Abstract
Advances in genetic epidemiology have increased understanding of common, polygenic preventable diseases such as type 2 diabetes. As genetic risk testing based on this knowledge moves into clinical practice, we propose that genetic counselors will need to expand their roles and adapt traditional counseling techniques for this new patient set. In this paper, we present a genetic counseling intervention developed for a clinical trial [Genetic Counseling/Lifestyle Change for Diabetes Prevention, ClinicalTrials.gov identifier: NCT01034319] designed to motivate behavioral changes for diabetes prevention. Seventy-two phenotypically high-risk participants received counseling that included their diabetes genetic risk score, general education about diabetes risk factors, and encouragement to participate in a diabetes prevention program. Using two validated genetic counseling scales, participants reported favorable perceived control and satisfaction with the counseling session. Our intervention represents one model for applying traditional genetic counseling principles to risk testing for polygenetic, preventable diseases, such as type 2 diabetes.
Keywords: Genetic counseling, Type 2 diabetes, Polygenic risk counseling, Diabetes prevention
Introduction
In 2005 the National Society of Genetic Counselors (NSGC) defined genetic counseling as “the process of helping people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease.” The goals of genetic counseling as articulated by the NSGC are to integrate the interpretation of genetic test results with medical and family history, to provide education and management information for genetic disease, and to promote informed patient choices (NSGC 2005). While upholding these principles, the everyday practice of genetic counseling also requires continuous adaptation to the new challenges presented by our rapidly expanding knowledge of genetics.
One such area of tremendous advancement is in our understanding of the genetic contributions to common polygenic diseases such as type 2 diabetes (T2D). Despite knowing there is a significant genetic basis for T2D (with heritable factors estimated as high as 40%) (Kaprio et al. 1992), genetic counseling in clinical practice is rarely offered to patients at increased risk for T2D because individual genetic locus analysis currently provides only modest information about heritable risk. Now though, new approaches to assess T2D genetic risk that aggregate multiple risk loci into a single T2D risk score (de Miguel-Yanes et al. 2011; Hivert et al. 2011) are advancing methods to predict diabetes genetic risk. Although the predictive ability of current approaches is modest at best, rapid advances in the field raise questions regarding how to effectively translate “personalized” diabetes genetic risk information to the clinic and primary care settings.
Limited information exists whether genetic risk information can motivate individuals to adopt healthier behaviors and so far, results have been mixed. One study demonstrated short-term increased motivation to quit smoking in subjects who received genetic test results related to lung cancer risk but failed to demonstrate long-term impact (Sanderson et al. 2008a). Other studies focused on diabetes genetic risk testing have found that individuals already at risk for T2D report interest in receiving individualized genetic risk information and predict such testing would motivate them to prevent diabetes (Grant et al. 2009; Markowitz, et al. 2011). However, the question remains unanswered whether personalized genetic risk information can synergize with existing phenotypic risk factors and an effective lifestyle modification program to alter behavior.
To examine this potential impact of genetic risk information, the Genetic Counseling/Lifestyle Change for Diabetes Prevention Study (GC/LC Study) (ClinicalTrials.gov identifier: NCT01034319) is underway. Specifically, the GC/LC Study aims to test whether the additional knowledge of one’s T2D genetic risk information, a risk factor not under individual control, can motivate diabetes prevention behaviors. Should genetic information provide extra incentive, either by increasing a sense of risk or by reframing existing information about risk, then subjects at high phenotypic risk for T2D may be more likely to adhere to an effective lifestyle modification diabetes prevention program (Crandall et al. 2008). In developing our intervention, we relied on evidence from studies of risk communication in genetic counseling. For example, patients receiving breast and ovarian cancer counseling preferred to receive their risk in quantitative, rather than qualitative, terms (Hallowell et al. 1997). Similarly, we drew from several literature reviews on how risk information is perceived and interpreted (Austin 2010; Sivell et al. 2008). Despite the presence of risk counseling literature, there are currently no published examples of polygenic counseling interventions designed to motivate long-term behavior change among phenotypically at-risk individuals (Scheuner et al. 2008).
In this report we describe a framework for developing the diabetes genetic risk counseling intervention created for the GC/LC Study and also provide some initial feedback from study participants based on their experience with this counseling intervention. Although the ultimate success of our intervention will be demonstrated by the outcomes of the GC/LC Study, the information we report here introduces an approach that can be expanded to other multifactorial conditions beyond T2D. As genetic counselors become increasingly involved in the care of these individuals, new models for genetic counseling will need to be developed for this role. We conclude by describing some of the potential implications of the counseling model described here.
Methods
The GC/LC Study was designed to test the hypothesis that diabetes genetic risk information could motivate at-risk patients to adopt healthy lifestyle changes for diabetes prevention. Additional details on the GC/LC Study design are provided elsewhere (Grant et al. 2009). Participants were at increased risk for developing T2D, based on phenotypically-assessed diabetes risk (overweight and with at least 3 of 5 elements of the Metabolic Syndrome: abnormal fasting glucose, waist circumference, HDL, triglycerides, and blood pressure) (Ford et al. 2008). Eligible patients were identified through a hospital-based research registry and contacted through primary care offices (Hivert et al. 2009). From this pool of potentially eligible participants, 12% were successfully enrolled into the GC/LC Study. Subjects allocated to diabetes genetic risk testing whose results placed them at either “higher” or “lower” diabetes genetic risk (see details below) received the diabetes genetic risk counseling intervention. By design of the GC/LC Study, participants with “average” diabetes genetic risk results did not receive counseling. This study was approved by the Massachusetts General Hospital Institutional Review Board.
Calculating the T2D Relative Genetic Risk Score
Over three dozen genetic loci, present at >5% allelic frequency, have been identified and validated as independent T2D risk factors (de Miguel-Yanes et al. 2011). Our group and others have implemented the approach of aggregating the small individual risks defined by each T2D-associated single nucleotide polymorphism (SNP) into a single overall diabetes genetic risk score (de Miguel-Yanes et al. 2011; Meigs et al. 2008).
For the GC/LC Study, each intervention subject received a single overall diabetes genetic risk score based on his or her 36-SNP profile. The scores (ranging from 0 – 72 based on the 36 total alleles in the genetic testing panel) allowed categorization of participants into “higher” (top quartile), “average” (middle two quartiles), and “lower” (bottom quartile) genetic risk for developing diabetes. Individuals falling in the “average” risk category had 34–38 higher risk alleles.
Diabetes risk for the “higher” and “lower” quartiles (relative to the middle quartiles) was calculated using data from the Framingham Offspring Study. This highly-phenotyped epidemiologic research cohort is both demographically and geographically similar to participants in our study. It included 3,471 genotyped individuals followed for a mean of 8.2 (± 1.0) years in whom 446 cases of diabetes were diagnosed. Among those in the “higher” genetic risk category (genotype risk scores >38), 17.9% developed diabetes while only 9.9% of those with a “lower” genetic risk score (genotype risk scores <34) developed diabetes. In the “average” genetic risk group, 12.2% developed diabetes. This demonstrated a 46% relative increase for the “higher” risk group and an 18% relative decrease from the “lower” risk group when compared to those with “average” risk scores.
The genotype risk scores were then modified by the existing phenotypic risk for all participants: their diagnosis of Metabolic Syndrome. Prior work by our group has shown that patients in our system with Metabolic Syndrome have an 11% absolute risk of developing diabetes over the next three-years. Using the relative risks derived from the Framingham cohort, an estimate of post-test absolute risk could be derived from the baseline phenotypic risk. For example, a subject falling into the “higher” genetic risk category would have a 46% greater relative risk of developing diabetes compared to someone in the “average” risk category so their 11% baseline risk would be adjusted (11% * 1.46) to yield an estimated absolute risk for diabetes of 17%.
Framework for the Genetic Counseling Intervention
To communicate diabetes genetic risk results, we focused on traditional goals of genetic counseling: conveying risk information and helping individuals understand that risk. Based on support from the literature, we aimed to present the risk information in a way that reflected Leventhal’s Common Sense Model of Illness perception (Sivell et al. 2008), with emphasis on risk perception and self-efficacy. Additionally, one important aspect of developing our intervention was the need to address the potential impact of phenotypically high-risk participants receiving a result that may decrease their motivation. Our goal therefore was to develop a message that was tailored to the test result and in either the case of a “higher risk” or “lower risk” result, emphasized motivation. Lastly, we chose a short, 15-min time length for the counseling session to maximize clinical applicability to the large primary care population estimated to be at increased T2D risk (Centers for Disease Control and Prevention 2011). All sessions were one-on-one and the participants were free to ask questions.
Visual Aid Development
Visual aids to convey the primary messages of our genetic counseling intervention were developed after reviewing published approaches on the presentation of relative and absolute risk and on the development of counseling aids (Sivell et al. 2008; Drake et al. 1999; Baty et al. 1997). We intentionally chose to use each group’s absolute risk for the figures, rather than relative risk, for two reasons. First, absolute risk is often easier for individuals to understand but also, we wished to avoid participants misunderstanding what seemed to be a larger number and thereby creating false impressions regarding the magnitude and potential impact of the genetic risk. The aids were then refined following feedback from phenotypically high-risk individuals not participating in the GC/LC Study (Markowitz et al. 2011).
Measurements for Assessing the Genetic Counseling Model
To determine if the counseling message was well-received and whether participants reported an increased sense of control over their health, we administered two validated surveys after the completion of each counseling session: 1) the Perceived Personal Control (PPC) Questionnaire (Smets et al. 2006) and 2) the Genetic Counseling Satisfaction Survey (GCSS)(DeMarco et al. 2004). The PPC measures the patient’s sense of cognitive and behavioral control, while the GCSS allows the patient to evaluate counselor knowledge and understanding, as well as the overall value of the session (Kasparian et al. 2007).
Results
The GC/LC Study Genetic Counseling Intervention
The genetic counseling intervention that we developed and implemented addressed three core topics: genetic education (consisting of review of basic genetic principles and discussion of the subject’s personal diabetes genetic risk score); diabetes modifiable risk factor education; and a motivational message intended to help participants use their diabetes genetic risk information as a stimulus to adopt diabetes lifestyle prevention behaviors.
The content of genetics and diabetes education was essentially the same for all recipients, while the motivational message was tailored based on whether the participant received a “higher” or “lower” diabetes genetic risk result (Fig. 1). Specifically, the motivational message had 2 elements: the first element was invariant in that all participants were taught the link between modifiable risk factors (e.g. weight, diet, exercise) and diabetes prevention. The second element of the message was determined by the subject’s genetic risk score. For subjects with “higher” diabetes genetic risk results, the increased genetic risk was presented as a motivational tool to adopt these behavioral changes. Conversely, for subjects with “lower” diabetes genetic risk results, the greater relative influence of modifiable environmental factors was emphasized.
Fig. 1.
The genetic counseling intervention. The counseling intervention developed for the GC/LC Study included both an educational component and a motivational message. The motivational message consisted of two parts: an invariant message emphasizing lifestyle change and a second message tailored to the individual’s genetic risk result
Visual Materials and Counseling Messages
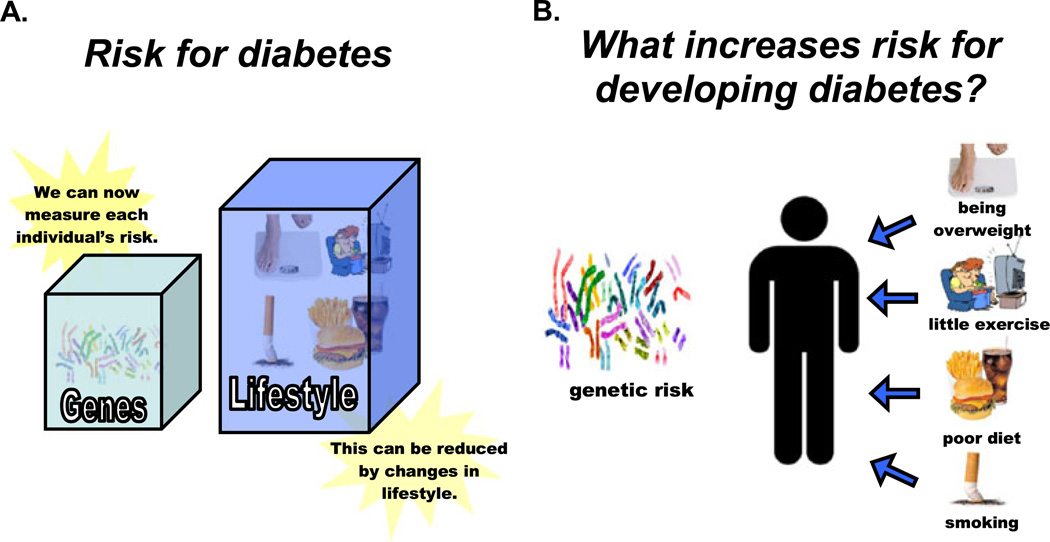
After an initial explanation of basic genetic principles (Figure not shown), study participants viewed a pair of figures (Figs. 2a and b) specific to diabetes counseling that depicted the contribution of both genetic and modifiable environmental factors to development of T2D. Figure 2a showed a simplified schematic of the relative proportions of each type of risk, while Fig. 2b visually emphasized a separation of risks into those under the participant’s control (environmental) versus those not under their control (genetics).
Fig. 2.
Counseling figures depicting environmental & genetic risk factors for T2D. This set of pictures illustrated that both genetic and environmental factors contributes to T2D risk. Panel 2a depicted the relative proportions of genetic and lifestyle risk, while Panel 2b demonstrated distinctions between risks under individual control versus those not under individual control
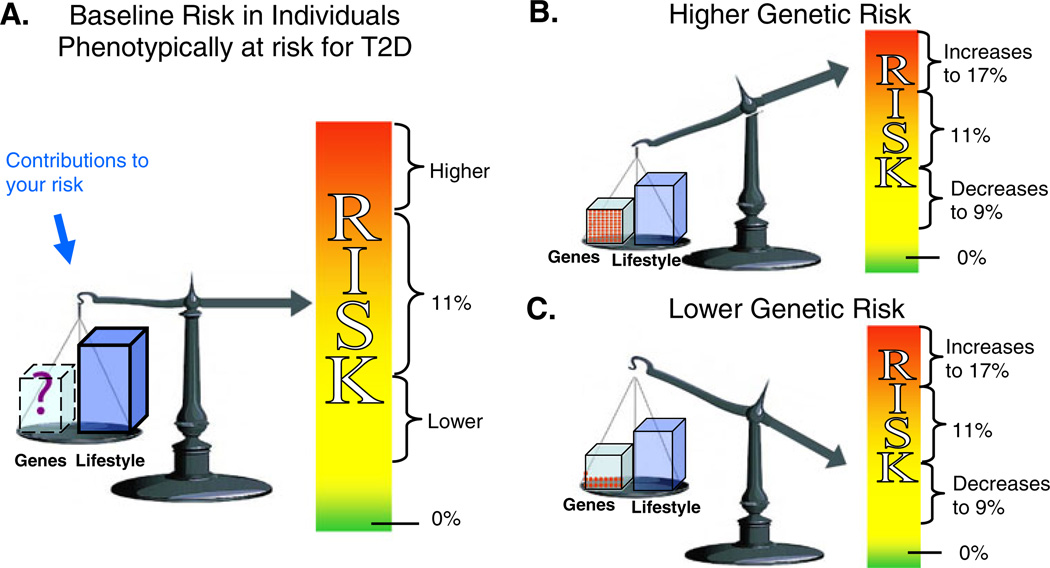
The third and final set of visual aids (Fig. 3) allowed the participants to compare pre- and post-genetic test diabetes risk numbers. Subjects whose genotype score placed them in the “higher” diabetes genetic risk group viewed Fig. 3a then b; Fig. 3b depicted an increased personal genetic risk using features such as an up-pointing arrow directed at the more urgent (the red) end of the color scale. To minimize a sense of genetic fatalism, these participants were also provided a message that emphasized the individual’s ability to reduce his or her overall risk by modifying behavioral risk factors. Alternatively, participants whose haplotype placed them in the “lower” diabetes genetic risk group viewed Fig. 3a and then Fig. 3c, and saw a down-pointing arrow indicating a decreased (but not eliminated) contribution from individual genetic risk. To emphasize the residual risk, the “lower risk” arrow remained in the mid-risk range of the color scale (yellow) instead of in the minimal or no risk range (green). The additional counseling message for this group stressed the importance of factors under personal control (such as diet and exercise) in an effort to reduce a sense of genetic invulnerability that could have been created by receiving a “lower” diabetes genetic risk score.
Fig. 3.
Risk for developing diabetes in the next 3 years. This set of figures compared a baseline risk for T2D (a) with a risk adjusted according to the individual’s genetic test result (b and c). We counseled an adjusted risk of 17% (b) for those subjects in the “higher genetic risk” group, while a 9% risk (c) was provided for those subjects in the “lower genetic risk” group
The GC/LC Study Participants
The genetic counseling intervention recipients (n = 72) included both men and women and represented a broad range of income levels (Table 1). The cohort was predominately white, most had some college education, and 55.6% had a family history of T2D. At baseline (prior to diabetes genetic risk testing), nearly two-thirds (65.8%) of participants felt they had a moderate to high risk of developing diabetes in 5 years. This proportion declined to 22.3% when participants were asked to estimate their 5-year diabetes risk if they adopted healthy lifestyle changes, suggesting that study participants as a group were amenable to the concept of behavior change for diabetes prevention.
Table 1.
Description of GC/LC Study participants. Numbers are provided for the entire group as well as broken down by genetic test result
| Total, n = 72 | High Risk, n = 40 | Low Risk, n = 32 | |
|---|---|---|---|
| Men, n (%) | 41 (56.9) | 22 (55.0) | 19 (59.4) |
| Age, mean (SD) | 58.1 (10.8) | 56.1 (9.1) | 60.8 (12.3) |
| White, Non-Hispanic, n (%) | 60 (83.3) | 30 (75.0) | 30 (93.8) |
| Education, n (%) | |||
| 12th Grade or GED | 17 (23.6) | 7 (17.5) | 10 (31.3) |
| 1–3 Years of College | 19 (26.4) | 10 (25.0) | 9 (28.1) |
| 4 or More Years of College | 36 (50.0) | 23 (57.5) | 13 (40.6) |
| Employed (Full or Part-Time), n (%) | 48 (66.7) | 28 (70.0) | 20 (62.5) |
| Income, n (%) | |||
| Less than $50,000 | 20 (27.8) | 10 (25.0) | 10 (31.3) |
| $50,000–$100,000 | 21 (29.2) | 9 (22.5) | 12 (37.5) |
| More than $100,000 | 28 (38.9) | 20 (50.0) | 8 (25) |
| Not Reported | 3 (4.2) | 1 (2.5) | 2 (6.3) |
| Family History of DM, n (%) | 40 (55.6) | 21 (52.5) | 19 (59.4) |
| Satisfaction w/Genetic Counseling Survey, n (%) completely agree with all 5 questions | 33 (45.8) | 20 (50.0) | 13 (40.6) |
| Genetic Counseling Satisfaction Scale, n (%) agree strongly to 5 or 6 questions | 31(43.1) | 15 (37.5) | 16 (50.0) |
Participant Evaluations of the Counseling Intervention
Participants reported high levels of support for the genetic counseling framework (Table 1). Subjects reported high perceived personal control (mean PPC score: 1.78, scale 0–2) and satisfaction with the genetic counseling sessions (mean GCSS score: 4.32, scale 1–5) with no difference between groups based on a t-test of the two means (PPC p = 0.73; GCSS p = 0.70). The average length of the counseling sessions was 10 min 8 s, SD = 3 min, 3 s. This average length was not statistically different between “higher” and “lower” diabetes genetic risk groups based on a t-test of the two means.
Discussion
Type 2 diabetes (T2D) is a common, multi-factorial chronic disease with both genetic and environmental determinants. Recent identification of multiple genetic variants that modify risk for T2D has enabled the creation of personalized diabetes genetic risk scores for individuals without diagnosed diabetes. Results from such testing are becoming increasingly available to the general public via direct-to-consumer advertising despite little precedent for how such results should be conveyed or how they might be received (Foster et al. 2008; O’Daniel et al. 2010; Sanderson et al. 2008b).
As part of the GC/LC clinical trial, we developed and implemented a model for sharing personalized genetic risk information with individuals at risk for a common polygenic disorder. Our approach sought to capitalize on the potential that providing genetic risk information might augment the impact of a proven lifestyle change program. Our highly structured and focused design sought to educate the subject about multifactorial inheritance (Figs. 1 and 2), provide general and individualized diabetes risk counseling (Figs. 2 and 3), and, finally, provide a motivational message all while minimizing possible negative impact of genetic testing for T2D. Our intervention was packaged as a concise counseling session, thereby, making it more feasible to broadly incorporate into primary care or behavioral programs. Although additional evaluation is needed, particularly in a realistic clinical setting, results from the PPC and GCSS provide initial support for our counseling framework. Study participants expressed high levels of satisfaction and high levels of perceived control, two important (although not sole) measures of the appropriateness of a genetic counseling session.
The intervention described herein offers specific suggestions for applying traditional genetic counseling to a common disorder by utilizing an approach based on a polygenetic testing panel offering both specific clinical recommendations and encouraging a healthy lifestyle. Although the elements of this approach are not entirely new to genetic counseling, the combination makes our model appropriate to apply to emerging genetic counseling situations like T2D.
Many genetic counseling roles today include helping individuals make decisions after receiving a genetic test result for which there is no single medical course of action, such as the identification of Down syndrome prenatally or a breast cancer mutation. Still, genetic counselors have always encountered scenarios, like diet recommendations for an individual with phenylketonuria (PKU), where medical standards of care exist and directive counseling is appropriate (Resnik 2003; Weil 2003; Weil et al. 2006). Based on our own experience working with T2D, in combination with the existence of accepted medical therapies, we believe that T2D is now another in a steadily growing number of situations (e.g. inborn errors of metabolism, alcohol consumption during pregnancy, pharmcogenetics) where it is appropriate to provide clear recommendations for subsequent medical care.
Further justification for providing such specific recommendations can be derived from the circumstances of our particular counseling scenario. First, the genetic test result provided to participants in our study has a limited predictive value. Accordingly, it was particularly imperative that participants did not interpret the result in a way that would actually cause more harm. This was made even more important by the fact that all of our participants had a high a priori risk for T2D and would benefit from a lifestyle change regardless of the genetic test result. We therefore, were particularly cautious about the need to avoid the extreme mindsets related to feelings of fatalism or invulnerability. Experience from other conditions has shown that a high-risk genetic profile can instill feelings of resignation in an individual, thereby reducing any motivation to change or prevent the onset of the condition (Bennett et al. 2008; Markowitz et al. 2011; Marteau and Lerman 2001). Conversely, individuals receiving a lower risk result may view this as protective and be less motivated to adopt significant lifestyle changes (Markowitz et al. 2011; Marteau and Lerman 2001). Encouragingly, there is evidence that providing the appropriately balanced message can pre-empt or minimize both extremes. Specifically, for those at lower genetic risk, the message emphasized personal control despite the presence of both environmental and genetic risk factors, while for those at higher genetic risk, the message focused on the potential to prevent T2D by lifestyle modification regardless of their genetic risk (Gamm et al. 2004). To that end, our highly specific message was actually necessary to avoid harm to the participants.
The second aspect of our intervention used the genetic test result as a tool to help motivate participants and therefore incorporated elements more common to motivational counseling. Although genetic counseling theory and motivational counseling theory have the same roots (Hettema et al. 2005; Weil 2000), genetic counselors do not typically attempt to increase or direct motivation. Our model uses the educational aspects of the genetic counseling as a “teachable moment” to encourage behavior change.
Although current studies disagree over the impact of genetic information on behavior (Marteau et al. 2010), there are unique qualities to the GC/LC Study that increase the possibility of changing behavior. First, all of our participants are already at increased phenotypic risk and thus have an a priori increased level of motivation, which as Markowitz et al. (2011) have shown, may increase the impact of genetic risk information. Second, the diabetes genetic risk counseling in our intervention has been coupled with a proven, structured curriculum that provides participants with the means to make the lifestyle changes required for preventing diabetes.
The role of genetic risk information in the management of common disorders, such as T2D, awaits definitive evidence that such information can provide an effective adjunct to alter behavior and promote health lifestyle changes. To this end, several research studies, in addition to the GC/LC Study, are currently underway to assess the clinical impact of diabetes genetic risk testing on patient behavior (Clinicaltrials.gov: NCT01034319; NCT00849563; NCT01060540; NCT01186354).
As advances in genetic risk assessment for common chronic diseases continue, the model developed for our study will likely become increasingly applicable. While the current knowledge of genetic risk prediction for T2D is limited and only offers small to modest predictive information, future studies are likely to uncover additional specific genetic factors contributing to T2D’s overall 40% heritability estimate. Accordingly, this will enable an at-risk individual to receive a more accurate, and possibly even more predictive, risk score. We anticipate future improvements and adaptations will be made to the visual aid materials so that they more precisely represent the contribution of genetic and environmental factors. These improvements are likely help eliminate any potential misconceptions caused by the oversimplified representation we used for genetic and environmental factors that does not take into consideration the genetic components for which testing is or is not available. In the meantime, it will be necessary for the expertise of the counselor, the “give and take” during the one-on-one counseling session, and the actual content of the session to provide this insight.
The specific approach we developed and applied to T2D highlights opportunities for new applications of the everyday practice of genetic counseling. This framework mirrors preventative care models which allow for education and motivation techniques to be incorporated into treatment plans, and it introduces a role for genetic counselors along side other health care providers such as primary care providers, dieticians, or diabetes educators. The flexibility and brevity of this model are further strengths when considering the limited number of genetic counselors relative to number of individuals potentially eligible for “personalized” genetic risk testing.
O’Daniel (2010) has already emphasized the need and opportunity for genetic counseling to branch out into the field of genome-guided preventive medicine and argues that genetic counselors should be actively involved in the development of efforts to incorporate genomic information into primary care practice. Our approach provides one example of the practicalities of integrating genetic counseling, one that could be applied not only by genetic counselors but also by other health care providers.
As this field moves forward, some consideration needs to be given to how the traditional genetic counseling framework will be applied to common diseases such as T2D, coronary disease, and emphysema. These developments provide the opportunity to explore new applications of genetic counseling and ways of broadening roles for genetic counselors. Capitalizing on the potential ability to prevent or ameliorate disease by motivating patient behavior change by coupling genetic risk counseling with a lifestyle change program, we offer in this report a concrete example of one such genetic counseling framework.
Acknowledgements
This work was funded by the National Institutes of Health (NIDDK R21 DK84527). Dr. Meigs is supported by NIDDK K24 DK080140.
Contributor Information
Jessica L. Waxler, Department of Pediatrics, Massachusetts General Hospital, Boston, MA, USA 175 Cambridge St, Room 502, Boston, MA 02114, USA, jwaxler@partners.org.
Kelsey E. O’Brien, Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA
Linda M. Delahanty, Massachusetts General Hospital Diabetes Center, Massachusetts General Hospital, Boston, MA, USA Harvard Medical School, Boston, MA, USA.
James B. Meigs, Division of General Medicine, Massachusetts General Hospital, Boston, MA, USA Harvard Medical School, Boston, MA, USA.
Jose C. Florez, Massachusetts General Hospital Diabetes Center, Massachusetts General Hospital, Boston, MA, USA Center for Human Genetic Research, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Elyse R. Park, Mongan Institute for Health Policy, Massachusetts General Hospital, Boston, MA, USA Harvard Medical School, Boston, MA, USA.
Barbara R. Pober, Department of Pediatrics, Massachusetts General Hospital, Boston, MA, USA Harvard Medical School, Boston, MA, USA.
Richard W. Grant, Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA
References
- Austin J. Reconceptualizing risk in genetic counseling: Implications for clinical practice. The Journal of Genetic Counseling. 2010;19(3):228–234. doi: 10.1007/s10897-010-9279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty B, Venne V, McDonald J, Croyle R, Halls C, Nash J, et al. BRCA1 testing: Genetic counseling protocol development and counseling issues. The Journal of Genetic Counseling. 1997;6(2):223–244. doi: 10.1023/A:1025620404473. [DOI] [PubMed] [Google Scholar]
- Bennett P, Wilkinson C, Turner J, Brain K, Edwards RT, Griffith G, et al. Psychological factors associated with emotional responses to receiving genetic risk information. Journal of Genetic Counseling. 2008;17:234–241. doi: 10.1007/s10897-007-9136-x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Atlanta Georgia: U.S. Department of Health and Human services, Centers for Disease Control and Prevention; 2011. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. [Google Scholar]
- Crandall JP, Knowler WC, Kahn SE, Marrero D, Florez JC, Bray GA, et al. The prevention of type 2 diabetes. Nature Clinical Practice: Endocrine & Metabolism. 2008;4(7):382–393. doi: 10.1038/ncpendmet0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel-Yanes JM, Shrader P, Pencina MJ, Fox CS, Manning AK, Grant RW, et al. Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care. 2011;34(1):121–125. doi: 10.2337/dc10-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco TA, Peshkin BN, Mars BD, Tercyak KP. Patient satisfaction with cancer genetic counseling: a psychometric analysis of the genetic counseling satisfaction scale. Journal of Genetic Counseling. 2004;13(4):293–304. doi: 10.1023/b:jogc.0000035523.96133.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake E, Engler-Todd L, O’Connor A, Surh L, Hunter A. Development and evaluation of a decision aid about prenatal testing for women of advanced maternal age. The Journal of Genetic Counseling. 1999;8(4):217–233. doi: 10.1023/A:1022998415890. [DOI] [PubMed] [Google Scholar]
- Ford E, Li C, Sattar N. Metabolic syndrome and incident diabetes. Diabetes Care. 2008;31(9):1898–1904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MW, Mulvihill JJ, Sharp RR. Evaluating the utility of personal genomic information. Genetics in Medicine. 2008;11(8):570–574. doi: 10.1097/GIM.0b013e3181a2743e. [DOI] [PubMed] [Google Scholar]
- Gamm JL, Nussbaum RL, Biesecker BB. Genetics and alcoholism among at-risk relatives I: Perceptions of cause, risk, and control. American Journal of Medical Genetics Part A. 2004;128:133–150. doi: 10.1002/ajmg.a.30082. [DOI] [PubMed] [Google Scholar]
- Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: A patient and physician survey. Diabetologia. 2009;52(11):2299–2305. doi: 10.1007/s00125-009-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallowell N, Statham H, Murton F, Green J, Richards M. “Talking about chance”: The presentation of risk information during genetic counseling for breast and ovarian cancer. The Journal of Genetic Counseling. 1997;6(3):269–286. doi: 10.1023/A:1025624221369. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller MR. Motivational interveiwing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Hivert MF, Grant RW, Shrader P, Meigs JB. Identifying primary care patients at risk for future diabetes and cardiovascular disease using electronic health records. BioMed Central Health Services Research. 2009;9:170. doi: 10.1186/1472-6963-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hivert MF, Jablonski KA, Perreault L, Saxena R, McAteer JB, Franks PW, et al. An updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the Diabetes Prevention Program. Diabetes. 2011;60(4):1340–1348. doi: 10.2337/db10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K, Reunanen A, Eriksson J, et al. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35(11):1060–1067. doi: 10.1007/BF02221682. [DOI] [PubMed] [Google Scholar]
- Kasparian NA, Wakefield CE, Meiser B. Assessment of psychosocial outcomes in genetic counseling research: An overview of available measurement scales. Journal of Genetic Counseling. 2007;16:693–712. doi: 10.1007/s10897-007-9111-6. [DOI] [PubMed] [Google Scholar]
- Markowitz SM, Park ER, Delahanty LM, O’Brien KE, Grant RW. Perceived impact of diabetes genetic risk testing among patients at high phenotypic risk for type 2 diabetes. Diabetes Care. 2011;34(3):568–573. doi: 10.2337/dc10-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Lerman C. Genetic risk and behavioral change. British Medical Journal. 2001;322:1056–1059. doi: 10.1136/bmj.322.7293.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviors (review) Cochrane Database of Systemic Reviews. 2010;10:1–77. doi: 10.1002/14651858.CD007275.pub2. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. New England Journal of Medicine. 2008;359(21):2208–2219. doi: 10.1056/NEJMoa0804742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Society of Genetic Counselors. Definition/FAQ’s. [Retrieved 5/27/2011];2005 from http://www.nsgc.org/About/FAQsDefinitions/tabid/97/Default.aspx.
- O’Daniel JM. The prospect of genome-guided preventive medicine: a need and opportunity for genetic counselors. Journal of Genetic Counseling. 2010;19(4):315–327. doi: 10.1007/s10897-010-9302-4. [DOI] [PubMed] [Google Scholar]
- O’Daniel JM, Haga SB, Willard HF. Considerations for the impact of personal genome information: A study of genomic profiling among genetics and genomics professionals. Journal of Genetic Counseling. 2010;19(4):387–401. doi: 10.1007/s10897-010-9297-x. [DOI] [PubMed] [Google Scholar]
- Resnik DB. Genetic testing and primary care: a new ethic for a new setting. New Genetics and Society. 2003;22(3):245–256. doi: 10.1080/1463677032000147207. [DOI] [PubMed] [Google Scholar]
- Sanderson SC, Humphries SE, Hubbart C, Hughes E, Jarvis MJ, Wardle J. Psychological and behavioural impact of genetic testing smokers for lung cancer risk: A phase II exploratory trial. Journal of Health Psychology. 2008a;13(4):481–494. doi: 10.1177/1359105308088519. [DOI] [PubMed] [Google Scholar]
- Sanderson SC, Wardle J, Humphries SE. Public health genomics and genetic test evaluation: The challenge of conducting behavioral research on the utility of lifestyle-genetic tests. Journal of Nutrigenetics and Nutrigenomics. 2008b;1:223–231. doi: 10.1159/000149826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner MT, Sieverding P, Shekell PG. Delivery of genomic medicine for common chronic adult diseases. A systemic review. Journal of the American Medical Association. 2008;299(11):1320–1334. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- Sivell S, Elwyn G, Gaff CL, Clarke AJ, Iredale R, Shaw C, et al. How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: Systemic review. The Journal of Genetic Counseling. 2008;17(1):30–63. doi: 10.1007/s10897-007-9132-1. [DOI] [PubMed] [Google Scholar]
- Smets EM, Pieterse AH, Aalfs CM, Ausems MG, van Dulmen AM. The Perceived Personal Control (PPC) Questionnaire as an outcome of genetic counseling: Reliability and validity of the instrument. American Journal of Medical Genetics Part A. 2006;140:843–850. doi: 10.1002/ajmg.a.31185. [DOI] [PubMed] [Google Scholar]
- Weil J. Psychosocial genetic counseling. New York, NY: Oxford University Press, Inc; 2000. [Google Scholar]
- Weil J. Psychosocial genetic counseling in the post-nondirective era: A point of view. Journal of Genetic Counseling. 2003;12(3):199–211. doi: 10.1023/a:1023234802124. [DOI] [PubMed] [Google Scholar]
- Weil J, Ormond K, Peters J, Peters K, Biesecker BB, LeRoy B. The relationship of nondirectiveness to genetic counseling: Report of a workshop at the 2003 NSGC Annual Education Conference. Journal of Genetic Counseling. 2006;15(2):85–93. doi: 10.1007/s10897-005-9008-1. [DOI] [PubMed] [Google Scholar]