Abstract
Objective
To assess the effect of the addition of coronary artery bypass grafting (CABG) to medical therapy on mode of death in heart failure.
Background
While CABG therapy is widely used in ischemic cardiomyopathy patients, there is no prospective clinical trial data on mode of death.
Methods
The Surgical Treatment for Ischemic Heart Failure Trial (STICH) compared the strategy of CABG plus medical therapy to medical therapy alone in 1212 ischemic cardiomyopathy patients with reduced ejection fraction. A clinical events committee adjudicated deaths using pre-specified definitions for mode of death.
Results
In STICH, there were 462 deaths over a median follow-up of 56 months. The addition of CABG therapy tended to reduce cardiovascular deaths (HR 0.83; CI (0.68, 1.03),p=0.09) and significantly reduced the most common modes of death: sudden death (HR 0.73; CI (.54–.99) p=0.041) and fatal pump failure events (HR 0.64; CI (.41–1.00) p=0.05). Time-dependent estimates indicate that the protective effect of CABG principally occurred after 24 months in both categories. Deaths post- cardiovascular procedures were increased in CABG patients (HR 3.11 CI (1.47–6.60), but fatal myocardial infarction deaths were lower (HR 0.07 CI (0.01–0.57). Non- cardiovascular deaths were infrequent and did not differ between groups.
Conclusion
In STICH, the addition of CABG to medical therapy reduced the most common modes of death: sudden death and fatal pump failure events. The beneficial effects were principally seen after 2 years. Post-procedure deaths were increased in patients randomized to CABG while myocardial infarction deaths were decreased.
Keywords: heart failure, mode of death, surgical
INTRODUCTION
Chronic congestive heart failure with reduced ejection fraction is a progressive disease in which cardiac dysfunction is associated with increased hospitalization and shortened survival (1). Advances in medical therapy have improved outcomes, but the burden of disease remains excessive (2–7). In industrialized societies, coronary artery disease is the most common etiology of heart failure with reduced ejection fraction and there has been great interest in the use of coronary revascularization to improve outcomes (8). The recently completed Surgical Treatment for IsCHemic heart failure study (STICH) compared the addition of coronary artery bypass grafting (CABG) to medical therapy vs. medical therapy alone (9). While surgical therapy did not statistically reduce the primary outcome of all-cause mortality, there was significant benefit seen on secondary outcomes including cardiovascular death, and composites of all-cause mortality or hospitalizations whether for any cause or for either cardiovascular or heart failure. These findings suggested a need to better understand the all-cause mortality events.
Mode of death analysis in heart failure trials has enhanced our understanding of fatal outcomes associated with this disease and suggested potential therapeutic approaches (10–12). Multiple lines of evidence have suggested potential benefit of CABG therapy on the most common modes of death in chronic heart failure: Sudden death and pump failure (13–20).
The purpose of the current analysis is to determine the effect of CABG added to medical therapy on mode of death compared to medical therapy alone.
METHODS
The current manuscript reports outcomes on 1212 patients randomized in a comparison of CABG therapy plus medical therapy (610 patients) to medical therapy (602 patients) alone with a median follow-up of 56 months. The study design, subject demographics, and main outcomes of STICH have been previously described (21).
Adjudication Process and Definitions
A 7-member adjudication committee comprised of 6 heart failure cardiologists and one cardiac surgeon reviewed deaths. Committee members reviewed source documents and mode of death categorizations were assigned by majority vote during meetings. Deaths were considered cardiovascular unless a specific non-cardiovascular cause was present or unknown if there was insufficient information for adjudication. The major cardiovascular mode of death sub-classifications included the following definitions: Sudden death - death that occurred suddenly and unexpectedly; pump failure death - new or worsening symptoms and/or signs of heart failure usually involving a hospitalization; myocardial infarction – death due to an event with symptoms, EKG, cardiac marker/enzyme evidence, or autopsy data; complication of cardiac procedure – death occurring during or related to a cardiovascular procedure. The full adjudication definitions are in the appendix. The category labeled unknown consisted of a small number of patients in whom documentation of the mode of death was insufficient for the committee to render a specific classification. The mortality analyses presented in this report are based strictly on classifications of the events committee except for supportive hospitalization data adjudicated by investigators for all-cause, cardiac, and heart failure categories.
Statistical Analysis
Baseline patient characteristics were descriptively summarized using medians with 25th and 75th percentiles for continuous variables and frequencies and percentages for categorical variables. Comparisons of the distributions of continuous or ordinal baseline variables between patient groups were performed using the Wilcoxon rank-sum test, and categorical variables were compared using the chi-square test or Fisher’s Exact Test.
Event–rate estimates in each treatment arm for different mode of death categories were calculated using the Kaplan-Meier method (22) and statistically compared using the log-rank test (23). Relative risks, expressed as hazard ratios with associated 95% confidence intervals, were derived using the Cox regression model (24). All treatment comparisons were performed with the treatment groups defined as randomized (i.e., intention-to-treat).
RESULTS
Total Mortality and Demographics
There were 462 deaths in STICH reported during the follow-up period. From the baseline characteristics, patients who died differed from the 750 subjects who did not in the following (Table 1): they were older, with higher New York Heart Association class, worse renal function, and a worse Duke CAD score, lower left ventricular ejection fraction (LVEF), higher end-systolic volume index (ESVI) and end-diastolic volume index (EDVI), and more mitral regurgitation. Among baseline medications, those who died tended to be less commonly treated with a beta blocker, but were more commonly treated with amiodarone, loop diuretics, and insulin. Overall 2.4% had an ICD at baseline. Baseline characteristics of patients who experienced the two major modes of death in STICH, sudden-death and pump failure, are also seen in Table 1: their characteristics differed little from those of the overall death group though cardiac volumes were largest in the fatal pump failure group.
Table 1.
Baseline Characteristics of STICH Hypothesis 1 Patients by Mortality Status (including sudden death and pump failure sub-classifications)
| Characteristics1 | Patients Survived (N= 750) |
Patients Died (N= 462) |
P value3 | ||
|---|---|---|---|---|---|
| Sudden Deaths (N=173) |
Pump Failure Deaths (N=82) |
All-cause Deaths2 (N=462) |
|||
| Age (years) | 59 (53, 66) | 59 (53, 67) | 61 (56, 69) | 62 (54, 69) | <0.001 |
| White race | 66% | 64% | 78% | 72% | 0.017 |
| Previous stroke | 6% | 9% | 10% | 10% | 0.008 |
| Renal insufficiency | 5% | 9% | 17% | 12% | <0.001 |
| Atrial fibrillation | 10% | 15% | 16% | 17% | 0.001 |
| Current NYHA4 HF Class I / II III / IV |
13% / 53% 32% / 2% |
9% / 52% 35% / 5% |
9% / 46% 40% / 5% |
10% / 49% 37% / 5% |
0.003 |
| Creatinine (mg/dL) | 1.1 (0.9, 1.2) | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.4) | 1.1 (1.0, 1.4) | <0.001 |
| BUN (mg/dL) | 22 (16, 35) | 24 (16, 37) | 25 (19, 43) | 24 (17, 39) | 0.004 |
| LV EF (%) | 28 (23, 34) | 25 (20, 31) | 24 (20, 30) | 25 (20, 32) | <0.001 |
| ESVI5 (ml/m2) | 75 (57, 97) | 86 (67, 111) | 96 (70, 128) | 86 (65, 118) | <0.001 |
| EDVI6 (ml/m2) | 108 (86, 132) | 120 (97, 144) | 127 (102, 169) | 120 (97, 154) | <0.001 |
| Mitral regurgitation Mild or less Moderate/severe |
85% 15% |
81% 19% |
65% 35% |
76% 24% |
<0.001 |
| Duke CAD Index (1–100) | 52 (39, 77) | 65 (39, 77) | 65 (39, 77) | 65 (39, 77) | 0.018 |
| Beta-blocker | 87% | 88% | 79% | 83% | 0.046 |
| Amiordarone | 8% | 13% | 12% | 13% | 0.005 |
| Diuretic (loop/thiazide) | 61% | 69% | 77% | 72% | <0.001 |
| Diuretic (loop/thiazide or potassium sparing) | 71% | 78% | 88% | 82% | <0.001 |
| Previous ICD | 2% | 1% | 2% | 2% | 0.983 |
Data shown are median and inter-quartile range or proportions as a percentage.
All-cause deaths include sudden deaths, pump failure deaths and deaths from other causes.
P-values are based on comparisons between patients survived (N=750) and patients died (N=462).
NYHA=New York Heart Association Functional Class.
LVEF= left ventricular ejection fraction
ESVI=End-systolic volume index.
EDVI=End-diastolic volume index.
Mode of Death
For committee adjudicated fatal events, there were 351 deaths (29.0% of patients, 76.0% of overall deaths) categorized as cardiovascular while 67 (5.5% of patients, 14.5% of deaths) were assessed as non-cardiovascular. There were 44 deaths adjudicated as unknown (3.6% of patients, 9.5% of deaths) (Table 2).
Table 2.
Clinical Events Committee (CEC) Adjudicated Cause of Death
| Mortality Distribution N (% of Patients) |
Treatment | Hazard Ratio (95% CI) |
P value | ||
|---|---|---|---|---|---|
| CABG (N=610)1 |
MED (N=602) |
Total (N=1212) |
|||
| All-cause death | 218 (35.7) | 244 (40.5) | 462 (38.1) | 0.86 (0.72, 1.04) | 0.12 |
| Cardiovascular (CEC) | 162 (26.6) | 189 (31.4) | 351 (29.0) | 0.83 (0.68, 1.03) | 0.09 |
| Non-cardiovascular (CEC) | 35 (5.7) | 32 (5.3) | 67 (5.5) | 1.03 (0.64, 1.67) | 0.90 |
| Unknown (CEC) | 21 (3.4) | 23 (3.8) | 44 (3.6) | 0.87 (0.48, 1.58) | 0.67 |
These are total number of patients.
Compared to medical therapy, the addition of CABG was associated with a non-significant decrease in cardiovascular mortality (HR 0.83, CI 0.68–0.1.03 p= 0.09) but no difference was seen in the non-cardiovascular and unknown categories.
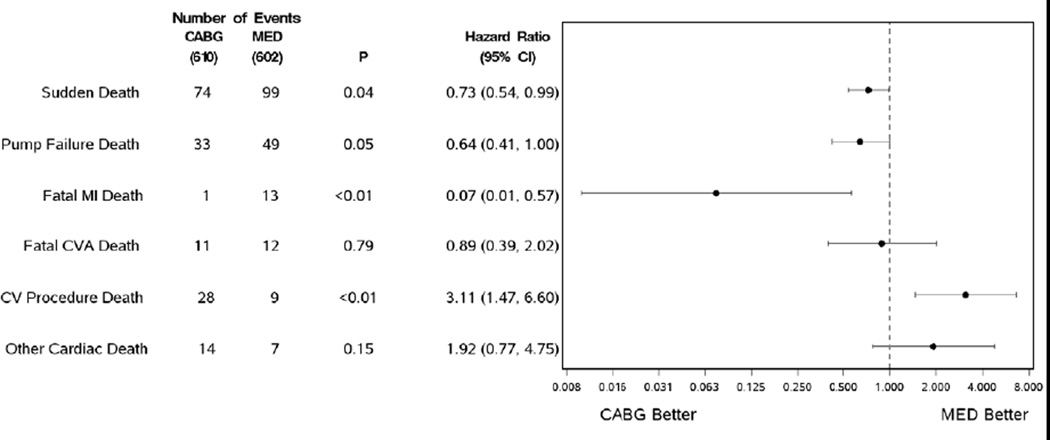
Among the cardiovascular sub-classification categories (Table 3), there were differences between the treatment groups in the following: sudden deaths were significantly reduced by CABG therapy, 74 CABG vs. 99 MED (HR 0.73, CI 0.54–0.99 p= 0.04) with a similar effect on pump failure deaths, 33 vs. 49 events, that was nominally significant (HR 0.64 CI 0.41–1.00 p= 0.05). Fatal myocardial infarctions were infrequent, but also decreased by the addition of CABG therapy, 1 vs.13 (HR 0.07, CI 0.01–0.57 p= <0.01), while cardiovascular procedure deaths were increased in the surgical group, 28 vs. 9 (HR 3.11, CI 1.47–6.60 p<0.01). Fatal cardiovascular procedure events are listed in detail in the Appendix. A forest plot of the major cardiovascular categories is seen in Figure 1. Within the non-cardiovascular category there were also no differences seen for any specific sub-classification of event (Table 4). With unadjudicated data, CABG therapy significantly reduced all-cause hospitalizations as well as cardiac and heart failure subcategories (Table 5).
Table 3.
Cause of Death: Cardiovascular Sub-classifications
| Mortality Distribution (% of Patients, % of Deaths) |
Treatment | ||
|---|---|---|---|
| CABG (N=610, 218)1 |
MED (N=602, 244) |
Total (N=1212, 462) |
|
| Cardiovascular (CEC) | 162 (26.6, 74.3) | 189 (31.4, 77.5) | 351 (29.0, 76.0) |
| Sudden | 74 (12.1, 33.9) | 99 (16.4, 40.6) | 173 (14.3, 37.4) |
| Pump failure | 33 (5.4, 15.1) | 49 (8.1, 20.1) | 82 (6.8, 17.7) |
| MI | 1 (0.2, 0.5) | 13 (2.2, 5.3) | 14 (1.2, 3.0) |
| CVA | 11 (1.8, 5.0) | 12 (2.0, 4.9) | 23 (1.9, 5.0) |
| Cardiac procedure | 28 (4.6, 12.8) | 9 (1.5, 3.7) | 37 (3.1, 8.0) |
| Other cardiovascular | 14 (2.3, 6.4) | 7 (1.2, 2.9) | 21 (1.7, 4.5) |
These are total number of patients and total number of deaths.
Figure 1.
Cardiovascular modes of death: Hazard ratios and confidence intervals
Table 4.
Cause of Death: Non-cardiovascular Sub-classifications
| Mortality Distribution (% of Patients, % of Deaths) |
Treatment | ||
|---|---|---|---|
| CABG (N=610, 218)1 |
MED (N=602, 244) |
Total (N=1212, 462) |
|
| Non-cardiovascular (CEC) | 35 (5.7, 16.1) | 32 (5.3, 13.1) | 67 (5.5, 14.5) |
| Infection | 7 (1.1, 3.2) | 5 (0.8, 2.0) | 12 (1.0, 2.6) |
| Neurologic | 0 (0.0, 0.0) | 2 (0.3, 0.8) | 2 (0.2, 0.4) |
| Pulmonary | 2 (0.3, 0.9) | 2 (0.3, 0.8) | 4 (0.3, 0.9) |
| Renal | 4 (0.7, 1.8) | 0 (0.0, 0.0) | 4 (0.3, 0.9) |
| Malignancy | 17 (2.8, 7.8) | 18 (3.0, 7.4) | 35 (2.9, 7.6) |
| Other non-cardiovascular | 5 (0.8, 2.3) | 5 (0.8, 2.0) | 10 (0.8, 2.2) |
These are total number of patients and total number of deaths.
Table 5.
Major Categories of Hospitalization: Site adjudicated
| Clinical endpoint | CABG (N=610) |
MED (N=602) |
Hazard Ratio (95% CI) |
P-value |
|---|---|---|---|---|
| Hospitalization (all cause) | 290 (48%) | 340 (56%) | 0.77 (0.66, 0.90) | 0.001 |
| Hospitalization (cardiac) | 221 (36%) | 294 (49%) | 0.65 (0.55, 0.78) | <0.001 |
| Hospitalization (heart failure) | 127 (21%) | 169 (28%) | 0.70 (0.56, 0.88) | 0.003 |
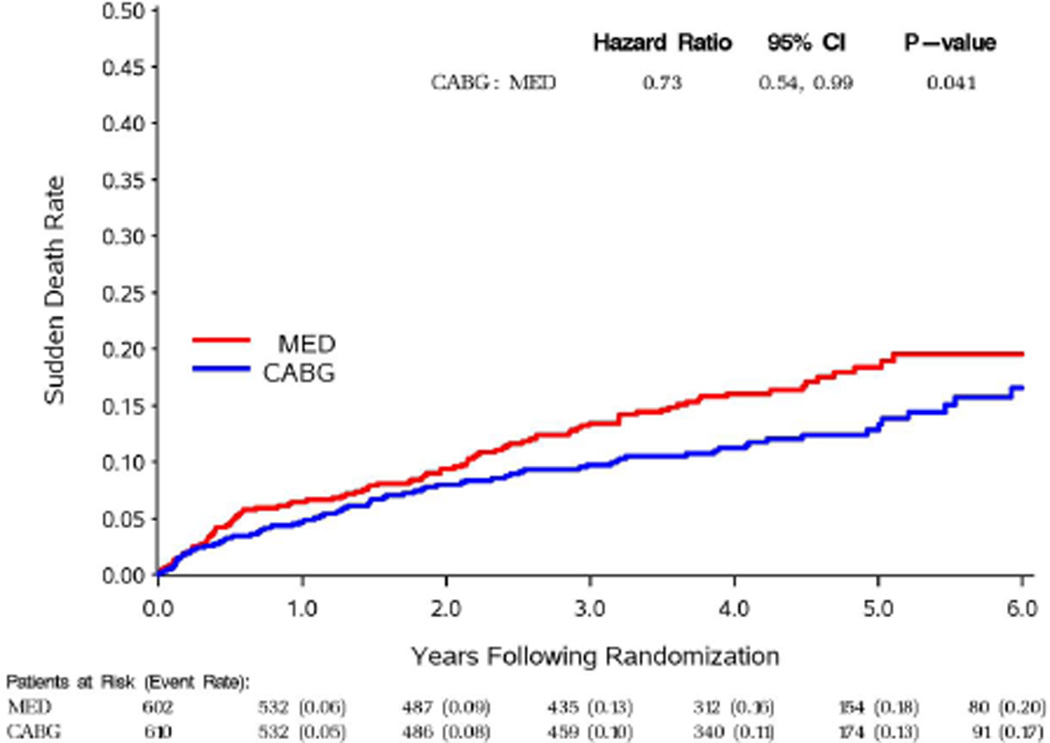
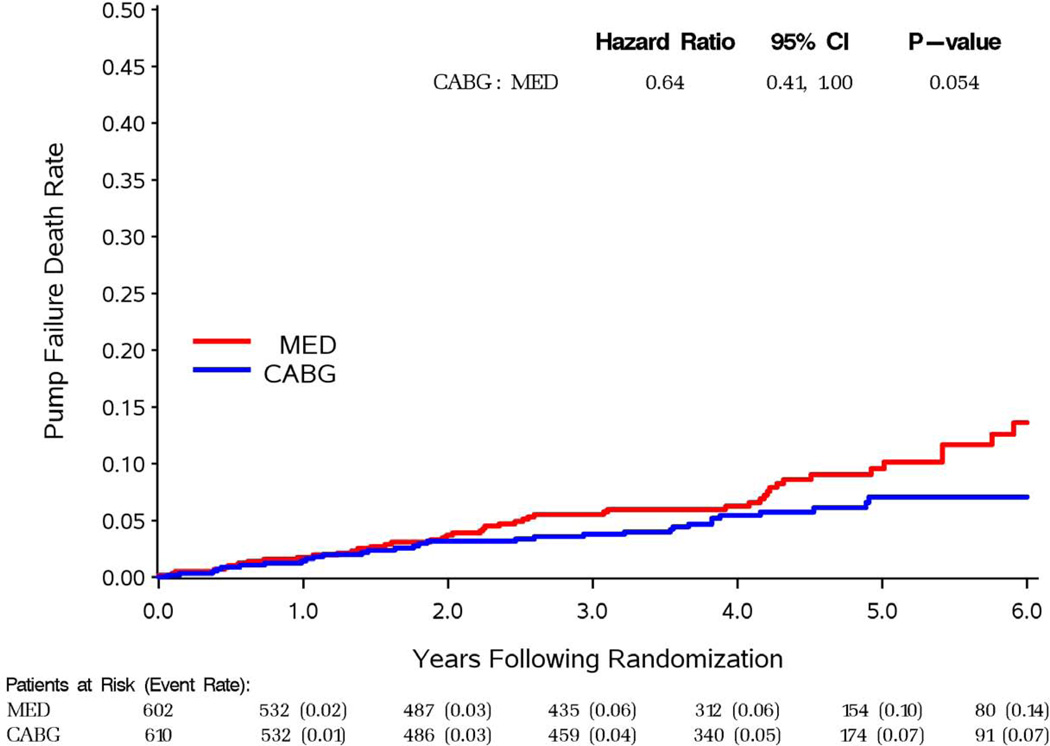
For the two most common sub-classifications of mode of death, sudden and pump failure, time to event curves are shown by treatment group in figures 2 and 3. For both modes of death, there was a later separation of the curves with fewer events in the CABG group. Table 6 indicates a trend for benefit of CABG therapy within 6 months for sudden death, and significant benefit beyond 24 months for both modes of death.
Figure 2.
Sudden death KM curves
Figure 3.
Pump failure KM curves
Table 6.
Sudden Deaths and Pump Failure Deaths by Treatment Group in Time Intervals of 0–6, 6–24, and >24 Months
| Events | Time Intervals Following Randomization |
Number of Events | Hazard Ratio (CABG:MED) |
95% CI of Hazard Ratio (Lower, Upper) |
P-value | |
|---|---|---|---|---|---|---|
| CABG (N=610) |
MED (N=602) |
|||||
| Sudden Death | 0 – 6 Months | 19 | 26 | 0.74 | (0.41, 1.34) | 0.321 |
| 6 – 24 Months | 26 | 28 | 0.93 | (0.55, 1.59) | 0.801 | |
| >24 Months | 29 | 45 | 0.60 | (0.38, 0.96) | 0.032 | |
| Overall | 74 | 99 | 0.73 | (0.54, 0.99) | 0.041 | |
| Pump Failure Death | 0 – 6 Months | 5 | 6 | 0.84 | (0.26, 2.76) | 0.778 |
| 6 – 24 Months | 12 | 13 | 0.93 | (0.42, 2.03) | 0.848 | |
| >24 Months | 16 | 30 | 0.49 | (0.27, 0.90) | 0.021 | |
| Overall | 33 | 49 | 0.64 | (0.41, 1.00) | 0.054 | |
Effect of Functional Class and Ventricular Function on Mode of Death
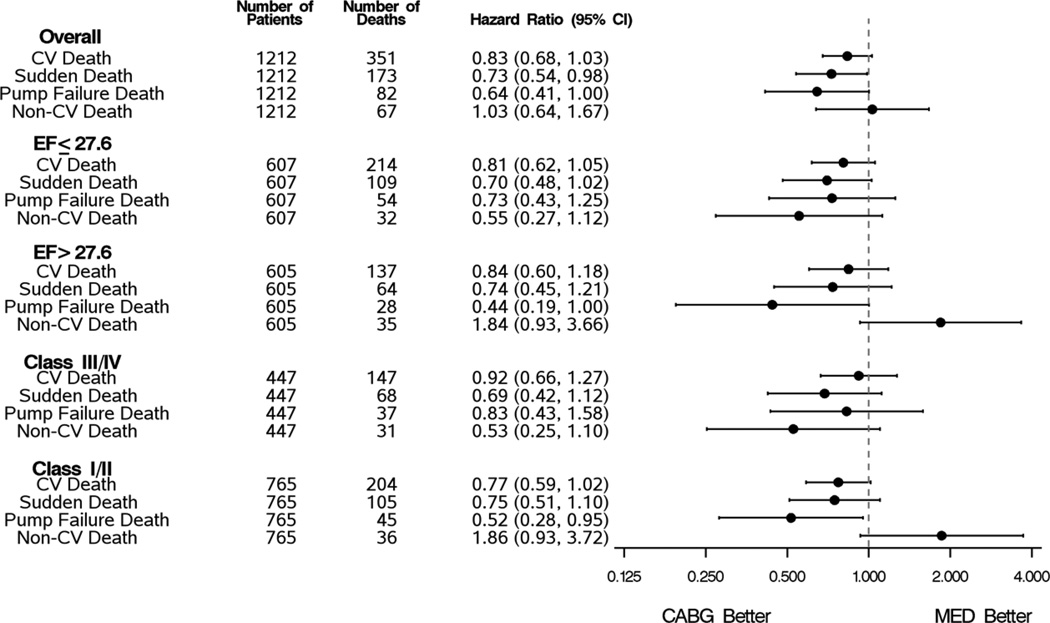
We further analyzed mode of death in subgroups by severity of LV dysfunction or New York Heart Association class (Figure 4). The addition of CABG continued to be directionally favorable but the fewer events within each subgroup resulted in a loss of statistical significance in the sudden and pump failure sub-classifications. Non-cardiovascular deaths tended to be less frequent in those treated with medical therapy alone in the above median LVEF subgroup and in the NYHA class I-II subgroup but no significant differences were seen.
Figure 4.
Cardiovascular, Sudden Death, pump failure, and Non-cardiovascular deaths by treatment group for LVEF and NYHA subgroups
DISCUSSION
The STICH trial presents the opportunity to examine the effect of CABG added to medical therapy on mode of death. The results indicate that the majority of deaths were cardiovascular, with sudden and pump failure deaths being the two most common sub-classifications. Using CEC adjudicated data only, the addition of CABG therapy reduced cardiovascular deaths, though not significantly. Bypass surgery significantly reduced sudden deaths as well as deaths due to myocardial infarction with a nominally significant effect on fatal pump failure events. Cardiovascular procedure deaths were increased partially offsetting these findings. The benefits of bypass surgery on mode of death were supported by significant reductions in hospitalizations. Effects on the two major modes of death were principally seen after 2 years. Defining subgroups by median LVEF or NYHA class did not alter the distribution of cardiovascular deaths or the direction of the CABG therapeutic result. These data represent the only prospective clinical trial experience evaluating the effect of CABG on mode of death in ischemic cardiomyopathy patients. It is also one of the larger databases using committee adjudicated results to examine mode of death in CABG patients.
Mode of Death in Ischemic Cardiomyopathy Patients in STICH
In STICH, the majority of deaths adjudicated by the CEC were due to a cardiovascular cause, which is in agreement with other databases in heart failure with reduced ejection fraction (3–6, 10–12, 25–26). The number of cardiovascular deaths differs slightly in this paper compared to the main STICH paper (9) due to a difference in methodology in assignment for “unknown” deaths. For this analysis, deaths were adjudicated as “unknown” when the CEC believed that there was insufficient data to adjudicate. The main manuscript used a “best available” methodology, pre-specified prior to analyses, that combined committee adjudicated cardiovascular deaths (excluding vascular deaths, n=348) with those deaths the committee adjudicated as “unknown” but the investigator assessed as “cardiovascular” (n=21). Among all deaths classified by the site as cardiovascular, the event committee’s adjudicated assessment was concordant with the site in 92% of cases (Kappa Coefficient = 0.67, 95% CI 0.59, 0.75 p<0.001).
The treatment comparison with respect to cardiovascular death in the current manuscript remains directionally favorable for the addition of CABG therapy and has a similar effect size as that reported in the primary manuscript, but a slightly wider confidence interval and hence a slightly larger p-value due to fewer cardiovascular deaths. This directional effect is also consistent with the favorable effect of bypass surgery on the secondary outcome of all-cause mortality or cardiovascular hospitalization in the main paper (9).
Deaths assessed as non-cardiovascular have been relatively infrequent (7–29%) in heart failure with reduced ejection fraction heart failure studies (3–6, 10–12, 25–6).
As noted above, sudden deaths and fatal pump failure events were the most common specific sub-classification of death in STICH, in comparable proportion to other recent heart failure trials (4,25–27). Myocardial infarction deaths were infrequent in STICH as seen in other chronic heart failure trials including those with an ischemic cohort (26, 28).
Effect of Surgical Therapy on Sub-classification of Mode of Death in STICH
Compared to medical therapy alone, the addition of CABG in STICH significantly reduced sudden cardiac deaths. Previous reports have noted this finding, although those studies comprised small numbers of patients (15) or were retrospective analyses (29–30). In the largest retrospective analysis, of the SOLVD database, Veenhuyzen et al reported that patients with previous CABG were associated with a lower risk of sudden death than those without prior CABG (18) though it was noted that interpretation of retrospective studies involving historical procedures must be interpreted cautiously (31).
For the benefit seen in the prospective STICH trial, the pathophysiologic benefit with CABG may be related to a reduction in severe ischemic events, including sudden coronary occlusions, which would reduce the substrate for the development of reentrant ventricular arrhythmias. Although there are small event numbers, the reduction in myocardial infarction deaths in STICH in the CABG treatment group provide support for reduction in fatal ischemic events as part of the sudden death benefit. While myocardial infarction deaths were uncommon in STICH and other chronic heart failure databases, coronary thromboses have been reported in sudden cardiac deaths in autopsy studies as well as angiography in SCD survivors. In chronic heart failure, Uretsky et al (32) reported coronary thromboses and undiagnosed myocardial infarctions in deaths adjudicated as sudden from the ATLAS study. The favorable effect on sudden death by bypass surgery may also be related to a salutary impact on heart failure progression, as indicated by a reduction in both fatal pump failure events.
There was a low use of ICDs in STICH, which is common among international heart failure trials with enrollment predominantly outside of the United States and Western Europe (26, 33). Whether widespread ICD use would have changed the reduction in sudden death seen with bypass surgery cannot be answered with currently available clinical trial data. The CABG Patch trial tested the addition of ICD therapy to bypass surgery patients but interpretation of this relatively small trial is hampered by a low arrhythmic death rate (16–17). Conceptually, a trial containing both bypass surgery and ICDs would result in even lower arrhythmic event rates as these modalities influence different pathways to sudden deaths and neither therapy could be expected to completely eliminate such events as noted in STICH for bypass surgery, in SCD-Heft for ICDs (34), and in CABG Patch for both therapies. It is of interest that the favorable effect of CABG was more prominent after 24 months which is consistent with a temporal relationship reported for the efficacy of ICD therapy and coronary revascularization in the MADIT-II database (35). Furthermore, a continued late benefit on mortality has been noted previously in CABG trials (13–14).
Surgical therapy also reduced pump failure deaths, though this effect was only marginally significant due to a low number of events. It is interesting that this finding was present considering the concept of "competing risk": a favorable effect on sudden death could increase patients at risk for fatal pump failure events. There is no other prospective data on the CABG effect on pump failure deaths, and the SOLVD retrospective analysis described above did not show a favorable association of previous bypass surgery with pump failure deaths (18). The explanation for benefit on pump failure outcomes is not certain. Previous data on ventricular function indicated an association with revascularization for viable myocardium (19–20) though the sub-study in STICH did not indicate benefit associated with this pathway for CABG therapy (36). However, the favorable effect on pump failure deaths is consistent with the secondary outcome in STICH of all-cause mortality or heart failure hospitalizations (9).
Myocardial infarction deaths were also decreased by CABG therapy in STICH although this was a small number of fatal events. As indicated above, this data is concordant with the reduction in sudden death in that at least some of these events may represent severe ischemia or coronary thrombosis.
Cardiac procedure deaths were increased in the surgical group compared to medical therapy, a finding that would not be surprising in a population of patients with predominantly NYHA class II-III heart failure and depressed ejection fraction.
Effect of CABG Therapy on Mode of Death for Patients Stratified by Ejection Fraction and NYHA Class
In this analysis, patients with more impaired ventricular function and worse NYHA class had higher event rates but the proportion of sudden and pump failure deaths or the direction of therapeutic effect did not change. There is no previous prospective data assessing the effect of bypass surgery on cause specific outcomes in ischemic cardiomyopathy patients stratified by either ventricular function or heart failure severity. The CASS study did indicate a relationship of ventricular function to risk for sudden death as well as an association with CABG therapy in a multivariable model, but it did not include patients below with LVEF < 34%, and there was no assessment of response to bypass surgery by NYHA class. The retrospective SOLVD analysis reported a consistent reduction of LVEF on sudden death rate in CABG patients.
Limitations
Interpreting the results of secondary end points and subgroup analyses in clinical trials in which the primary outcome has not been met needs to be done with caution (37). However, the mode of death results on concordant directionally and in degree with the favorable effects previously reported on composite outcomes involving hospitalizations and in this paper on major nonfatal hospitalization categories.
The adjudication of clinical endpoints is dependent upon the availability, accuracy, and quality of clinical information that is available from sites. The adjudication process in STICH involved standardized definitions used in other heart failure trials, as well as the participation of experienced physicians. In the case of international clinical trials, there are limitations on availability of clinical material and there may be some loss of information due to translation or patient follow-up. However STICH had excellent follow-up with missing status on only 5 patients (9). All clinical trials in heart failure must deal with the difficulties of sudden death events in that such events, thought to be predominantly arrhythmic, usually lack evidence of exact causality and therefore this categorization always contains an inherent uncertainty. Deaths assessed, as “unknown” by the clinical event committee remained a separate category in this analysis, alternative approaches include classifying these as “cardiovascular”.
CONCLUSION
In STICH, CABG therapy reduced the most common modes of death, sudden and pump failure. Fatal myocardial infarctions were also reduced but the beneficial effects of bypass surgery on cardiovascular mortality were offset by an increase in post procedure deaths. Non-cardiovascular deaths were relatively infrequent and did not differ between treatment groups. The beneficial effects on the two major modes of death were principally seen after 2 years. The durability of these findings is currently being tested in the STICH Extension Study.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Vanessa Moore for her assistance in preparation of the manuscript, Thomas Barfield for administrative activities in STICH, and James Hill M.D. and the publications committee for their review of the manuscript.
This work supported by: Supported by grants (U01HL69015 and U01HL69013) from the National, Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 3.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 4.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 5.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 6.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 7.Jhund PS, Macintyre K, Simpson CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202–1213. doi: 10.1161/CIRCULATIONAHA.106.623199. [DOI] [PubMed] [Google Scholar]
- 9.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn JN, Johnson G, Ziesche S, Cobb F, et al. Enalapril vs. Hydralazine-Isosorbide Dinitrate in the Treatment of Chronic Congestive Heart Failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor CM, Carson PE, Belkin RN, Cropp AB, et al. Effect of Amlodipine on Mode of Death in Severe Chronic Heart Failure: The PRAISE Trial. American Journal of Cardiology. 1998;82:881–888. doi: 10.1016/s0002-9149(98)00496-2. [DOI] [PubMed] [Google Scholar]
- 12.Carson P, Anand I, Jaski B, Steinberg J, et al. Mode of Death in Advanced Heart Failure – The COMPANION. J Am Coll Cardiol. 2005 Dec 20;46(12):2329–2334. doi: 10.1016/j.jacc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Coronary Artery Surgery Study (CASS): a randomized trial of coronary artery bypass surgery: survival data. Circulation. 1983;68:939–950. doi: 10.1161/01.cir.68.5.939. [DOI] [PubMed] [Google Scholar]
- 14.Passamani E, Davis KB, Gillespie MJ, Killip T. A randomized trial of coronary artery bypass surgery: survival of patients with a low ejection fraction. N Engl J Med. 1985;312:1665–1671. doi: 10.1056/NEJM198506273122603. [DOI] [PubMed] [Google Scholar]
- 15.Holmes DR, Jr, Davis KB, Mock MB, et al. The effect of medical and surgical therapy on subsequent sudden cardiac death in patients with coronary artery disease: a report from the Coronary Artery Surgery Study. Circulation. 1986;73:1254–1263. doi: 10.1161/01.cir.73.6.1254. [DOI] [PubMed] [Google Scholar]
- 16.Bigger JT., Jr Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. N Engl J Med. 1997;337:1569–1575. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 17.Bigger JT, Jr, Whang W, Rottman JN, et al. Mechanisms of death in the CABG Patch Trial: a randomized trial of implantable cardiac defibrillator prophylaxis in patients at high risk of death after coronary artery bypass graft surgery. Circulation. 1999;99:1416–1421. doi: 10.1161/01.cir.99.11.1416. [DOI] [PubMed] [Google Scholar]
- 18.Veenhuyzen GD, Singh SN, McAreavey D, et al. Prior coronary artery bypass surgery and risk of death among patients with ischemic left ventricular dysfunction. Circulation. 2001;104:1489–1493. doi: 10.1161/hc3801.096335. [DOI] [PubMed] [Google Scholar]
- 19.Allman KC, Shaw LJ, Hachamovitch R, et al. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–1158. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 20.Elefteriades JA, Tolis G, Jr, Levi E, et al. Coronary artery bypass grafting in severe left ventricular dysfunction: excellent survival with improved ejection fraction and functional state. J Am Coll Cardiol. 1993;22:1411–1417. doi: 10.1016/0735-1097(93)90551-b. [DOI] [PubMed] [Google Scholar]
- 21.Velazquez EJ, Lee KL, O'Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 23.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Second Edition. New York: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 24.Cox DR. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 25.Solomon SD, Wang D, Finn P, et al. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2004;110:2180–2183. doi: 10.1161/01.CIR.0000144474.65922.AA. [DOI] [PubMed] [Google Scholar]
- 26.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 27.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor CM, Gottlieb S, Bourque JM BEST Investigators. Impact of nonfatal myocardial infarction on outcomes in patients with advanced heart failure and the effect of bucindolol therapy. Am J Cardiol. 2005 Mar 1;95(5):558–564. doi: 10.1016/j.amjcard.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Pigott JD, Kouchoukos NT, Oberman A, et al. Late results of surgical and medical therapy for patients with coronary artery disease and depressed left ventricular function. J Am Coll Cardiol. 1985;5:1036–1045. doi: 10.1016/s0735-1097(85)80003-6. [DOI] [PubMed] [Google Scholar]
- 30.Hammermeister KE, DeRouen TA, Murray JA, et al. Effect of aortocoronary saphenous vein bypass grafting on death and sudden death: comparison of nonrandomized medically and surgically treated cohorts with comparable coronary disease and left ventricular function. Am J Cardiol. 1977;39:925–934. doi: 10.1016/s0002-9149(77)80048-9. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub WS. Revascularization Versus Implantable Cardioverter-Defibrillators to Prevent Sudden Death in Patients With Severe Left Ventricular Dysfunction. Circulation. 2001;104:1457–1458. [PubMed] [Google Scholar]
- 32.Uretsky BF, Thygesen K, Armstrong PW, et al. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the Assessment of Treatment with Lisinopril and Survival (ATLAS) trial. Circulation. 2000;102:611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 33.Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 34.Packer DL, Prutkin JM, Hellkamp AS, et al. Impact of Implantable Cardioverter-Defibrillator, Amiodarone, and Placebo on the Mode of Death in Stable Patients With Heart Failure: Analysis From the Sudden Cardiac Death in Heart Failure Trial. Circulation. 2009;120:2170–2176. doi: 10.1161/CIRCULATIONAHA.109.853689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenberg I, Moss AJ, McNitt S, et al. Time dependence of defibrillator benefit after coronary revascularization in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:1811–1817. doi: 10.1016/j.jacc.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 36.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freemantle N. Interpreting the results of secondary endpoints in subgroup analyses in clinical trials: should we lock the crazy aunt in the attic? BMJ. 2001;322(7292):989–991. doi: 10.1136/bmj.322.7292.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.