Abstract
Background
Web-based personal health records (PHRs) have been advocated as a means to improve type 2 diabetes mellitus (DM) care. However, few Web-based systems are linked directly to the electronic medical record (EMR) used by physicians.
Methods
We randomized 11 primary care practices. Intervention practices received access to a DM-specific PHR that imported clinical and medications data, provided patient-tailored decision support, and enabled the patient to author a “Diabetes Care Plan” for electronic submission to their physician prior to upcoming appointments. Active control practices received a PHR to update and submit family history and health maintenance information. All patients attending these practices were encouraged to sign up for online access.
Results
We enrolled 244 patients with DM (37% of the eligible population with registered online access, 4% of the overall population of patients with DM). Study participants were younger (mean age, 56.1 years vs 60.3 years; P< .001) and lived in higher-income neighborhoods (median income, $53 784 vs $49 713; P<.001) but had similar baseline glycemic control compared with nonparticipants. More patients in the intervention arm had their DM treatment regimens adjusted (53% vs 15%; P< .001) compared with active controls. However, there were no significant differences in risk factor control between study arms after 1 year (P=.53).
Conclusions
Previsit use of online PHR linked to the EMR increased rates of DM-related medication adjustment. Low rates of online patient account registration and good baseline control among participants limited the intervention's impact on overall risk factor control.
Despite the demonstrated efficacy of medical therapy in controlled clinical trials,1-5 most patients with type 2 diabetes mellitus (DM) cared for in the community do not reach recommended treatment goals for glycemic, blood pressure, or low-density lipoprotein cholesterol (LDL-C) control.6,7 Fundamental changes in how DM care is delivered may be needed to overcome the current “quality chasm” seen in DM management.8
Increasing patients' knowledge about their current risk factor levels9,10 and facilitating collaboration between patients and physicians11-13 have both been demonstrated to improve clinical outcomes. Personal health records (PHRs) that enable patients to interact directly with their own clinical records represent one innovative means to achieve these goals.14–16 Older studies have used patient-carried paper reminder cards and mini records17,18 to engage patients in their care. More recent studies have used computerized systems to provide patients with personalized hemoglobin Alc (HbA1c) reports,19 Internet-based glucose monitoring systems,20 telephone-based case management,21 peer-supported behavioral self-management,22 and Web-based case management.23
To date, there have been no large-scale studies of interventions that integrate PHRs directly with the electronic medical records (EMRs) used by patients' own primary care physicians (PCPs). Given the central role PCPs play in the medical management of DM, we hypothesized that a link that enabled patients to both read (eg, real-time access to laboratory results, guidelines, and medication lists) and write (eg, medication list edits and DM-related comments) to the EMR was crucial to achieve measurable changes in DM control. In addition, although Web-based interventions have had modest success among highly selected research participants, it is currently not known to what extent such services will be adopted by the general population.
To address these questions, we conducted a multipractice randomized clinical trial to evaluate the impact of a DM-specific PHR that was linked directly to the EMR and was made available to all patients registered for care within the study practices (the Prepare for Care study). We hypothesized that use of the DM-specific PHR would result in improved care by increasing patient knowledge and engagement in their own care and by facilitating patient-physician communication. We compared changes in DM management and related clinical outcomes in the intervention arm with those of eligible patients with DM in control practices who were invited to use an online family history and health maintenance PHR. This active control design permitted direct comparison of the intervention effect among patients willing to engage in online PHR use.
Methods
Setting and Participants
This study was conducted in 11 primary care practices within the Partners HealthCare system. The study practices were staffed by 230 PCPs and were located in both hospital- and community-based settings in eastern Massachusetts. All participating clinical sites used the same EMR and central laboratory for all clinical care activities. Data from the EMR, patient registration, laboratory testing, radiology studies, clinic appointments, and billing were automatically stored in a common clinical data repository readily queried for research purposes. Informed consent was obtained from eligible patients prior to notification of practice randomization status. This study was approved by the Partners HealthCare institutional review board and was registered with ClinicalTrials.gov.
Criteria for Eligible Patients
First, patients had to have DM, defined based on review of problem lists, HbA1c level higher than 7.0% in the prior year, and/or an active prescription for a DM-specific medicine at time of study enrollment using a previously validated algorithm.24
Second, they had to have had at least 1 visit with their designated PCP in one of the study practices in the prior year. Patients self-designated their PCP when signing up for PG and confirmed their PCP when consenting to participate in the Prepare for Care study.
Third, they had to have an active account with the practice's online patient portal, Patient Gateway (PG), a password-enabled, online access system available since 2002 to all patients within the Partners HealthCare network. This system was developed to permit online access for basic tasks such as updating registration information, confirming upcoming appointments, sending non urgent clinical messages, and requesting prescription refills.25 “Active accounts” were defined as registered PG accounts for which patients had logged on to the PG Web site at least once; PG served as the underlying framework for implementing the advanced PHR modules in this study.
Intervention
After providing informed consent, eligible patients with DM in the intervention arm practices were invited to use a DM-specific PHR prior to scheduled clinic visits. The DM PHR included a medications module component that allowed patients to review their medication lists from the EMR, edit inaccuracies, and answer several brief questions regarding adherence barriers and adverse effects of medication. Patient-updated data for medications specific to DM care (eg, for the treatment of hyperglycemia, hypertension, and hyperlipidemia) were then used to populate specific fields within the DM PHR. Separate domains within the DM PHR for glucose, blood pressure, LDL-C, and preventive care allowed patients to view their most recent results and current treatments, and to respond to specific questions about barriers to current therapy. Check boxes and free text boxes within the PHR encouraged patients to enter therapy concerns and requests to address specific care limitations (eg, out-of-date cholesterol test, under-treated hypertension).
The conceptual framework, design and development, and features of the DM PHR are described in detail elsewhere.26 In brief, the functions and goals of the DM module were to (1) provide patients with their own clinical information linked to tailored decision support (to engage patients in their care), (2) take patients through a series of simple and direct questions designed to identify areas requiring clinical action (to encourage patients to take a greater role in their DM management), and (3) generate a “Diabetes Care Plan” based on patients' responses to share with their PCP at the upcoming clinic visit (to facilitate communication and reduce clinical inertia). This care plan was submitted directly to the EMR used by the patient's PCP and could also be printed by the patient and brought to the upcoming appointment.
After providing informed consent, eligible patients with DM in the control arm practices were invited to participate in a novel PHR that allowed them to review and update their family medical history and to review cancer screening and other non–DM-specific preventive services prior to their upcoming appointment. Updates and requests entered by patients within this PHR were then electronically submitted to the EMR used by the patient's PCP. Patients were unaware of their PHR assignment when consenting to enroll in the study. Because both intervention and control groups used PG and received additional PHR modules, the primary distinction between the 2 study arms was the content of the modules.
Recruitment Strategy
This study was designed to evaluate the impact of a Web-based PHR in the general primary care population. Because patients were required to have online access via PG to be eligible for the experimental PHR modules, we implemented a coordinated effort to increase registration for PG accounts within our network through mailed postcards to all patients registered for care with study practices, posters and signs for PG in waiting or examination rooms, and voicemail recordings played to patients telephoning the practices. Some practices also offered onsite enrollment.
To increase adoption of the Prepare for Care study modules within the study practices, study personnel (1) identified physician champions at each practice to facilitate deployment, (2) presented the modules to physicians at practice meetings to introduce their functionality to the physicians, (3) provided on-site and online support, (4) provided marketing materials to practices and returned to practices to hear feedback after rollout, and (5) sent reminders through PG to all patients who had consented to participate in the study to prompt them to review their PHR modules prior to an eligible upcoming visit.
Clinical Trial Objectives
The primary goal of this study was to improve the clinical management of DM in primary care practices through the use of a Web-based DM PHR. We hypothesized that this PHR-based intervention would result in more effective treatment of DM-related risk factors (hyperglycemia, hypertension, and hyperlipidemia) compared with control patients with access to a non–DM-specific PHR. We also had a priori concerns that the so-called digital divide might lead to reduced participation in the study among poorer and older patients.27 Thus, as a secondary goal we examined demographic and clinical differences between intervention users and nonusers within the study practices.
Outcomes
The primary outcomes for this study were changes from baseline in 3 key measures of DM management: HbA1c, blood pressure, and LDL-C, comparing all eligible patients with DM in the 2 study arms in an intention-to-treat analysis. One-year baseline and follow-up periods were uniquely defined for each eligible patient in both study arms based on the date of their first clinic visit during the study period. For patients with no clinic visits during the study period, the midpoint between the date their practice enrolled in the study and the study end date was used to define their 12-month baseline and follow-up periods.
We prespecified 2 additional analyses: (1) We compared demographic and clinical characteristics between study participants and nonparticipants (ie, all patients with DM attending the study practices who did not enroll in the study) to gain further insight into online PHR adoption in the general primary care population. (2) We identified any DM-related medication initiation or intensification at the first episode of care after journal submission, comparing patients in the 2 trial arms who submitted journals, to investigate whether patients using the DM-specific PHR would be more likely to have subsequent management changes for glycemic, blood pressure, and LDL-C control compared with patients using the health maintenance and family history PHR. An episode of care was defined as the next PCP clinic visit and/or any associated patient letters, telephone calls, or e-mails.
Randomization
Because this was a system-level intervention that involved patients, their physicians, and the clinical support staff at each practice, we randomized at the practice level. Practices were grouped in 4 mutually exclusive strata (women's health practices, low-income urban practices, large suburban practices, and smaller suburban practices), and practices within each independent stratum were randomly assigned to 1 of 2 study arms (for 16 possible randomization combinations). All primary and other clinical outcomes were collected directly from the electronic clinical data repository, and these data were obtained solely as part of usual clinical care.
Statistical Analysis
Baseline and follow-up clinical variables were compared between study arms using χ2 tests for categorical variables and t tests for normally distributed continuous variables. Results of our primary outcome analyses were not significantly different after adjusting for imbalanced baseline variables in multivariate models (data not shown). Patients newly diagnosed as having DM during the course of the study were not included in any analyses. Two-sided P values <.05 were considered significant. We used SAS statistical software (version 9.0; SAS Inc, Cary, North Carolina) for all analyses.
Results
Participant Flow
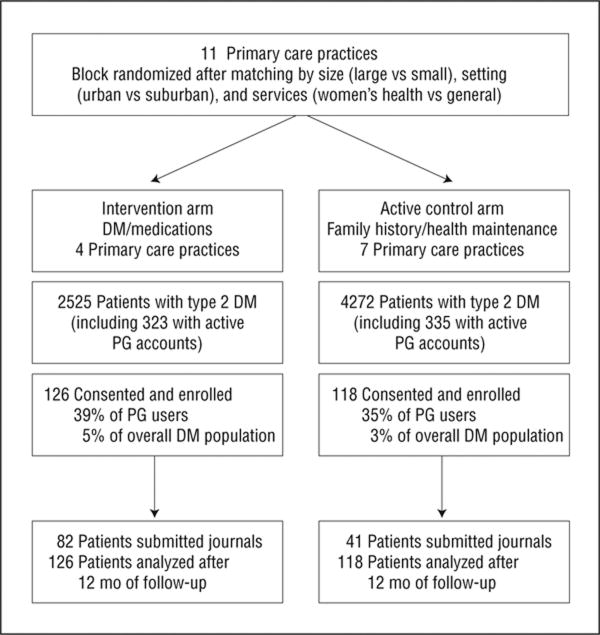
Figure 1 illustrates the number of primary care practices randomized and the flow of patients with DM in each study arm. Use of the parent PG secure Web portal by patients with DM ranged from 7% to 14% of each practice population. Among patients with active PG accounts, the rate of consent to enroll in the advanced patient portal clinical trial was 39% in the DM PHR intervention arm and 35% in the family history and health maintenance PHR active control arm. Practices were enrolled beginning September 30, 2005, and follow-up ascertainment was completed when the study was formally closed March 22, 2007.
Figure 1.
Flowchart of a clinic-randomized trial comparing type 2 diabetes mellitus (DM) management among patients receiving access to a DM and medications personal health record (intervention arm) vs a family history and health maintenance personal health record (active control arm). Eligible participants had DM and an active Patient Gateway (PG) account for secure Internet access within the health network.
Baseline Data
The Table presents baseline data for all patients with DM cared for in the 11 study practices. Patients with DM who enrolled in the Prepare for Care study were younger (mean age, 56.1 years vs 60.3 years; P<.001), and a greater proportion were white (89% vs 67%; P<.001), commercially insured (72% vs 47%; P< .001), and at or below their HbA1c goal (54% vs 47%; baseline HbA1c level, <7.0%; P=.04) compared with nonparticipating subjects. Study participants in the 2 treatment arms had moderate demographic differences but had similar health care utilization and rates of glycemic, blood pressure, and LDL-C control at baseline (Table).
Table. Baseline Characteristics Comparing Study Participants vs Nonparticipants and Comparing Study Participants by Study Arm.
| Characteristic | All Patients (n=6797)a | Study Participants (n=244) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Study Participants | Nonparticipants | P Valueb | Intervention Arm | Controls | P Valuec | |
| Patients with type 2 DM, No. | 244 | 6553 | 126 | 118 | ||
| Age, mean (SD), y | 56.1 (11.6) | 60.3 (14.8) | <.001 | 58.8 (10.1) | 53.3 (12.3) | <.001 |
| Women, % | 49 | 54 | .16 | 43 | 56 | .04 |
| Nonwhite race or ethnicity, % | 12 | 33 | <.001 | 7 | 16 | .04 |
| Insurance status, % | ||||||
| Private insurance | 72 | 47 | <.001 | 67 | 77 | .002 |
| Medicare | 25 | 39 | 33 | 16 | ||
| Medicaid or free care | 3 | 14 | 0 | 7 | ||
| Neighborhood income, $d | 53 784 | 49 713 | <.001 | 54 950 | 52 529 | .06 |
| HbA1c level, mean (SD), % | 7.3 (1.5) | 7.5 (1.6) | .14 | 7.3 (1.5) | 7.4 (1.6) | .69 |
| At goal (<7.0), % | 54 | 47 | .04 | 60 | 55 | .42 |
| LDL-C level, mean (SD), mg/dL | 83.8 (29) | 88.7 (33) | .09 | 81.4 (27) | 86.7 (31) | .33 |
| <100 mg/dL, % | 74 | 69 | .24 | 73 | 68 | .48 |
| BP, mean (SD), mm Hg | 126 (13)/75 (8) | 129 (16)/74 (10) | .08/.17 | 127 (14)/74 (9) | 126 (13)/76 (9) | .71 |
| <130/80 mm Hg, % | 48 | 46 | .50 | 51 | 47 | .49 |
| PCP visits during prior year, mean (SD), No. | 2.6 (2.9) | 2.7 (2.9) | .67 | 2.6 (2.1) | 2.7 (3.1) | .82 |
Abbreviations: BP, blood pressure; DM, diabetes mellitus; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; PCP, primary care physician.
SI conversion factors: To convert LDL cholesterol to millimoles per liter, multiply by 0.0259; to convert HbA1c to a proportion of total hemoglobin, multiply by 0.01.
“Study participants” were defined as patients with type 2 DM with registered online hospital accounts who consented to participate in the study. “Nonparticipants” were defined as all other patients with type 2 DM receiving primary care at one of the study sites.
P values compare all study participants (n=244) with all nonparticipants (n =6553).
P values compare the intervention arm (n=126 patients) with controls (n=118 patients).
Neighborhood income was based on federal tax returns by patients' zip code.
Comparison of active account users between study arms
Study participants had relatively good glycemic control at baseline with modest improvement over the study period that did not differ by treatment arm (0.16% vs 0.26% decline in HbA1c levels between arms; P=.62). Patients in the intervention arm (n=126) and controls (n=118) had similar mean HbA1c levels after 1 year of follow-up (7.1% vs 7.2%; P=.45), with nearly three-quarters of all patients at goal (73% vs 68% among control patients; P=.53). A similar pattern of good baseline control and statistically similar improvement over time in both study arms was also seen for both blood pressure and LDL-C control (data not shown).
When we limited our analyses to patients with HbAlc level greater than 7.0% at baseline, patients in the intervention arm were more likely to reach HbA1c goal at study end compared with controls (45% vs 25%, 79 patients with available data; P=.07). Although patients with baseline elevations of LDL-C level or blood pressure in the intervention arm also tended to do better relative to controls, the differences were small (data not shown).
Analysis of treatment intensification
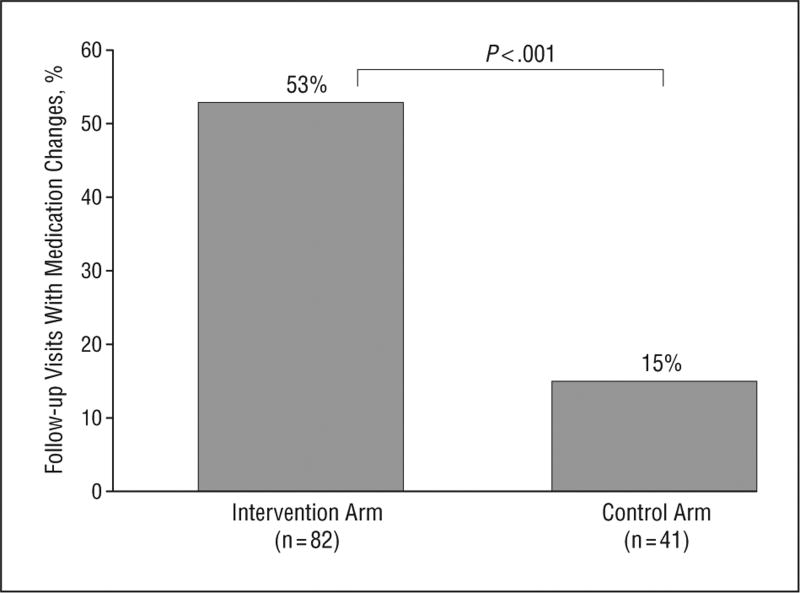
One-half of patients in the intervention arm (51%) who completed a DM PHR Care Plan prior to an upcoming visit indicated in their care plans that they wished to improve their blood glucose control; 32%, blood pressure control; and 28%, LDL-C control. These patient comments were reflected in the higher overall rate of medication intensification compared with control patients, with over half of subsequent care episodes in the intervention arm resulting in DM-related medication changes (53% vs 15% among controls submitting PHR journals; P<.001; Figure 2). Patients who submitted DM PHR Care Plans (intervention arm, n = 82) were more likely to have a medication initiation or dosage adjustment for hyperglycemia (29% vs 15%; P=.10), hypertension (13% vs 0%; P=.02), or hyperlip-idemia (11% vs 0%; P=.03) during the subsequent episode of care compared with the patients with DM who submitted family history and health maintenance journals (active control arm, n=41).
Figure 2.
Proportion of follow-up visits with diabetes mellitus–related medication changes among patients who submitted personal health record journals to their physician's electronic medical record (“on-treatment analysis” comparing intervention and control arms).
Although there were too few patients for meaningful comparisons by risk factor within the “on treatment” subset, in an exploratory analysis we found that patients with medication changes had clinically and statistically significant subsequent decreases in HbA1c level (mean [SD] decrease, 0.57%[1.0%]; paired t test, P=.009) and LDL-C level (32.1[31.9] mg/dL; P=.02) but not blood pressure (decrease of 0.6/5.2 mm Hg; P=.90). (To convert LDL-C to millimoles per liter, multiply by 0.0259.)
Comment
In this report, we present the results of a randomized controlled trial of a Web-based patient PHR linked directly to the physician EMR and conducted within the general primary care setting. We found that users of the DM-specific PHR were markedly more likely to have their medical regimens changed at their next clinic visit relative to patients with DM who used the non-DM PHRs. This finding suggests that, when used, our intervention may have worked to improve the process of DM care by reducing barriers to medication change at the clinic visit.
The lack of an overall impact on DM-related risk factor levels, however, can be attributed to 2 major barriers: (1) Despite the large, multipractice population covered by the study, the power to detect differences was reduced because only a small proportion of potentially eligible patients signed up for access to the parent PG secure Web portal. (2) Patients with poor metabolic control were less likely to enroll in the Prepare for Care study. We believe these limitations hold important lessons for future efforts to broadly implement Web-based strategies for changing DM care.
Effective translation of new innovations into improved DM care remains a difficult challenge for current research efforts.28 The results of our study underscore a number of critical points for future work in this area. First, evaluating the impact of new technologies and new strategies for care requires a rigorous study design. Our use of an active control study design provided the advantage of allowing us to compare 2 groups of patients who were equally inclined to enroll in a clinical trial of Web-based PHR interventions. Thus, the comparison of primary outcomes between “active account” study arms reflects the impact of the DM PHR on clinical care among patients equally inclined to engage in online PHR clinical interactions (eliminating “confounding by participation,” a methodological shortcoming in which the true effect of a patient-oriented DM intervention is difficult to isolate from its mode of delivery).
Second, because we used our network's existing online access system and linked our intervention directly to the EMR used for all patient care within our network, all patients with DM within the 11 primary care practices could theoretically have participated in the study. Although the low rates of enrollment limit the interpretation of the “real world” effect of our intervention, our results do clearly demonstrate that many patients with DM chose not to sign up for these services when presented the opportunity to engage in online access to their PCPs. Understanding this lack of enthusiasm becomes a crucial question that must be answered if we are to fully achieve the potential benefit of online PHRs.
Third, our exploratory analysis of medication changes among PHR users revealed that patients using the DM PHR were much more likely to have significant medication changes at the next clinical visit leading to corresponding declines in risk factor levels (P< .001). These clinically significant declines in DM-related risk factor levels provide evidence that medication initiation or dosage adjustment represents a valid intermediate measure for effective DM management and also support to the idea that DM-specific patient portals linked directly to physicians' EMRs can have an important impact on reducing clinical inertia.29,30
Our study participants were younger, less likely to belong to a racial or ethnic minority group, and less likely to live in a poor neighborhood compared with nonparticipants, evidence that the digital divide remains an important barrier to the adoption of new health information technologies. Moreover, barriers to engaging in new health informatics tools may exist even among patients with existing Internet access. A recent survey conducted within our network found that nearly 50% of patients with type 2 DM currently use the Internet.31 Given that patients had markedly higher levels of general online use than PG use (52% used the Internet vs 10% PG account registration in study practices), further research is needed to identify and overcome barriers to adoption of patient health portals beyond the physical availability of an Internet connection.
The current generation of informatics tools have had only a limited and inconsistent impact on improving DM care.32-34 The DM-specific patient portal we developed included an innovative patient interface that presented key clinical information and individualized patient decision support, grouped results, and current medications within each risk factor domain (hyperglycemia, hypertension, and hyperlipidemia), and facilitated the development of a care plan that was automatically shared with the PCP as a patient-authored note in the medical record. We believe that this organizational format created a necessary first step toward the ideal patient DM portal. Missing from this intervention, however, were methods to (1) easily upload clinical information from home (eg, blood glucose level, blood pressure, weight, and exercise activities from wireless monitors) and (2) effectively integrate these data with the clinical workflow.
There are several limitations of our study to consider. Because of the novel and potentially disruptive impact on usual care of inviting patients to provide nonvisit care plan updates to their physicians, in this initial study we sought to minimize the amount of additional work required of PCPs. Prior surveys had documented physician concerns and resistance to any interventions that increased demands on their already limited time.35 Thus, we did not undertake any formal training of patients and physicians with regard to creating and acting on the care plans, and we did not seek to significantly change the ways in which PCPs currently practiced. In addition, we did not collect patient measures such as DM knowledge, level of engagement in care, or confidence with patient-physician communication that may have provided further insight into the effect of our intervention on study participants. Moreover, given the lack of impact on key clinical outcomes, we did not undertake a formal cost analysis of the program as currently designed. Finally, our study was not designed to test the marginal effect of different components of the DM PHR (eg, medications review, decision support for blood test results, creation of a DM care plan).
In summary, we adapted an existing clinical informatics system that included a password-protected patient portal to conduct a rigorous, population-level controlled trial of a novel “first generation” DM tool designed to engage and activate primary care patients toward achieving goals of care. Our findings suggest that the close link between the PHR and the patients' physicians' EMRs may have facilitated the process of medication initiation and dosage adjustment. However, despite enrolling 11 primary care practices, our study was limited by a “ceiling effect” among participants and ultimately underpowered to show differences between study consenters in the intervention vs control study arms. Success of future Web-based patient portals will require broader patient enrollment and may also benefit from significant “redesign” of current clinical practice to more effectively engage both physicians and patients (particularly older and low-income patients) in collaborative, non–visit-based care.36
Acknowledgments
Funding/Support: This study was supported in part by a grant from the Agency for Healthcare Research and Quality (AHRQ R01 HS013660-02: Shared Online Health Records for Patient Safety and Care). Dr Grant is also supported by a National Institute of Diabetes and Digestive and Kidney Diseases Career Development Award (K23 DK067452).
Footnotes
Financial Disclosure: None reported.
Trial Registration: clinicaltrials.gov Identifier: NCT00251875
Author Contributions: Study concept and design: Grant, Wald, Schnipper, Gandhi, Poon, Volk, and Middleton. Acquisition of data: Grant, Williams, Volk, and Middleton. Analysis and interpretation of data: Grant, Gandhi, Poon, Orav, Volk, and Middleton. Drafting of the manuscript: Grant, Williams, and Middleton. Critical revision of the manuscript for important intellectual content: Wald, Schnipper, Gandhi, Poon, Orav, and Volk. Statistical analysis: Grant and Orav. Obtained funding: Schnipper, Gandhi, Poon, and Middleton. Administrative, technical, and material support: Poon, Williams, Volk, and Middleton. Study supervision: Grant, Volk, and Middleton.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 3.Curb JD, Pressel SL, Cutler JA, et al. Systolic Hypertension in the Elderly Program Cooperative Research Group Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276(23):1886–1892. [PubMed] [Google Scholar]
- 4.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a subgroup analysis of the Scandinavian Simva-statin Survival Study (4S) Diabetes Care. 1997;20(4):614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Vedel P, Larsen N, Jensen GVH, Parving HH, Pedersen O. Multifacto-rial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 6.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 7.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KM. A diabetes report card for the United States: quality of care in the 1990's. Ann Intern Med. 2002;136(8):565–574. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine Crossing the Quality Chasm: A New Health System for the 21st Century Health Care Services. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 9.Heisler M, Piette JD, Spencer M, Kieffer E, Vijan S. The relationship between knowledge of recent HbA1c values and diabetes care understanding and self-management. Diabetes Care. 2005;28(4):816–822. doi: 10.2337/diacare.28.4.816. [DOI] [PubMed] [Google Scholar]
- 10.Berikai P, Meyer PM, Kazlauskaite R, Savoy B, Kozik K, Fogelfeld L. Gain in patients' knowledge of diabetes management targets is associated with better glycemic control. Diabetes Care. 2007;30(6):1587–1589. doi: 10.2337/dc06-2026. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield S, Kaplan SH, Ware JE, Jr, et al. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–457. doi: 10.1007/BF02595921. [DOI] [PubMed] [Google Scholar]
- 12.Rachmani R, Levi Z, Slavachevski I, Avin M, Ravid M. Teaching patients to monitor their risk factors retards the progression of vascular complications in highrisk patients with type 2 diabetes mellitus: a randomized prospective study. Dia-bet Med. 2002;19(5):385–392. doi: 10.1046/j.1464-5491.2002.00701.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM, Funnell MM, Butler PM, Arnold MS, Fitzgerald JT, Feste CC. Patient empowerment: results of a randomized controlled trial. Diabetes Care. 1995;18(7):943–949. doi: 10.2337/diacare.18.7.943. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CC, Starren J. Patient participation in electronic medical records. JAMA. 2001;285(13):1765. [PubMed] [Google Scholar]
- 15.Tang PC, Ash JS, Bates DW, Overhage JM, Sands DZ. Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc. 2006;13(2):121–126. doi: 10.1197/jamia.M2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masys D, Baker D, Butros A, Cowles KE. Giving patients access to their medical records via the Internet: the PCASSO experience. J Am Med Inform Assoc. 2002;9(2):181–191. doi: 10.1197/jamia.M1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner RC, Waivers LE, O'Brien K. The effect of patient-carried reminder cards on the performance of health maintenance measures. Arch Intern Med. 1990;150(3):645–647. [PubMed] [Google Scholar]
- 18.Dickey LL, Petitti D. A patient-held minirecord to promote adult preventive care. J Fam Pract. 1992;34(4):457–463. [PubMed] [Google Scholar]
- 19.Levetan CS, Dawn KR, Robbins DC, Ratner RE. Impact of computer-generated personalized goals on HbA1c. Diabetes Care. 2002;25(1):2–8. doi: 10.2337/diacare.25.1.2. [DOI] [PubMed] [Google Scholar]
- 20.Cho JH, Chang S, Kwon H, et al. Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability. Diabetes Care. 2006;29(12):2625–2631. doi: 10.2337/dc05-2371. [DOI] [PubMed] [Google Scholar]
- 21.Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasgow RE, Boles SM, McKay HG, Feil EG, Barrera M., Jr The D-Net diabetes self-management program: long-term implementation, outcomes, and generalization results. Prev Med. 2003;36(4):410–419. doi: 10.1016/s0091-7435(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 23.McMahon GT, Gomes HE, Hickson Hohne S, Hu TM, Levine BA, Conlin PR. Web-based care management in patients with poorly controlled diabetes. Diabetes Care. 2005;28(7):1624–1629. doi: 10.2337/diacare.28.7.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant RW, Hamrick HE, Sullivan CM, Cagliero E, Meigs JB. Impact of population management with direct physician feedback on care of patients with type 2 diabetes. Diabetes Care. 2003;26(8):2275–2280. doi: 10.2337/diacare.26.8.2275. [DOI] [PubMed] [Google Scholar]
- 25.Wald JS, Middleton B, Bloom A, et al. A patient-controlled journal for an electronic medical record: issues and challenges. Medinfo. 2004;11(pt 2):1166–1170. [PubMed] [Google Scholar]
- 26.Grant RW, Wald JS, Poon EG, et al. Design and implementation of a Web-based patient portal linked to an ambulatory care electronic health record: patient gateway for diabetes collaborative care. Diabetes Technol Ther. 2006;8(5):576–586. doi: 10.1089/dia.2006.8.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang BL, Bakken S, Brown SS, et al. Bridging the digital divide: reaching vulnerable populations. J Am Med Inform Assoc. 2004;11(6):448–457. doi: 10.1197/jamia.M1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garfield SA, Malozowski S, Chin MH, et al. Diabetes Mellitus Interagency Coordinating Committee (DIMCC) Translation Conference Working Group Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26(9):2670–2674. doi: 10.2337/diacare.26.9.2670. [DOI] [PubMed] [Google Scholar]
- 29.Grant RW, Buse JB, Meigs JB. Quality of diabetes care in US academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28(2):337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlin JB, Pogach LM. Improving the outcomes of metabolic conditions: managing momentum to overcome clinical inertia. Ann Intern Med. 2006;144(7):525–527. doi: 10.7326/0003-4819-144-7-200604040-00012. [DOI] [PubMed] [Google Scholar]
- 31.Watson AJ, Bell AG, Kvedar JC, Grant RW. Re-evaluating the digital divide: current lack of Internet use is not a barrier to adoption of novel health information technology. Diabetes Care. 2008;31(3):433–435. doi: 10.2337/dc07-1667. [DOI] [PubMed] [Google Scholar]
- 32.Jackson CL, Bolen S, Brancati FL, Batts-Turner ML, Gary TL. A systematic review of interactive computer-assisted technology in diabetes care. J Gen Intern Med. 2006;21(2):105–110. doi: 10.1111/j.1525-1497.2005.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linder JA, Ma J, Bates DW, Middleton B, Stafford RS. Electronic health record use and the quality of ambulatory care in the United States. Arch Intern Med. 2007;167(13):1400–1405. doi: 10.1001/archinte.167.13.1400. [DOI] [PubMed] [Google Scholar]
- 34.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 35.Kittler AF, Carlson GL, Harris C, et al. Primary care physician attitudes towards using a secure Web-based portal designed to facilitate electronic communication with patients. Inform Prim Care. 2004;12(3):129–138. doi: 10.14236/jhi.v12i3.118. [DOI] [PubMed] [Google Scholar]
- 36.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]