yablonsky F, Roussel S, MacKenzie ET, Vivien D, Buisson A. A transforming growth factor-b antagonist unmasks the neuroprotective role of this endogenous cytokine in excitotoxic and ischemic brain injury. J Cereb Blood Flow Metab. 1999;19:1345–1353. doi: 10.1097/00004647-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 120.Johnston RE, Dillon-Carter O, Freed WJ, Borlongan CV. Trophic factor secreting kidney cell lines: In vitro characterization and functional effects following transplantation in ischemic rats. Brain Res. 2001;900:268–276. doi: 10.1016/s0006-8993(01)02327-7. [DOI] [PubMed] [Google Scholar]
- 121.Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen YH. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–426. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, Chen YH, Shi Y. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol. 2009;16:89–90. doi: 10.1038/nsmb.1522. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Wei X, Liu L, Liu S, Wang Z, Zhang B, Fan B, Yang F, Huang S, Jiang F, Chen YH, Yi F. TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem. 2012;287:32546–32555. doi: 10.1074/jbc.M112.348755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kooijman R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 2006;17:305–323. doi: 10.1016/j.cytogfr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 125.Liu XF, Fawcett JR, Hanson LR, Frey WH. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13:16–23. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 126.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 127.Kooijman R, Sarre S, Michotte Y, DeKeyser J. Insulin-like growth factor I: a potential neuroprotective compound for the treatment of acute ischemic stroke? Stroke. 2009;404:e83–88. doi: 10.1161/STROKEAHA.108.528356. [DOI] [PubMed] [Google Scholar]
- 128.Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117:312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 129.De SA, Brouns R, Uyttenboogaart M, De RS, Moens M, Wilczak N, Luijckx GJ, De KJ. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42:2180–2185. doi: 10.1161/STROKEAHA.110.600783. [DOI] [PubMed] [Google Scholar]
- 130.Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van NJM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 131.Bsibsi M, Ravid R, Gveric D, Van NJM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 132.Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 133.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 134.Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neurosci. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H503–H509. doi: 10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 136.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 137.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Comm. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 138.Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like recep-tor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Comm. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- 140.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 141.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kinouchi H, Sharp FR, Hill MP, Koistinaho J, Sagar SM, Chan PH. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993;13:105–115. doi: 10.1038/jcbfm.1993.13. [DOI] [PubMed] [Google Scholar]
- 143.Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007;103:590–603. doi: 10.1111/j.1471-4159.2007.04788.x. [DOI] [PubMed] [Google Scholar]
- 144.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 145.Liu H, Xin L, Chan BPL, Teoh R, Tang BL, Tan YH. Interferon beta administration confers a beneficial outcome in a rabbit model of thromboembolic cerebral ischemia. Neurosci Lett. 2002;327:146–148. doi: 10.1016/s0304-3940(02)00371-3. [DOI] [PubMed] [Google Scholar]
- 146.Veldhuis W, Derksen J, Floris S, Vander MP, de Vries H, Schepers J, Vos I, Dijkstra C, Kappelle L, Nicolay K, Bar P. Interferon-beta blocks infiltration of inflammatory cells and reduces infarct volume after ischemic stroke in the rat. J Cereb Blood Flow Metab. 2003;23:1029–1039. doi: 10.1097/01.WCB.0000080703.47016.B6. [DOI] [PubMed] [Google Scholar]
- 147.Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 148.Ferrell JE. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 149.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 150.Nithianandarajah-Jones GN, Wilm B, Goldring CE, Müller J, Cross MJ. ERK5: structure, regulation and function. Cell Signal. 2012;24:2187–2196. doi: 10.1016/j.cellsig.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 151.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 152.Sutton LN, Clark BJ, Norwood CR, Woodford EJ, Welsh FA. Global cerebral ischemia in piglets under conditions of mild and deep hypothermia. Stroke. 1991;22:1567–1573. doi: 10.1161/01.str.22.12.1567. [DOI] [PubMed] [Google Scholar]
- 153.Kamme F, Camp K, Wieloch T. Biphasic expression of the fos and jun families of transcription factors following transient forebrain ischaemia in the rat. Effect of hypothermia. Eur J Neurosci. 1995;7:2007–2016. doi: 10.1111/j.1460-9568.1995.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 154.Wang ZQ, Wu DC, Huang FP, Yang GY. Inhibition of MEK/ERK1/2 pathway reduces pro-inflammatory cytokine interleukin-1 expression in focal cerebral ischemia. Brain Res. 2004;996:55–66. doi: 10.1016/j.brainres.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 155.Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:6–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 156.Woo CH, Massett MP, Shishido T, Itoh S, Ding B, McClain C, Che W, Vulapalli SR, Yan C, Abe J. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor delta (PPARdelta) stimulation. J Biol Chem. 2006;281:32164–32174. doi: 10.1074/jbc.M602369200. [DOI] [PubMed] [Google Scholar]
- 157.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 158.Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 159.Guan QH, Pei DS, Zong YY, Xu TL, Zhang GY. Neuroprotection against ischemic brain injury by a small peptide inhibitor of c-Jun N-terminal kinase (JNK) via nuclear and non-nuclear pathways. Neurosci. 2006;139:609–627. doi: 10.1016/j.neuroscience.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 160.Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- 161.Benakis C, Bonny C, Hirt L. JNK inhibition and inflammation after cerebral ischemia. Brain Behav Immun. 2010;24:800–811. doi: 10.1016/j.bbi.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 162.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 163.Wang LW, Tu YF, Huang CC, Ho CJ. JNK signaling is the shared pathway linking neuroinflammation, blood-brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J Neuroinflammation. 2012;17:175. doi: 10.1186/1742-2094-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barone FC, Irving EA, Ray AM. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med Res Rev. 2001;21:129–145. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 165.Wang H, Xu L, Venkatachalam S, Trzaskos JM, Friedman SM, Feuerstein GZ, Wang X. Differential regulation of IL-1beta and TNF-alpha RNA expression by MEK1 inhibitor after focal cerebral ischemia in mice. Biochem Biophys Res Commun. 2001;286:869–874. doi: 10.1006/bbrc.2001.5482. [DOI] [PubMed] [Google Scholar]
- 166.Maddahi A, Edvinsson L. Enhanced expressions of microvascular smooth muscle receptors after focal cerebral ischemia occur via the MAPK MEK/ERK pathway. BMC Neurosci. 2008;9:85. doi: 10.1186/1471-2202-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang X, Wang H, Xu L, Rozanski DJ, Sugawara T, Chan PH, Trzaskos JM, Feuerstein GZ. Significant neuroprotection against ischemic brain injury by inhibition of the MEK1 protein kinase in mice: exploration of potential mechanism associated with apoptosis. J Pharmacol Exp Ther. 2003;304:172–178. doi: 10.1124/jpet.102.040246. [DOI] [PubMed] [Google Scholar]
- 169.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang RM, Zhang QG, Li J, Yang LC, Yang F, Brann DW. The ERK5-MEF2C transcription factor pathway contributes to anti-apoptotic effect of cerebral ischemia preconditioning in the hippocampal CA1 region of rats. Brain Re. 2009;1255:32–41. doi: 10.1016/j.brainres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 172.Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neurosci. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 173.Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–68. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 175.Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, Elango C, Singh AK, Singh I. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25:177–192. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- 176.Hill WD, Hess DC, Carroll JE, Wakade CG, Howard EF, Chen Q, Cheng C, Martin-Studdard A, Waller JL, Beswick RA. The NF-kappaB inhibitor diethyldithiocarbamate (DDTC) increases brain cell death in a transient middle cerebral artery occlusion model of ischemia. Brain Res Bull. 2001;55:375–86. doi: 10.1016/s0361-9230(01)00503-2. [DOI] [PubMed] [Google Scholar]
- 177.Smith CJ, Emsley HC, Vail A, Georgiou RF, Rothwell NJ, Tyrrell PJ, Hopkins SJ. Variability of the systemic acute phase response after ischemic stroke. J Neurol Sci. 2006;251:77–81. doi: 10.1016/j.jns.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 178.Palasik W, Fiszer U, Lechowicz W, Czartoryska B, Krzesiewicz M, Lugowska A. Assessment of relations between clinical outcome of ischemic stroke and activity of inflammatory processes in the acute phase based on examination of selected parameters. Eur Neurol. 2005;53:188–193. doi: 10.1159/000086355. [DOI] [PubMed] [Google Scholar]
- 179.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 181.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 182.McGill JK, Gallagher L, Carswell HV, Irving EA, Dominiczak AF, Macrae IM. Impaired functional recovery after stroke in the stroke-prone spontaneously hypertensive rat. Stroke. 2005;36:135–141. doi: 10.1161/01.STR.0000149629.32525.b7. [DOI] [PubMed] [Google Scholar]
- 183.Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- 184.Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke Lancet. 2003;362:1211–1224. doi: 10.1016/S0140-6736(03)14544-8. [DOI] [PubMed] [Google Scholar]
- 185.Krakauer JW. The complex dynamics of stroke onset and progression. Curr Opin Neurol. 2007;20:47–50. doi: 10.1097/WCO.0b013e328013f86b. [DOI] [PubMed] [Google Scholar]
- 186.Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- 187.Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 188.Ruehl Mary L, Orozco Jose A, Stoker Matthew B, McDonagh Paul F, Coull Bruce M, Ritter Leslie S. Protective effects of inhibiting both blood and vascular selectins after stroke and reperfusion. Neurological Research. 2002;24:226–232. doi: 10.1179/016164102101199738. [DOI] [PubMed] [Google Scholar]
- 189.Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, Anderson DC. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26:1438–1442. doi: 10.1161/01.str.26.8.1438. [DOI] [PubMed] [Google Scholar]
- 190.Chopp M, Zhang RL, Chen H, Li Y, Jiang N, Rusche JR. Postischemic administration of an anti-Mac-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in rats. Stroke. 1994;25:869–875. doi: 10.1161/01.str.25.4.869. [DOI] [PubMed] [Google Scholar]
- 191.Ma M, Ma Y, Yi X, Guo R, Zhu W, Fan X, Xu G, Frey WH, 2nd, Liu X. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 2008;10:117. doi: 10.1186/1471-2202-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 193.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ. Acute Stroke Investigators: A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA ASTIN Study Investigators. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- 196.Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18:s18–22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- 197.http://clinicaltrials.gov/ct2/show/NCT00630396?term=Minocycline+stroke&rank=3
- 198.http://clinicaltrials.gov/ct2/show/NCT00229177?term=ONO-2506+stroke&rank=1
- 199.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 200.Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke:current status and future perspectives. Int J Mol Sci. 2012;13:11753–11772. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mergenthaler P, Meisel A. Do stroke models model stroke? Dis Model Mech. 2012;5:718–725. doi: 10.1242/dmm.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zaremba J, Losy J. Early TNF-α levels correlate with ischemic stroke severity. Acta Neurol Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]