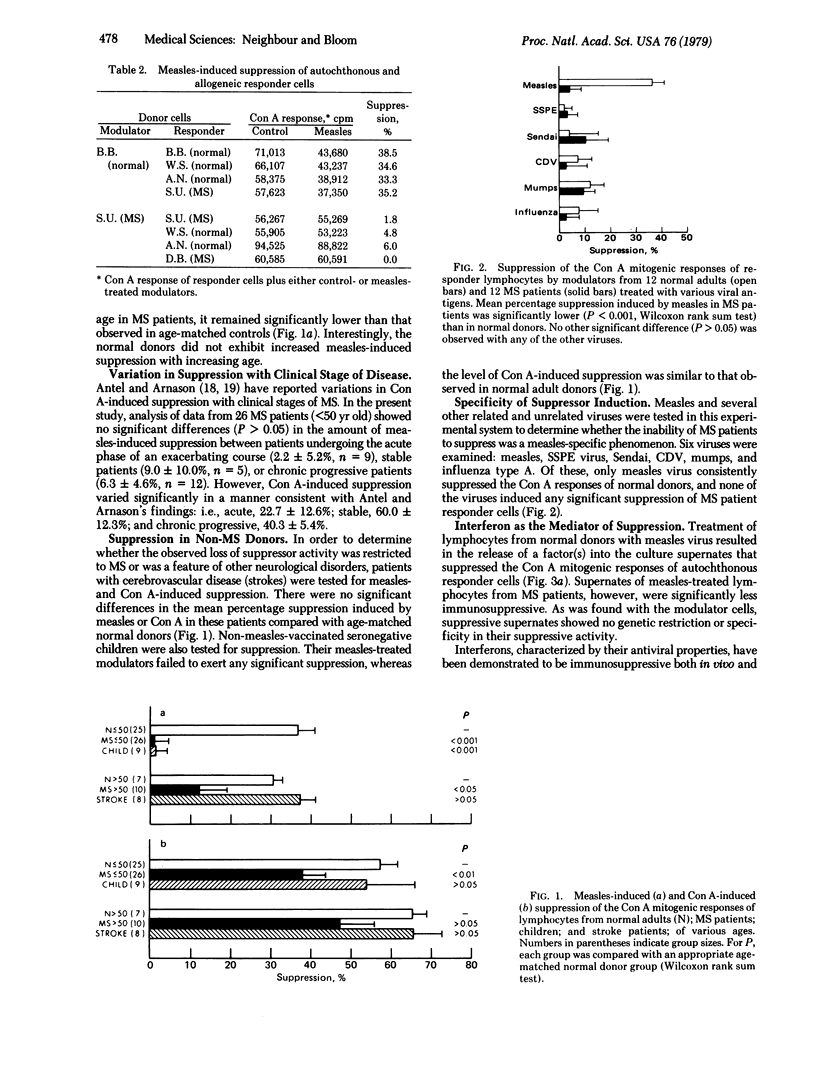
Abstract
Lymphocytes from normal adult donors exposed in vitro to inactivated measles virus were found to exert significant suppression (33.9%) of the concanavalin A responses of cryopreserved, autochthonous responder cells. In marked contrast, lymphocytes from multiple sclerosis patients exhibited significantly reduced suppression (1.5%), and in 80% of cases failed to suppress at all. The degree of suppression increased slightly with age of the patient but did not vary with the clinical stage of disease. There was no apparent genetic restriction of suppressor activity. Although specificity of this phenomenon for measles virus has not been established, no differences in the responses of lymphocytes from normal or multiple sclerosis patient donors were found with subacute sclerosing panencephalitis, Sendai, canine distemper, mumps, or influenza viruses.
Supernates of measles-treated lymphocytes from normal donors possessed both suppressive and antiviral activities. Both activities were resistant to pH 2 treatment and were neutralized by an anti-human leukocyte interferon antiserum, strongly suggesting that interferon (probably type I) was the mediator of suppression. Consistent with their inability to suppress concanavalin A responses, lymphocytes from multiple sclerosis patients failed to produce significant amounts of interferon in response to measles challenge in vitro. These results extend previous observations that multiple sclerosis patients are unable to respond appropriately to measles virus antigen in vitro.
Keywords: cell-mediated immunity, suppressor cell, viral antigens, slow neurologic diseases
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS J. M., IMAGAWA D. T. Measles antibodies in multiple sclerosis. Proc Soc Exp Biol Med. 1962 Dec;111:562–566. doi: 10.3181/00379727-111-27855. [DOI] [PubMed] [Google Scholar]
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Antel J. P., Weinrich M., Arnason B. G. Circulating suppressor cells in man as a function of age. Clin Immunol Immunopathol. 1978 Jan;9(1):134–141. doi: 10.1016/0090-1229(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Antel J. P., Weinrich M., Arnason B. G. Mitogen responsiveness and suppressor cell function in multiple sclerosis. Influence of age and disease activity. Neurology. 1978 Oct;28(10):999–1003. doi: 10.1212/wnl.28.10.999. [DOI] [PubMed] [Google Scholar]
- Bean M. A., Kodera Y., Cummings K. B., Bloom B. R. Occurrence of restricted suppressor T-cell activity in man. J Exp Med. 1977 Nov 1;146(5):1455–1460. doi: 10.1084/jem.146.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Mechanism of the suppressive effect of interferon on antibody synthesis in vivo. J Immunol. 1975 Apr;114(4):1323–1328. [PubMed] [Google Scholar]
- Byington D. P., Castro A. E., Burnstein T. Adaptation to hamsters of neurotropic measles virus from subacute sclerosing panencephalitis. Nature. 1970 Feb 7;225(5232):554–555. doi: 10.1038/225554b0. [DOI] [PubMed] [Google Scholar]
- Cook S. D., Dowling P. C. A possible association between house pets and multiple sclerosis. Lancet. 1977 May 7;1(8019):980–982. doi: 10.1016/s0140-6736(77)92281-4. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., McMichael A. J., Batey M. E., McDevitt H. O. A suppressor T cell of the mixed lymphocyte reaction in man specific for the stimulating alloantigen. Evidence that identity at HLA-D between suppressor and responder is required for suppression. J Exp Med. 1978 Jan 1;147(1):137–146. doi: 10.1084/jem.147.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., McMichael A. J., McDevitt H. O. Suppression of the mixed lymphocyte reaction in man by a soluble T-cell factor. Specificity of the factor for both responder and stimulator. J Exp Med. 1978 Apr 1;147(4):1037–1043. doi: 10.1084/jem.147.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Hallgren H. M., Yunis E. J. Suppressor lymphocytes in young and aged humans. J Immunol. 1977 Jun;118(6):2004–2008. [PubMed] [Google Scholar]
- Havell E. A., Berman B., Ogburn C. A., Berg K., Paucker K., Vilcek J. Two antigenically distinct species of human interferon. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2185–2187. doi: 10.1073/pnas.72.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Johnson H. M. Differentiation of the immunosuppressive and antiviral effects of interferon. Cell Immunol. 1978 Mar 15;36(2):220–230. doi: 10.1016/0008-8749(78)90266-6. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. The possible viral etiology of multiple sclerosis. Adv Neurol. 1975;13:1–46. [PubMed] [Google Scholar]
- Knowles M., Saunders M. Lymphocyte stimulation with measles antigen in multiple sclerosis. Neurology. 1970 Jul;20(7):700–702. doi: 10.1212/wnl.20.7.700. [DOI] [PubMed] [Google Scholar]
- Koprowski H., ter Meulen V. Multiple sclerosis and parainfluenza 1 virus. History of the isolation of the virus and expression of phenotypic differences between the isolated virus and Sendai virus. J Neurol. 1975;208(3):175–190. doi: 10.1007/BF00630631. [DOI] [PubMed] [Google Scholar]
- Levy N. L., Auerbach P. S., Hayes E. C. A blood test for multiple sclerosis based on the adherence of lymphocytes to measles-infected cells. N Engl J Med. 1976 Jun 24;294(26):1423–1427. doi: 10.1056/NEJM197606242942604. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Ubels-Postma J., Galama J. M., Rezee A. Studies on the mechanism of measles virus-induced suppression of lymphocyte functions in vitro: lack of a role for interferon and monocytes. Cell Immunol. 1978 May;37(2):448–458. doi: 10.1016/0008-8749(78)90212-5. [DOI] [PubMed] [Google Scholar]
- Miörner H., Landström L. E., Larner E., Larsson I., Lundgren E., Strannegård O. Regulation of mitogen-induced lymphocyte DNA synthesis by human interferon of different origins. Cell Immunol. 1978 Jan;35(1):15–24. doi: 10.1016/0008-8749(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff J. M., Enders J. F. Poliovirus replication and cytopathogenicity in monolayer hamster cell cultures fused with beta propiolactone-inactivated Sendai virus. Proc Soc Exp Biol Med. 1968 Jan;127(1):260–267. doi: 10.3181/00379727-127-32668. [DOI] [PubMed] [Google Scholar]
- Offner H., Konat G., Clausen J. Effect of phytohemagglutinin, basic protein and measles antigen on myo-(2-3H)inositol incorporation into phosphatidylinositol of lymphocytes from patients with multiple sclerosis. Acta Neurol Scand. 1974;50(6):791–800. doi: 10.1111/j.1600-0404.1974.tb02819.x. [DOI] [PubMed] [Google Scholar]
- Rozes K. R., Lee S. H., Ngan J. Effect of priming on interferon inhibition of con A induced spleen cell blastogenesis. Nat New Biol. 1973 Sep 5;245(140):16–18. doi: 10.1038/newbio245016a0. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER G. A., BEEBE G., KIBLER R. F., KURLAND L. T., KURTZKE J. F., MCDOWELL F., NAGLER B., SIBLEY W. A., TOURTELLOTTE W. W., WILLMON T. L. PROBLEMS OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS: REPORT BY THE PANEL ON THE EVALUATION OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS. Ann N Y Acad Sci. 1965 Mar 31;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- Santoli D., Moretta L., Lisak R., Gilden D., Koprowski H. Imbalances in T cell subpopulations in multiple sclerosis patients. J Immunol. 1978 Apr;120(4):1369–1371. [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J Immunol. 1978 Aug;121(2):532–538. [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. The immunosuppressive effect of type II mouse interferon preparations on antibody production. Cell Immunol. 1977 Dec;34(2):193–206. doi: 10.1016/0008-8749(77)90243-x. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D., Dee R. R., Knowles B. B. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation. J Exp Med. 1978 May 1;147(5):1299–1313. doi: 10.1084/jem.147.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermohlen V., Farmer J., Kornbluth J., Kornstein M. The relationship between direct migration inhibition with measles antigen and E rosettes in normals and patients with multiple sclerosis. Clin Immunol Immunopathol. 1978 Jan;9(1):63–66. doi: 10.1016/0090-1229(78)90121-6. [DOI] [PubMed] [Google Scholar]
- Utermohlen V., Zabriskie J. B. A suppression of cellular immunity in patients with multiple sclerosis. J Exp Med. 1973 Dec 1;138(6):1591–1596. doi: 10.1084/jem.138.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdirmarsson H., Agnarsdottir G., Lachmann P. J. Measles virus receptor on human T lymphocytes. Nature. 1975 Jun 12;255(5509):554–556. doi: 10.1038/255554a0. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Cherry J., McIntosh K. Decreased lymphocyte transformation to vaccinia virus in multiple sclerosis. Neurology. 1978 May;28(5):415–420. doi: 10.1212/wnl.28.5.415. [DOI] [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]



