Abstract
Background
Informatics tools may help support hypertension management.
Objective
To design, implement and evaluate a web-based system for patient anti-hypertensive medication self-titration.
Methods
Study stages included: six focus groups (50 patients) to identify barriers/facilitators to patient medication self-titration, software design informed by qualitative analysis of focus group responses and a six-month single-arm pilot study (20 patients) to assess implementation feasibility.
Results
Focus groups emphasised patient need to feel confident that their own primary care providers were directly involved and approved of the titration protocol. Physicians required 3.3 ± 2.8 minutes/patient to create individualised six-step medication pathways for once-monthly blood pressure evaluations. Pilot participants (mean age of 51.5 ± 11 years, 45% women, mean baseline blood pressure 139/84 ± 12.2/7.5 mmHg) had 5 medication increases, 2 non-adherence self-reports, 52 months not requiring medication changes, 24 skipped months and 17 months with no evaluations due to technical issues. Four pilot patients dropped out before study completion. From baseline to study completion, blood pressure decreased among the 16 patients remaining in the study (8.0/4.7 mmHg, p = 0.03 for both systolic and diastolic pressures).
Conclusions
Lessons learned included the benefit of qualitative patient analysis prior to system development and the feasibility of physicians designing individual treatment pathways. Any potential clinical benefits were offset by technical problems, the tendency for patients to skip their monthly self-evaluations and drop outs. To be more widely adopted such systems must effectively generalise to a wider range of patients and be integrated into clinical workflow.
Keywords: blood pressure, home monitoring, hypertension, medication adjustment, medication safety, self-management
Introduction
Almost one-third of US adults have been diagnosed with hypertension.1 Despite the availability of a spectrum of potent medications, 63% of the US hypertensive population remains suboptimally controlled.2 Poor blood pressure control, in turn, has been shown to increase the risk for myocardial infarction, renal disease, stroke and premature death.3 The failure to satisfactorily address hypertension management within our current healthcare system requires the development of novel care strategies.
To date, most interventions to transform care have focused primarily on clinicians and clinical practice systems and have had only marginal benefit.4–7 The patient, however, is increasingly recognised as the most important member of the healthcare team.8–10 Prior research has demonstrated that increasing patients’ involvement in their care improves control of chronic diseases such as hypertension and diabetes.11–14 A relatively untested innovation in chronic disease management is to expand the patient’s role in the medication titration process.
There are few published trials of medication self-titration for blood pressure control15–17 and limited experience in implementing such approaches using a health IT infrastructure. We hypothesised that enabling patients to implement a predefined hypertension medication treatment pathway designed by their primary care physician (PCP) would result in more timely treatment changes and more effective blood pressure control. In this report, we describe the design, implementation and feasibility evaluation of the Medication Self-Titration and Evaluation Program (Med-STEP) for blood pressure control.
Methods
Conceptual framework for the Med-STEP system
A review of the published literature indicated two prevalent barriers to optimal hypertension management: (1) clinical inertia (the observation that medication changes frequently are not made during clinic visits despite elevated blood pressure levels),18–21 and (2) lack of patient engagement with treatment plans.22–24 To address these two barriers, we conceived of a system in which: (1) a sequence of medication changes (medication treatment pathway) prespecified by the patient’s PCP could be followed independent of clinic visits; and (2) patients directed their own medication management through home blood pressure self-monitoring, health IT-supported monthly evaluation of blood pressure results and medication self-titration based on PCP-defined medication treatment pathways.
Based on this conceptual framework, we created the Med-STEP intervention. The development process had three sequential phases: (1) focus group discussions with patients to identify both perceived benefits and concerns related to the self-titration of chronic disease medications; (2) development of the Med-STEP web-based interface linked to an existing system of home blood pressure electronic data collection; and (3) piloting the system in a single primary care practice to assess its feasibility prior to wider implementation.
Patient focus groups
Six 90-minute patient focus groups were conducted from March 2008 to May 2008. Patients diagnosed with both diabetes and hypertension were recruited from the primary care practices of the Massachusetts General Hospital Practice-based Research Network, Boston, MA.25 All patients were currently taking hypertension and/or diabetes-related medicines. After eliciting their views regarding their experiences with starting and adjusting medications over time, participants were asked their views about adjusting their own medical regimens. To help convey the concept, we showed participants a paper-based example of a medication treatment pathway for a hypothetical patient depicting a sequence of potential future medication changes over time (Figure 1). Group interviews were recorded digitally and transcribed for qualitative content analysis by three coders using NVivo 7.0 software (SdG Associates, London, UK). The moderator reviewed the transcripts for accuracy. All participants received a $40 stipend.
Figure 1.
Paper hand-out used in focus group discussions to explain medication self-titration concept
Med-STEP system development
Our health system has an existing informatics platform to help manage home blood pressure readings (Blood Pressure Connect). Components of this platform include: automated data upload and central storage from home blood pressure cuffs, a patient web-based interface that graphically represents blood pressure trends, a provider web-based interface that lists patients enrolled in the Blood Pressure Connected health programme and allows review of individual patient results, and a secure messaging system for patients and providers.
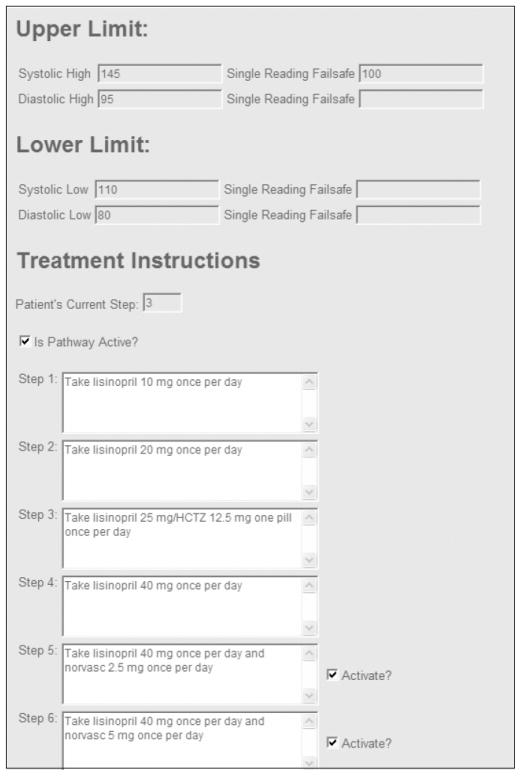
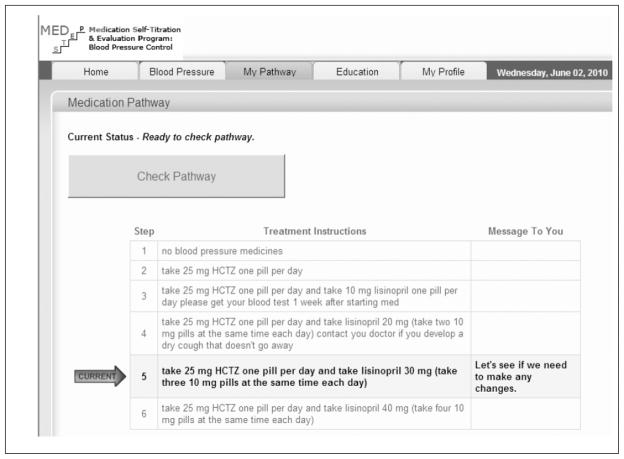
With this platform as our starting point, we added two novel components: a stand-alone medication treatment pathway entry form to input the PCP-defined treatment algorithms (blood pressure thresholds, medication steps, and any corresponding laboratory safety monitoring tests if required, see Figure 2); and a new ‘my treatment pathway’ page within the existing patient Blood Pressure Connect web application to guide patients through the process of blood pressure medication adjustment over time (see Figure 3). As described in the Results, development of these two new components was informed by the results of the focus group analysis.
Figure 2.
Screenshot of the ’medication treatment pathway’ algorithm entry form used to record PCP-planned medication changes
Figure 3.
Screenshot of ’my treatment pathway’ page to guide patients through the process of blood pressure medication adjustment over time
Pilot clinical study
To assess the feasibility of the Med-STEP intervention, we collaborated with a primary care practice within our hospital network that expressed interest in interventions to address quality of hypertension management. The five PCPs in this practice all endorsed the Med-STEP approach and referred suitable patients to our study staff. In addition to PCP referrals, we also used available electronic registry data to identify other potentially eligible patients. Patient eligibility criteria included: age > 18 years, elevated blood pressure (> 140/90 mmHg when last measured, or on treatment and referred by PCP), prescribed 0 or 1 blood pressure medications, access to an Internet connection and a compatible analogue telephone land line and willingness to adjust own medicines. Patients participating in the original focus group sessions were not included in the pilot trial.
The study coordinator met eligible subjects at the practice to explain the Med-STEP intervention and obtain written informed consent. At this research visit, subjects also completed a baseline survey designed to assess their views about hypertension management.
Once a study participant had successfully logged-on to the website from home and had uploaded at least one blood pressure reading, a medication treatment pathway was defined by the subject’s PCP. The principal investigator collected the following data from the PCP to be entered into the medication treatment pathway algorithm entry form (Figure 2): the minimum number of home blood pressure readings required before a change would be considered; PCP-designated blood pressure thresholds for increasing or decreasing the regimen; and a sequence of four to six medication changes (and any corresponding laboratory testing) that this patient would follow. These pathways all began with ‘Step 1’ (no medicines) and progressed in single management increments (e.g. dose adjustment or addition of new medicine) up to ‘Step 6.’ Each patient had a unique pathway and could be enrolled in the study starting at any step.
During the first week for each calendar month of the six-month clinical trial, patients used a home blood pressure monitoring device to automatically upload blood pressure readings. Based on the PCP-defined algorithm, the web-based user interface advised patients to increase, decrease or remain at their current medication treatment pathway step. If a medication change was advised, a prescription was manually sent to the pharmacy, the medical record was manually updated, and the research coordinator followed up with the patient. Pilot trial participants received a $75 stipend. All phases of the study were approved by the Massachusetts General Hospital Institutional Review Board.
Results
Patient focus groups and software development
Most focus group participants had a positive impression of the concept underlying the Med-STEP intervention. The following major themes emerged from the discussions as benefits of medication self-titration.
Awareness: Participants reported that knowing the sequence of planned medication steps was very appealing because it would inform them of their disease process (e.g. ‘…you know whether you’re on the right track or not’) and could reduce anxiety (e.g. ‘…it eliminates the … anxiety you have when the doctor suddenly announces something to you’).
Engagement: Several participants suggested that the programme would make them feel more responsible for their hypertension management. One man stated, ‘It’s becoming participatory medicine’. Other similar comments included: the programme would ‘help me to take more responsibility for myself, to take better care of myself ’ and ‘We are, after all, all managers of our own health issues…. And this gives us the chance to do that.’
Motivation: Many participants expected that the programme would ultimately lead to better disease control through increased patient motivation. As one participant declared, the programme would provide ‘…incentive to try to get down to that Step 1.’
Convenience: Another participant suggested that, since patients would make medication adjustments between appointments with their physicians, the programme would eliminate some delay in care between visits: he noted, ‘Because at times, one is waiting for the doctor to help…’; One person said, ‘I would like if it… eliminated two out of four appointments.’
Because of these patient-reported benefits, we were encouraged to proceed with building the Med-STEP system. However, despite the many positive comments, participants voiced five important concerns that were key to informing the development process. Table 1 lists the five major concerns (detrimental effect on doctor–patient relationship, variability of individual blood pressure readings, difficulty in correctly following a pathway, concern about medication side effects and the effort required to participate) and the corresponding design features we implemented to address the concern. One major issue raised by participants was the concern that enrolling in a medication self-management programme could interfere with the current relationship the patients enjoyed with their PCPs. An innovative feature of our design in response to this concern was the implementation of individualised medication treatment pathways that were authored by each patient’s PCP rather than using a single, external treatment algorithm.
Table 1.
Focus group concerns and corresponding design features to address these concerns
| Concern | Design features |
|---|---|
| Detrimental effect on doctor–patient relationship |
|
| Variability in blood pressure readings |
|
| Correctly following pathway |
|
| Medication side effects |
|
| Too much effort/doesn’t want responsibility |
|
The Med-STEP pilot study
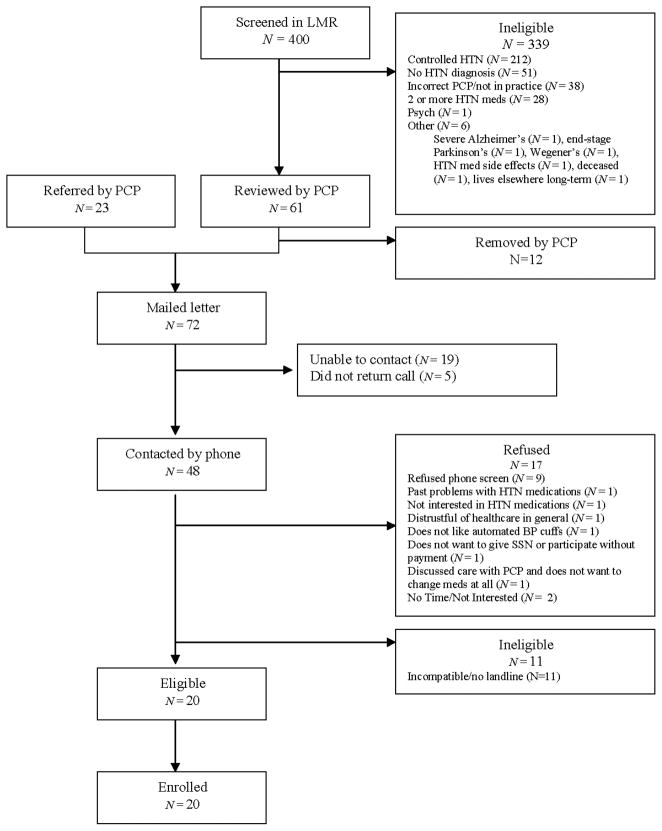
Pilot study participants were recruited both by PCP referral and by direct invitation (see Figure 4 for patient flow diagram). Of 48 patients successfully contacted by phone, 20 (42%) consented to participate (mean age 51.5 years, 45% women, 20% non-white ethnicity; Table 2). Participants were diagnosed with hypertension for a mean of 6.0 ±4.3 years, and 17 (85%) were on medicines prior to enrolling in the pilot. The two factors from the baseline survey that patients most often cited as motivating their desire to improve their hypertension management were ‘to avoid future medical problems’ (all 20 subjects rated this ‘very important’) and ‘to live a longer and healthier life’ (19 subjects rated this ‘very important’).
Figure 4.
Pilot clinical trial enrolment flow diagram. BP, blood pressure; HTN, hypertension; SSN, social security number
Table 2.
Characteristics of patients participating in the Med-STEP pilot study (n = 20)
| Characteristic | |
|---|---|
| Women, n (%) | 9 (45) |
| Age, years ± SD | 51.5 ± 11 |
| Non-white ethnicity, n (%) | 4 (20) |
| Employed, n (%) | 15 (75) |
| College educated, n (%) | 15 (75) |
| Household annual income > $100,000, n (%) | 4 (25) |
| Duration of hypertension ± SD (years) | 6.0 ± 4.3 |
| Baseline blood pressure ± SD (mmHg) | 139/84 ± 12.2/7.5 |
| Currently prescribed antihypertensive medicines, n (%) | 17 (85) |
| Baseline survey responses, n (%): | |
| Days taking blood pressure medicines in prior week | 6.9 ± 0.3 days |
| Confidence in being able to control blood pressure | 5 (25) ‘very confident’; 11 (55) ‘confident’ |
| Importance of improving blood pressure | 18 (90) ‘very important’; 2(10) ‘important’ |
| Generally feel confident in using new technology | 13 (65) ‘strongly agree’; 7 (35) ‘agree’ |
| Like to be involved in making decisions about medical treatment | 13 (65) ‘strongly agree’; (30) ‘agree’ |
| Believe it is primarily the patients responsibility to look after hypertension | 10 (50) ‘strongly agree’; 9 (45) ‘agree’ |
| Prefer to leave decisions about hypertension treatment to provider | 1 (5) ‘strongly agree’; 6 (30) ‘agree’ |
Medication treatment pathways were obtained from the five PCPs managing these 20 study subjects. Excluding the first ‘training’ pathway, PCPs required 3.3 ± 2.8 minutes to designate six-step medication pathways for each patient. These pathways were very patient specific, with 19 different sequences created for the 20 subjects.
Med-STEP pilot results
The 20 participants provided 100 patient-months of data for the Med-STEP algorithm (four patients withdrew before completing all six months). Patients successfully evaluated their medication treatment pathways for 59 study months, resulting in five increased medication dose recommendations (for three patients), two patient self-reports of non-adherence, and 52 readings that did not require medication changes. Pathways were not evaluated for 41 patient-months either because patients chose not to access the system (24 months) or due to system technical issues (17 months). Common technical problems included: digital voicemail interference with uploading blood pressures (n = 5), faulty modems (n = 4) and web password problems (n = 4). Of interest, for the three patients with recommendations to ‘step-up’ in their medication treatment pathway, all three had successful regimen intensification when instructed the first time, but neither of the two who were instructed to step-up the second time had successful regimen changes (one subject declined to increase her dose again and the other subject did not obtain the laboratory testing required prior to making the change).
There was a significant decrease in blood pressure among the 16 participants who completed the study (systolic blood pressure declined by 8.0 mmHg, p = 0.03; diastolic blood pressure declined by 4.7 mmHg, p = 0.025). The three subjects with medication increases had an average blood pressure decline of 8.7/5.7 mmHg.
End-of-study surveys were collected from 18 participants. Most of these patients (16/18) agreed that the system was useful to help them manage their hypertension and 17/18 would recommend the system to others, although several participants reported their frustration with technical issues.
Discussion
We designed a web-based system linking home blood pressure monitoring to PCP-defined medication treatment pathways with the goal of improving blood pressure control. In a six-month pilot study, we found that implementation of the system was feasible but was limited by technical issues, patient reluctance to make changes or self-monitor and relatively low enrolment rates.
Although we showed the system to be feasible for blood pressure management, there were several key lessons learned that we believe are crucial for subsequent efforts to generalise this model of care:
Patient–PCP connection
In response to concerns raised by many of our focus group participants, we designed our programme to strengthen rather than obscure the PCPs role. To achieve this goal, we opted to have PCPs design their patients’ medication treatment pathways rather than rely on published algorithms. We found that PCPs were able to quickly and efficiently design unique pathways for each patient. This process also increased PCP and patient confidence in the programme. Pilot participants confirmed the importance of their PCP knowing that changes were being made. Our results suggest that this strategy may have advantages over using predefined treatment algorithms.
Limitations of the ‘patient engagement’ model
It has become widely accepted that increasing patient engagement with their care can improve clinical outcomes.27,28 While our study does not disprove this principle, we found that several subjects were reluctant to make changes even when indicated by their PCP-designed medication treatment pathways. This result suggests that even among consented study participants, many patients with asymptomatic diseases conveying relatively minor short-term clinical risks may not feel comfortable making repeated medication changes over a relatively short time. It must be noted that patients in our pilot study were not directly involved in the design of their medication pathways. Thus, while pilot participants had an active role in their hypertension management, the Med-STEP pilot did not represent a truly collaborative, shared decision-making model of care as recommended by the chronic care model. Future research is needed into the potential benefits (and increased time requirements) of a shared decision-making approach.
Technology failings
Because of issues with connectivity and password access, several participants experienced repeated technical problems, which led to withdrawal from the programme. This is not a great insight, but it underscores the importance of implementing simple, high-fidelity health IT systems to avoid losing patient or provider buy-in.
Workflow
The primary care practice and associated PCPs were willing to participate in the study because the research team assumed responsibility for many of the tasks generated by the programme (e.g. programme enrolment and patient education, updating the clinic record when changes were made, ordering and following-up the results of safety labs triggered by medication changes, and sending new prescriptions to the patient’s pharmacy). While several of these functions could be automated in a more advanced version of the programme, the clinical benefit has to justify the model of non-visit-based care. With health payment reform, newer models of care (e.g. accountable care organisations) may be amenable to this programme.26 In addition, current care systems may lack the flexibility required for more collaborative care given the additional time that would be required to create medication pathways using a PCP-patient shared decision-making approach.
To date, there have been two small pilot studies of patient medication self-titration16,17 and one recent, large randomised trial.15 The earliest study randomised 31 hypertensive subjects to home blood pressure monitoring and medication self-titration using a paper-based algorithm.17 This initial pilot, conducted in 1997, reported modest blood pressure benefits but did not appear to be subsequently evaluated or adopted on a larger sale. A second pilot study conducted more recently in France focused on feasibility and safety.16 This short-term study (only eight weeks) combined home blood pressure monitoring, telemedicine contact with the research team and a single titration protocol for all participants. Patients on non-study medicines at study entry were converted to the study protocol. The authors report overall satisfaction with the programme by participants and decline from baseline in mean blood pressure by eight weeks.
Most recently, McManus et al reported the results of a large randomised trial (telemonitoring and self-management in the control of hypertension [TASMINH2]) conducted in the UK involving 24 primary care practices and 527 participants with hypertension (blood pressure > 140/90 mmHg despite anti-hypertensive treatment).15 As in our study, medication titration parameters were define by patients’ PCPs, although in TASMINH2 the treatment pathways were limited to two medication titrations over the 12-month study and changes were only made if blood pressure readings were elevated for two consecutive months. The protocol of medication adjustment was less aggressive than the monthly assessments in the Med-STEP study. Based on exit interviews from Med-STEP participants, we believe that fewer changes over a greater period of time (as with TASMINH2) may actually improve patient participation. Implementation of the TASMINH2 trial was resource intensive. Researchers invited 7637 patients to participate, and screened 1650 patients accepting the invitation to randomise 527 participants (of whom only 480 were ultimately included in the final analysis). Participants each received two intensive training sessions prior to enrolment to ensure competence in blood pressure measurement and data transfer via modem. Researchers also schedule visits at 6 and 12 months for data collection.
The results from our study and from TAMSINH2 and other pilot trials lead to several general conclusions and suggest important next steps. The data show that patients who can successfully self-titrate their blood pressure medications will likely have improved blood pressure control. Given the high prevalence and significant clinical impact of hypertension, efforts to implement this approach among patients with inadequately controlled blood pressure deserve strong consideration. As with many innovations that involve new technology and new patterns of care, however, translation of this concept into usual care will require addressing significant barriers to change such as current visit-based payment mechanisms, provider workflow and team composition,29 reliability of technology, and patient willingness to adopt a greater role in their disease management.
Acknowledgments
The authors wish to thank and acknowledge the efforts of the providers and staff at the MGH Back Bay Health Center.
FUNDING
This study was funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases (R03 DK080183) and by a Partners IS Research Grant award.
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing interests.
Contributor Information
Richard W Grant, Department of Medicine, Massachusetts General Hospital, Boston, USA.
Jennifer C Pandiscio, Job title?.
Hannah Pajolek, Mongan Institute for Health Policy, Massachusetts General Hospital, Boston, USA.
Alyssa Woulfe, Job title?.
Alexandra Pelletier, Partners Center for Connected Health, Boston, USA.
Joseph Kvedar, Partners Center for Connected Health, Boston and Harvard Medical School, Boston, USA.
Elyse R Park, Mongan Institute for Health Policy, Massachusetts General Hospital, Boston, and Harvard Medical School, Boston, USA.
References
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004 Oct;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007 Jan;49(1):69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 4.Phillips LS, Ziemer DC, Doyle JP, Barnes CS, Kolm P, Branch WT, et al. An endocrinologist-supported intervention aimed at providers improves diabetes management in a primary care site: improving primary care of African Americans with diabetes (IPCAAD) 7. Diabetes Care. 2005 Oct;28(10):2352–60. doi: 10.2337/diacare.28.10.2352. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, et al. Randomized effectiveness trial of a computer-assisted intervention to improve diabetes care. Diabetes Care. 2005 Jan;28(1):33–9. doi: 10.2337/diacare.28.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Jaspers MW, Smeulers M, Vermeulen H, Peute LW. Effects of clinical decision-support systems on practitioner performance and patient outcomes: a synthesis of high-quality systematic review findings. Journal of the American Medical Informatics Association. 2011 May 1;18(3):327–34. doi: 10.1136/amiajnl-2011-000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Annals of Internal Medicine. 2006 May 16;144(10):742–52. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 8.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Quarterly. 1996;74(4):511–44. [PubMed] [Google Scholar]
- 9.Davis K, Schoenbaum SC, Audet AM. A 2020 vision of patient-centered primary care. Journal of General Internal Medicine. 2005 Oct;20(10):953–7. doi: 10.1111/j.1525-1497.2005.0178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. Journal of the American Medical Association. 2002 Nov 20;288(19):2469–75. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 11.Rachmani R, Levi Z, Slavachevski I, Avin M, Ravid M. Teaching patients to monitor their risk factors retards the progression of vascular complications in high-risk patients with type 2 diabetes mellitus—a randomized prospective study. Diabetes Medicine. 2002 May;19(5):385–92. doi: 10.1046/j.1464-5491.2002.00701.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenfield S, Kaplan SH, Ware JE, Jr, Yano EM, Frank HJ. Patients’ participation in medical care: Effects on blood sugar control and quality of life in diabetes. Journal of General Internal Medicine. 1988 Sep-Oct;3(5):448–57. doi: 10.1007/BF02595921. [DOI] [PubMed] [Google Scholar]
- 13.Greenfield S, Kaplan S, Ware JE., Jr Expanding patient involvement in care. Effects on patient outcomes. Annals of Internal Medicine. 1985 Apr;102(4):520–8. doi: 10.7326/0003-4819-102-4-520. [DOI] [PubMed] [Google Scholar]
- 14.Glasgow RE, Funnell MM, Bonomi AE, Davis C, Beckham V, Wagner EH. Self-management aspects of the improving chronic illness care breakthrough series: implementation with diabetes and heart failure teams. Annals of Behavioral Medicine. 2002 Spring;24(2):80–7. doi: 10.1207/S15324796ABM2402_04. [DOI] [PubMed] [Google Scholar]
- 15.McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010 Jul 17;376(9736):163–72. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 16.Bobrie G, Postel-Vinay N, Delonca J, Corvol P SETHI Investigators. Self-measurement and self-titration in hypertension: a pilot telemedicine study. American Journal of Hypertension. 2007 Dec;20(12):1314–20. doi: 10.1016/j.amjhyper.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Zarnke KB, Feagan BG, Mahon JL, Feldman RD. A randomized study comparing a patient-directed hypertension management strategy with usual office-based care. American Journal of Hypertension. 1997 Jan;10(1):58–67. doi: 10.1016/s0895-7061(96)00305-6. [DOI] [PubMed] [Google Scholar]
- 18.Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, et al. Inadequate management of blood pressure in a hypertensive population. New England Journal of Medicine. 1998 Dec 31;339(27):1957–63. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 19.Grant RW, Cagliero E, Dubey AK, Gildesgame C, Chueh HC, Barry MJ, et al. Clinical inertia in the management of type 2 diabetes metabolic risk factors. Diabetes Medicine. 2004 Feb;21(2):150–5. doi: 10.1111/j.1464-5491.2004.01095.x. [DOI] [PubMed] [Google Scholar]
- 20.Grant RW, Buse JB, Meigs JB University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005 Feb;28(2):337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlin JB, Pogach LM. Improving the outcomes of metabolic conditions: Managing momentum to overcome clinical inertia. Annals of Internal Medicine. 2006 Apr 4;144(7):525–7. doi: 10.7326/0003-4819-144-7-200604040-00012. [DOI] [PubMed] [Google Scholar]
- 22.Marvel MK, Epstein RM, Flowers K, Beckman HB. Soliciting the patient’s agenda: Have we improved? Journal of the American Medical Association. 1999 Jan 20;281(3):283–7. doi: 10.1001/jama.281.3.283. [DOI] [PubMed] [Google Scholar]
- 23.Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? Journal of General Internal Medicine. 2003 Nov;18(11):893–902. doi: 10.1046/j.1525-1497.2003.21132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heisler M. Actively engaging patients in treatment decision making and monitoring as a strategy to improve hypertension outcomes in diabetes mellitus. Circulation. 2008 Mar 18;117(11):1355–7. doi: 10.1161/CIRCULATIONAHA.108.764514. [DOI] [PubMed] [Google Scholar]
- 25.Atlas SJ, Chang Y, Lasko TA, Chueh HC, Grant RW, Barry MJ. Is this ‘my’ patient? Development and validation of a predictive model to link patients to primary care providers. Journal of General Internal Medicine. 2006 Sep;21(9):973–8. doi: 10.1111/j.1525-1497.2006.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee PV, Berenson RA, Tooker J. Payment reform—the need to harmonize approaches in Medicare and the private sector. New England Journal of Medicine. 2010 Jan 7;362(1):3–5. doi: 10.1056/NEJMp0910459. [DOI] [PubMed] [Google Scholar]
- 27.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Social Science & Medicine. 2000 Oct;51(7):1087–110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 28.Bergeson SC, Dean JD. A systems approach to patient-centered care. Journal of the American Medical Association. 2006 Dec 20;296(23):2848–51. doi: 10.1001/jama.296.23.2848. [DOI] [PubMed] [Google Scholar]
- 29.Sciamanna CN, Marcus BH, Goldstein MG, Kipp L, Swartz S, Bock B, Graham AL, Ahern DK. Feasibility of incorporating computer-tailored health behaviour communications in primary care settings. Informatics in Primary Care. 2004;12(1):40–8. doi: 10.14236/jhi.v12i1.107. [DOI] [PubMed] [Google Scholar]