Abstract
Orthotopic liver transplantation (OLT) is the only proven effective treatment for both end-stage and metabolic liver diseases. Hepatocyte transplantation is a promising alternative for OLT, but the lack of available donor livers has hampered its clinical application. Hepatocyte-like cells (HLCs) differentiated from many multi-potential stem cells can help repair damaged liver tissue. Yet almost suitable cells currently identified for human use are difficult to harvest and involve invasive procedures. Recently, a novel mesenchymal stem cell derived from human menstrual blood (MenSC) has been discovered and obtained easily and repeatedly. In this study, we examined whether the MenSCs are able to differentiate into functional HLCs in vitro. After three weeks of incubation in hepatogenic differentiation medium containing hepatocyte growth factor (HGF), fibroblast growth factor-4 (FGF-4), and oncostain M (OSM), cuboidal HLCs were observed, and cells also expressed hepatocyte-specific marker genes including albumin (ALB), α-fetoprotein (AFP), cytokeratin 18/19 (CK18/19), and cytochrome P450 1A1/3A4 (CYP1A1/3A4). Differentiated cells further demonstrated in vitro mature hepatocyte functions such as urea synthesis, glycogen storage, and indocyanine green (ICG) uptake. After intrasplenic transplantation into mice with 2/3 partial hepatectomy, the MenSC-derived HLCs were detected in recipient livers and expressed human ALB protein. We also showed that MenSC-derived HLC transplantation could restore the serum ALB level and significantly suppressed transaminase activity of liver injury animals. In conclusion, MenSCs may serve as an ideal, easily accessible source of material for tissue engineering and cell therapy of liver tissues.
Keywords: Menstrual blood-derived mesenchymal stem cell (MenSC), Differentiation, Hepatocyte, Intrasplenic transplantation, Partial hepatectomy
1. Introduction
Orthotopic liver transplantation (OLT) represents the only reliable treatment for acute liver failure (ALF), liver failure associated with end-stage chronic liver diseases (CLDs) and non-metastatic liver cancer (Peleman et al., 1987). However, the shortage of suitable donor organs, high cost, and the requirement for immunosuppression largely restrict its application. Hepatocyte transplantation is a promising alternative for OLT in the treatment of both end-stage and metabolic liver diseases, because it is less invasive than the whole organ transplantation and can be performed repeatedly (Fox and Chowdhury, 2004). Recently, hepatocyte transplantation has been reported to improve metabolic abnormalities present in the animal models of Wilson’s disease, Crigler-Nijjar syndrome type I, and hereditary tyrosinaemia type 1 and lowered serum cholesterol levels in a rabbit model of homozygous familial hyper-cholesterolemia (Overturf et al., 1996; Gunsalus et al., 1997; Park et al., 2006). Furthermore, promising results have been also demonstrated from hepatocyte transplantation in human diseases including glycogen storage disease type 1a, Crigler-Najjar syndrome type I, argininosuccinate lyase deficiency, and inherited factor VII deficiency (Fox et al., 1998; Muraca et al., 2002; Dhawan et al., 2004; Stephenne et al., 2006). However, only less than 100 hepatocyte transplantation cases have been reported in humans up to date. The major reasons for this discrepancy are the success of OLT and shortage of available human hepatocytes. Therefore, it is critical to find easily available cell sources equivalent to primary hepatocytes for hepatocyte-like cell (HLC) transplantation.
Stem cells have been a focus of regenerative medicine for their multi-potential differentiation ability. It is attractive to develop stem-cell-based therapy or transplanting hepatocytes generated in vitro from stem cells for treatment of liver diseases. Many types of stem cells from different sources have been investigated for hepatic differentiation ability (Yamamoto et al., 2003; Banas et al., 2007; Chen et al., 2012). In addition, the in vivo transplantation of stem cell-derived HLCs improved liver functions of animal models undergoing liver damage (Yamamoto et al., 2003; Chen et al., 2012). Embryonic stem cells have enormous differentiation ability, but many limitations including teratoma formation after transplantation, immunogenicity and ethical issues are arresting their clinical application (Lin et al., 2011). Adult human stem cells are considered as promising candidates for cell therapy, overcoming ethical concerns and risks of rejection. Mesenchymal stem cells (MSCs) obtained from human bone marrow (BM), adipose tissue (AT), placenta, and umbilical cord blood (UCB) have been reported to differentiate in vitro into a variety of lineages, such as osteoblasts, adipocytes, chondrocytes, myoblasts and cardiomyocytes, neural cells, depending on the microenvironment in which they reside (Pittenger et al., 1999). Although MSCs from BW, UCB, and AT have been induced into a hepatic lineage, the question of whether these cells are the best sources for liver regeneration remains (Bieback et al., 2004; Banas et al., 2007; Chen et al., 2012).
Previous studies suggested that endometrium contains an MSC-like population (Schwab and Gargett, 2007). Menstrual blood-derived mesenchymal stem cells (MenSCs) possess stem cell-like characteristics, such as self-renewal, high proliferative potential, and a pluripotent differentiation ability in vitro (Meng et al., 2007). MenSCs have been widely used in regenerative medicine research. Recent studies have shown that MenSCs are able to differentiate into cardiocytes with the functions of beating spontaneously after induction and decreasing the myocardial infarction area in a rat model (Hida et al., 2008; Ikegami et al., 2010). MenSCs transferred dystrophin into dystrophied myocytes through cell fusion and transdifferentiation in vitro and in vivo (Cui et al., 2007; Toyoda et al., 2007). Furthermore, stem cells isolated from endometrium have been proven to be an excellent cell source for treating experimental disease models, such as critical limb ischemia, stroke, type I diabetes mellitus, Parkinson’s disease, and other neurodegenerative disorders (Murphy et al., 2008; Borlongan et al., 2010; Li H.Y. et al., 2010; Sanberg et al., 2011; Santamaria et al., 2011). These results indicated that MenSCs could be served as “seeder cells” regenerative medicine including treatment of liver diseases. We hypothesized that MSCs obtained from menstrual blood are able to differentiate into functional HLCs in vitro which can restore liver functions for liver injured animals.
2. Materials and methods
2.1. Ethics statement
This project was approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, China. Written informed consent was provided by donors. All the animal experiments were performed under the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All animal experiments were in accordance with legal regulations, including approval by the Animal Care and Use Committee of Zhejiang University, China.
2.2. Menstrual blood-derived stem cells isolation and culture
Human MenSCs were isolated from the menstrual blood of normal donors using a Divacup (S-Evans Biosciences, China) during the first day of menstruation as previously described by Meng et al. (2007). Briefly, mononuclear cells were separated by Ficoll-Paque (1.077 g/ml; Fisher Scientific, NH) density-gradient centrifugation according to the manufacturer’s instructions. The purified mononuclear cells were allowed to attach in the endometrial/menstrual stem cell culture medium (S-Evans Biosciences, China) overnight at 37 °C in 5% CO2. On Day 3, non-adherent cells were removed by washing with phosphate-buffered saline (PBS) while adherent cells were cultured until they reached 80%–90% confluence. Cells were trypsinized, subcultured, and used for experiments during passages 4 to 7.
2.3. Flow cytometry analysis
Surface marker expression levels of cultured MenSCs were analyzed using a flowcytometer (FC500MCL, Beckman Coulter, Fullerton, CA). The 2nd passage of MenSCs (1×105 cells) was incubated with direct phycoerythrin (PE)-conjugated monoclonal antibodies against CD29, CD34, CD45, CD73, CD90, CD105, and human leukocyte antigen (HLA)-DR (all purchased from eBioscience, San Diego, CA) for 1 h in the dark at 4 °C, followed by washing and resuspension in PBS. Immunoglobulin isotype (eBioscience, San Diego, CA) incubation was performed as a negative control. Data were analyzed using FlowJo Version 6.1 (Treestar, Ashland, OR).
2.4. Multi-lineage differentiation assays
To induce osteogenic differentiation, MenSCs were cultured in a commercially available osteogenesis differentiation medium (GIBCO, CA). On Day 21, calcium deposits were visualized by alizarin red S stain analysis. To induce adipogenic differentiation, MenSCs were cultured in a commercially available adipogenesis differentiation medium (GIBCO, CA). On Day 14, cells were stained with oil red O. To induce chondrogenic differentiation, MenSCs were cultured in a commercial available chondrogenesis differentiation medium (GIBCO, CA). Chondrogenesis was assessed by alcian blue stain analysis.
2.5. Hepatogenic differentiation
To induce hepatogenic differentiation, the 4th to 7th passages of MenSCs at 1.5×105/cm2 were cultured in a hepatogenesis differentiation medium for three weeks with medium changes twice weekly. Hepatogenesis differentiation medium consists of Iscove’s Modified Dulbecco’s Medium (IMDM; GIBCO, CA) supplemented with 20 ng/ml hepatocyte growth factor (HGF), 10 ng/ml fibroblast growth factor-4 (FGF-4), 10 ng/ml oncostain M (OSM) (Peprotech, UK), 40 μg/L dexamethasone (DEX; Sigma-Aldrich, MO), and 1× ITS+ Premix (BD Biosciences, CA). Hepatogenic differentiation was identified by cell morphology, reverse transcription polymerase chain reaction (RT-PCR) analysis, and in vitro biochemical functions. All were performed together with undifferentiated MenSCs.
2.6. Total RNA isolation and RT-PCR
RNA was isolated from MenSCs, differentiating cells, or differentiated cells using Trizol reagent (Invitrogen, CA) according to the manufacturer’s instruction. Total RNA (1.0 μg) was reverse transcribed to complementary DNA (cDNA) using the SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). cDNA was amplified using a Gene Amp PCR 9600 (PerkinElmer, Norwalk, CT) at 95 °C for 45 s, 56 °C for 60 s, and 72 °C for 45 s for 30 cycles, after initial denaturation at 95 °C for 3 min. Primers used are listed in Table 1.
Table 1.
Sequences of primers for reverse transcription polymerase chain reaction (RT-PCR) with fragment lengths
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) | Size (bp) |
| ALB | AAGGCAAGTCAGCAGGCATCTCATC | TGCTTGAATGTGCTGATGACAGGG | 163 |
| AFP | GAGATGTGCTGGATTGTC | GATACATAAGTGTCCGATAA | 644 |
| CK18 | GAACCACGAAGAGGAAGTAAA | CATCTGTAGGGCGTAGCG | 361 |
| CK19 | GCGACTACAGCCACTACTACACGA | TTCCTTCCCATCCCTCTACCC | 897 |
| CYP1A1 | AACATCGTCTTGGACCTCTTT | GCAATGGTCTCACCGATACAC | 464 |
| CYP3A4 | CCACCCACCTATGATACTGT | ATCCCTTGACTCAACCTTTA | 462 |
| β-Actin | TCACCACCACGGCCGAGCG | TCTCCTTCTGCATCCTGTCG | 350 |
ALB: albumin; AFP: α-fetoprotein; CK18/19: cytokeratin 18/19; CYP1A1/3A4: cytochrome P450 1A1/3A4
2.7. Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 10 min, and permeablized in 0.1% Triton X-100 (Sigma-Aldrich, MO) for 10 min. After blocking in 5% bovine serum albumin (BSA) solution, slides or dishes were incubated with primary antibodies against albumin (ALB), α-fetoprotein (AFP; Dako, Glostrup, Denmark), or human fumarylacetoacetate hydrolase (Fah; AbboMax, CA) at 4 °C overnight. Biotinylated secondary antibody was added and chromogenic reaction visualized using a DAB Horseradish Peroxidase Color Development Kit (Beyotime, China). Cells were counterstained with hematoxylin and examined using a phase contrast microscope.
2.8. Urea assay
The urea concentrations in the supernatants of the induction medium were measured using the Quantichrom Urea Assay Kit (Bioassay Systems, Brussels, Belgium) according to the manufacturer’s instruction. Fresh culture medium supplemented with 6 mmol/L NH4Cl was used as negative control.
2.9. Periodic acid-schiff (PAS) staining for glycogen
Differentiated cells were fixed with 4% formaldehyde for 10 min, and permeabilized with 0.1% Triton X-100 for 10 min. The samples were then oxidized in 1% periodic acid for 5 min, rinsed three times in de-ionized H2O (dH2O), treated with Schiff’s reagent (Sigma-Aldrich, MO) for 20 min in the dark, and rinsed in dH2O for 5 min. Finally, they were visualized under a light microscope.
2.10. Indocyanine green (ICG) uptake assay
Hepatogenesis differentiation medium was replaced with IMDM medium containing 1 mg/ml ICG (Aladdin, Shanghai, China). After incubation at 37 °C for 15 min, cells were rinsed three times with PBS and ICG uptake was measured using an inverted microscope. Dishes were refilled with endometrial/menstrual stem cell culture medium for 6 h and color changes were examined again.
2.11. Construction of liver injury mouse model with 2/3 partial hepatectomy (PH) and hepatocyte-like cell (HLC) transplantation
All animals were kept at 23–25 °C with a 12-h light/dark cycle and allowed standard chow and water until the time of the study. An acute liver injury mouse model with 2/3 PH was constructed as previously described (Mitchell and Willenbring, 2008). Twenty-five 3–4 weeks old male BALB/c mice were divided into the transplantation group (PH+HLC, 10 mice), the only PH group (PH, 10 mice), and the sham surgery group (Sham, 5 mice). MenSC-derived HLCs were trypsined into aliquots of 1.5×106 cells in 150 μl PBS for each animal and stored on ice until transplantation. HLCs were intrasplenically transplanted using a sterile syringe and needle immediately after PH. On Day 14, serum samples were collected and liver functional indicators such as ALB, alanine transaminase (ALT), and aspartate aminotransferase (AST) of the three groups were analyzed by an automatic biochemical analyzer (SHIMADZU, Japan). The livers were excised and fixed with 10% formaldehyde and embedded in paraffin for further study.
2.12. Statistical analysis
Data are expressed as mean±standard deviation (SD). The difference between groups was determined using Student’s t-test. P<0.05 was considered statistically significant.
3. Results
3.1. Isolation and characterization of MenSCs
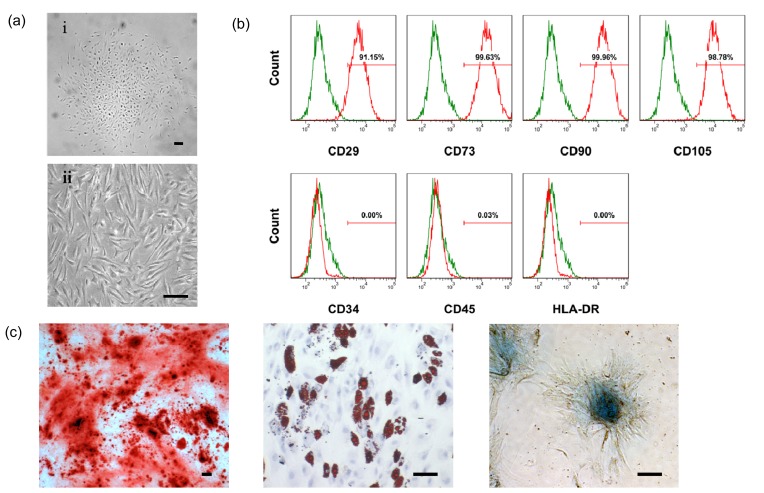
Fig. 1a shows that isolated cells from menstrual blood had a typical spindle shape, consistent with the morphology reported by others (Cui et al., 2007; Meng et al., 2007; Toyoda et al., 2007; Allickson and Xiang, 2012). The doubling time of the cultured cells was approximate 24 h. Clusters formed in the course of primary culturing and vortex-like cells appeared at 90% confluence in subcultures. These menstrual blood-derived cells were positive for MSC markers including CD29, CD73, CD90, and CD105 but were negative for hematopoietic stem cell markers such as CD34 and CD45, as well as the immune activation marker HLA-DR (Fig. 1b). Under the osteogenic, adipogenic, or chondrogenic condition, MenSCs could differentiate into osteocytes, adipocytes, or chondrocytes in vitro, respectively (Fig. 1c). These results indicated that the cells used in this study exhibited the classic MSC phenotype and multi-potential stem cell characteristics.
Fig. 1.
Characterization of human MenSCs in vitro
(a) Morphology of human MenSCs harvested from menstrual blood samples of healthy donors. MenSCs started to adhere and after about 5 d started to form colonies (i). MenSCs were subcultured and passaged twice a week and showed a fibroblast-like cell morphology at higher confluence (ii). (b) Flow cytometry analysis for expressions of mesenchymal, hematopoietic and immunologic markers: MenSCs showed strong positive expressions of mesenchymal markers (CD29, CD73, CD90, and CD105), but were negative for the hematopoietic lineage markers (CD34 and CD45) and MHC class II antigen (HLA-DR). (c) MenSCs are induced to differentiate into osteoblasts and stained positive for alizarin red S (left); under adipogenic condition, MenSCs accumulate neutral lipid vacuoles, which are positive for oil red O assay (middle); under chondrogenic condition, MenSCs differentiate into chrondrocyte-like cells and stained positive for alcian blue (right). Scale bar 50 μm
3.2. In vitro hepatogenic differentiation of MenSCs
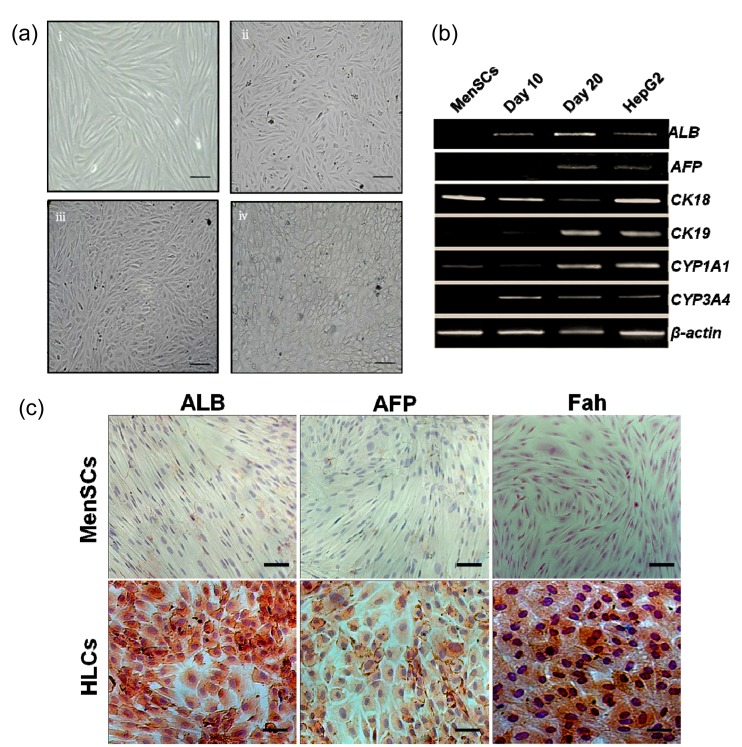
To examine the hepatogenic differentiation ability in vitro, MenSCs were cultured in a specific hepatogenesis differentiation medium containing HGF, FGF-4, and OSM. Cell morphology was monitored every 2–3 d using a phase-contrast microscope. During the differentiation procedure, cells gradually changed from spindle, bipolar, fibroblast-like cells to round or polygonal epithelioid cells (Fig. 2a). On Day 21, almost all induced cells showed epithelioid and cuboidal shapes, similar to primary human hepatocytes. Cells cultured in a medium without conditioned factors were set as control and showed no HLC morphology.
Fig. 2.
In vitro hepatogenic differentiation of MenSCs
(a) Sequential morphological changes from MenSCs to hepatocyte-like cells. Cells gradually changed from spindle, bipolar, fibroblast-like cells to round or polygonal epithelioid cells. (b) RT-PCR gene expressions of MenSCs and MenSC-derived hepatocyte-like cells for the hepatocyte markers albumin (ALB), α-fetoprotein (AFP), cytokeratin 18 (CK-18), CK-19, cytochrome P450 1A1 (CYP1A1), and CYP3A4. RNA was isolated from undifferentiated MenSCs and from MenSCs during hepatogenic induction on Days 10 and 20. HepG2 cells were used as positive control which constantly expresses hepatocyte-specific markers at the mRNA level. Gene expressions were normalized to β-actin. (c) Immunocytochemical staining analysis of hepatocyte-specific markers: on Day 21, cells were fixed and stained with monoclonal antibodies against ALB, AFP, and Fah. These hepatocyte-specific markers were positive in MenSC-derived HLCs but negative in undifferentiated MenSCs. Scale bar 50 μm
Cells on Days 10 and 20 post-induction were harvested for gene expression analysis. Hepatocyte-specific marker genes including ALB, AFP, CK18, CK19, CYP1A1, and CYP3A4 (Table 1) were evaluated by RT-PCR analysis. ALB, CK19, and CYP3A4 were expressed after 10 d of induction, and AFP was expressed after an additional 10 d (Fig. 2b). Undifferentiated cells showed no hepatocyte-specific gene expression except for CK18 and CYP1A1, which is consistent with previous studies (Lee et al., 2004; Chen et al., 2012).
In the next experiments, we tested whether MenSCs expressed hepatocyte-specific proteins after hepatogenic differentiation. Fig. 2c shows the MenSC-derived HLCs were positively stained for hepatocyte-specific markers such as ALB, AFP, and Fah. Undifferentiated cells were not stained positively for all these three markers.
3.3. Functional characterization of the MenSC-derived HLCs
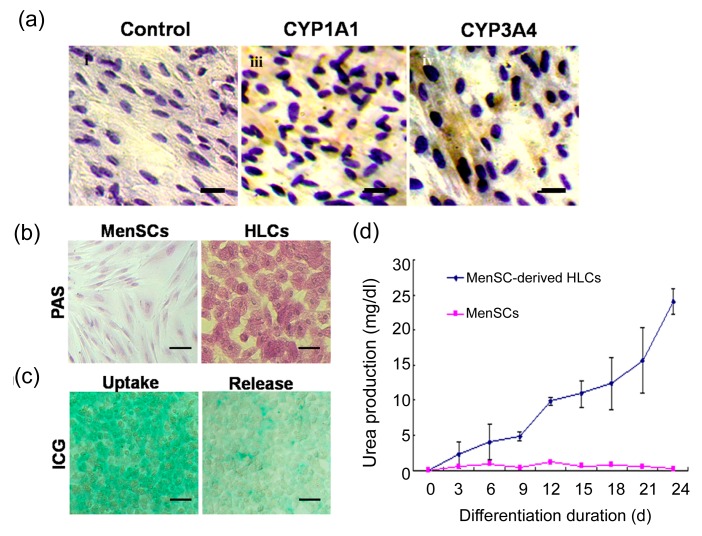
To assess the functional status of the human MenSCs-derived HLCs, we firstly examined their metabolic capacity-related protein cytochrome P450 (CYP450) enzyme expression using the immunocytochemistry method. As shown in Fig. 3a (Page 969), the MenSC-derived HLCs were positively stained for both of the most important enzyme isoforms, CYP3A4 and CYP1A1. Our results indicated that the differentiated cells expressed CYP450 enzyme and might have biological activity similar to primary human hepatocytes.
Fig. 3.
Functional analysis of the hepatocyte-like cells derived from MenSCs
(a) On Day 21, the differentiated HLCs were fixed and stained with monoclonal antibodies against CYP1A1/3A4. Immunocytochemical analysis results indicated that differentiated HLCs express functional hepatocyte-specific enzymes which are not expressed by undifferentiated cells. (b) PAS staining for glycogen showed that differentiated cell could store glycogen after hepatogenic induction for 21 d. Undifferentiated cells stained negative for glycogen storage. (c) Differentiated MenSCs were positive for ICG after incubation in ICG solution for 15 min. ICG in the differentiated cells was partly released 6 h after replacement ICG solution with hepatogenic induction medium. (d) Differentiated cells produced urea in a time-dependent manner. Data are expressed as mean±SD. Scale bar 50 μm
To further characterize the glycogen storage function of MenSC-derived HLCs, the presence of stored glycogen was determined using the PAS staining method. After hepatogenic induction for 3 weeks, glycogen was stained magenta and could be found in the differentiated cells, while no positive staining was observed in the undifferentiated cells (Fig. 3b). The ICG uptake assay, which also indicates the mature hepatocytes, was also used to examine the in vitro generated HLCs. As shown in Fig. 3c, most differentiated cells were positive for ICG after 15 min incubation (left panel). Six hours after refilling plates with endometrial/menstrual stem cell culture medium, ICG taken up by the differentiated cells was partially released (right panel). Undifferentiated cells were used as a negative control and showed no ICG uptake and release abilities (data not shown). Secretion of urea by the differentiated cells was monitored on Days 0, 3, 6, 9, 12, 15, 18, 21, and 24. Urea production was detectable on Day 3 and increased gradually during the hepatogenic differentiation (Fig. 3d).
3.4. Transplantation of MenSC-derived HLCs into mice with 2/3 PH
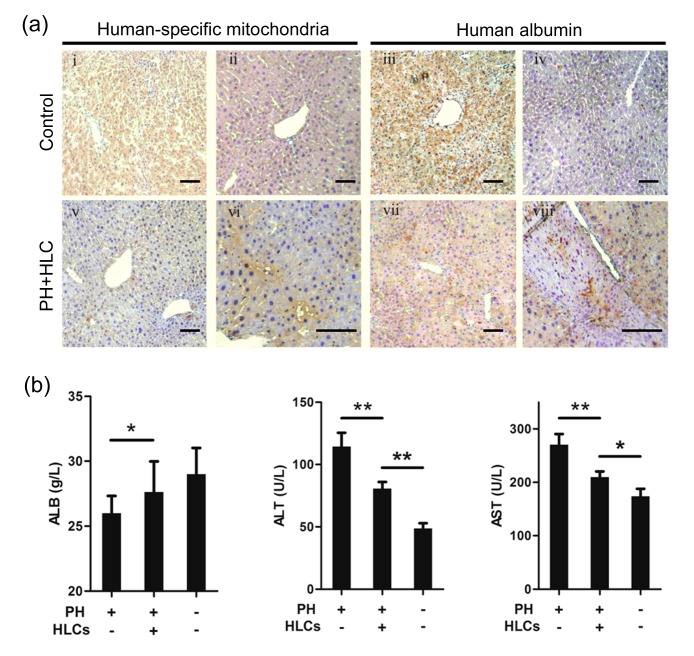
To assess the therapeutic potential of our generated HLCs derived from MenSCs, an acute liver injury mouse model with 2/3 PH was used. We transplanted 1.5×106 MenSC-derived HLCs directly into the splenic pulp of PH mice. We firstly examined whether the transplanted cells were engrafted into liver parenchyma of the recipients. Antibodies against human-specific mitochondria and albumin were used to detect human liver cells in mouse liver. Recipient mice that received intrasplenic transplantation of MenSC-derived HLCs were sacrificed 8 weeks after transplantation. The immunohistochemical staining demonstrated the presence of human mitochondria and albumin in the liver parenchyma of the recipient animals (Fig. 4a; Page 969). To further assess the effect of MenSC-derived HLC transplantation on the liver function of PH mice, serum levels of ALB, ALT, and AST were evaluated. As shown in Fig. 4b, the ALB levels of the PH+HLC group were higher than that of the only PH group, while the ALT and AST levels of the PH+HLC group were significantly lower than those of the only PH group. Although the liver function of the PH+HLC group was worse than that of the Sham group, our results indicated that MenSC-derived HLC transplantation could significantly improve liver function after traumatic liver injury.
Fig. 4.
Transplanted MenSC-derived HLCs integrate into the liver parenchyma and restore liver functions in liver injured mice with 2/3 partial hepatectomy (PH)
(a) MenSC-derived HLCs were transplanted into BALB/c mice with 2/3 PH and integrated into the liver parenchyma. Serial liver sections were stained for both human-specific mitochondria and ALB immunostaining. Human liver sections were used as positive control (i and iii) while mouse liver sections were used as negative control (ii and iv). (b) Serum levels of albumin (ALB), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in PH+HLC mice (n=10), only PH group mice (n=10), and sham surgery group (Sham, n=5). * P<0.05; ** P<0.01. Data are expressed as mean±SD. Scale bar 50 μm
4. Discussion
Most patients with end-stage liver diseases have no matched liver for transplantation. Hepatocyte transplantation might become more feasible, efficient and safe than whole organ transplantation to treat these patients. However, the shortage of matched primary hepatocytes and the difficulty of maintenance of hepatocyte functions in vitro largely hampered its clinical applications. Generation of functional hepatocytes from stem cells might overcome the limitations that hepatocyte transplantation faced and hold considerable promise for future clinical practices. The hepatogenic potential of stem cells from different sources has been reported previously (Yamamoto et al., 2003; Banas et al., 2007; Chen et al., 2012). Stem cells from an embryo face the hurdles of teratoma formation and the ethical and/or moral issues of the creation, usage and destruction of a human embryo (Lin et al., 2011; Allickson and Xiang, 2012). Another type of stem cells, named adult or somatic stem cells, has a controlled behavior and holds great promise for cell therapy and regenerative medicine (Allickson et al., 2011; Lin et al., 2011; Allickson and Xiang, 2012). Adult stem cells harvested from BW, AT, and UCB have shown multi-potent capacity and can be induced to differentiate into functional hepatocytes (Banas et al., 2007; Chen et al., 2012; Yu et al., 2012). However, BW- and AT-derived stem cells cannot be easily obtained by practical, cost-effective methods while obtaining UCB stem cells is limited to the time of birth (Patel and Silva, 2008). Endometrial tissue is a prolific source of stem cells that reoccur monthly (Allickson et al., 2011). Many studies have demonstrated that endometrial stem cells can be harvested from menstrual blood by a non-invasive method (Cui et al., 2007; Patel and Silva, 2008; Patel et al., 2008; Borlongan et al., 2010; Phuc et al., 2011; Allickson and Xiang, 2012). Consistent with previous reports, the MenSCs used in our study expressed classic MSC markers (CD29, CD73, CD90, and CD105) but did not express hematopoietic stem cell markers (CD34 and CD45) and immune activation markers (HLA-DR) (Cui et al., 2007; Patel and Silva, 2008; Patel et al., 2008; Borlongan et al., 2010; Phuc et al., 2011; Allickson and Xiang, 2012). As mentioned by Patel et al. (2008), the MenSCs are also positive for both Oct-4 and SSEA-4 markers expressed by human embryonic stem cells, which may explain their rapid expansion and multi-potent capability in this study (Patel and Silva, 2008; Patel et al., 2008). Meng et al. (2007) have obtained stem cells from menstrual blood and induced the cells to differentiate into 9 different cell lineages. Although positively stained for hepatocyte-specific marker albumin, the generated HLCs in Meng et al. (2007) were not further validated for their function in vitro and in vivo. In the present study, we tested whether the MenSCs could differentiate into functional hepatocytes in vitro and examined their treatment effect in vivo.
Strategies for transdifferentiation of stem cells into hepatocytes include induction by cocktails of cytokines, stimulation by chemicals, co-culture with primary liver cells or conditioned hepatocyte culture medium, and in vivo transplantation (Banas et al., 2007). Induction systems using differentiation condition mediums containing cytokines and growth factors have been widely studied (Lee et al., 2004; Banas et al., 2007; Chen et al., 2012). These systems do not require co-culture and avoid undesired cell-cell interactions. HGF, epidermal growth factor (EGF), FGF, and OSM have been shown to possess a potent effect on hepatic growth and differentiation in vitro. However, it is still not clear which of those stem cells would be the best source for hepatocyte generation or which would be the best protocol. BW-MSCs cultured with FGF-4 and HGF on Matrigel can differentiate into cells expressing several liver-specific markers and had functional characteristics of hepatocyte such as albumin and urea secretion and CYP450 activity (Schwartz et al., 2002). Other researchers achieved functional hepatocytes derived from BM-MSCs using sequential treatment with factors (Lee et al., 2004; Snykers et al., 2006; Li J. et al., 2010). These cells also had the characteristics of HLCs including albumin and urea secretion, glycogen storage, low-density lipoprotein (LDL) uptake, and CYP activity. As well as hematopoietic stem cells (HSCs), UCB-MSCs contain stem cells with the properties of MSCs. It has been reported that after treatment with HGF, DEX, and OSM, UCB-MSCs differentiate into HLCs (Lee et al., 2004). AT-MSCs are an attractive source of MSCs for stem cell-based therapy. The ability of AT-MSCs to differentiate into HLCs was first observed by Seo et al. (2005). Banas et al. (2007) reported hepatogenic differentiation of AT-MSCs from CD105+ AT-MSCs. Other MSCs from amniotic fluid and placenta have been also reported to differentiate into functional HLCs (Chien et al., 2006; de Coppi et al., 2007). In this study, MenSCs were induced to differentiate into heoatocytes with a cocktail of HGF, FGF-4, and OSM (Chen et al., 2007; Li J. et al., 2010). We demonstrated the ability of MenSCs to undergo hepatogenic differentiation, resulting in the achievement of functional and transplantable hepatocytes with high differentiation efficiency. The differentiated hepatocytes derived from MenSCs not only displayed HLC morphology and expressed hepatic cell markers, but also possessed mature hepatocyte-specific functions. In addition, the MenSC-derived hepatocytes expressed CYP1A1 and CYP3A4 enzymes, which are involved in drug, xenobiotic metabolism, and sterol and bile acid synthesis (Banas et al., 2007). Therefore, our findings also suggest that the MenSC-derived hepatocytes might be useful for in vitro preclinical drug investigations.
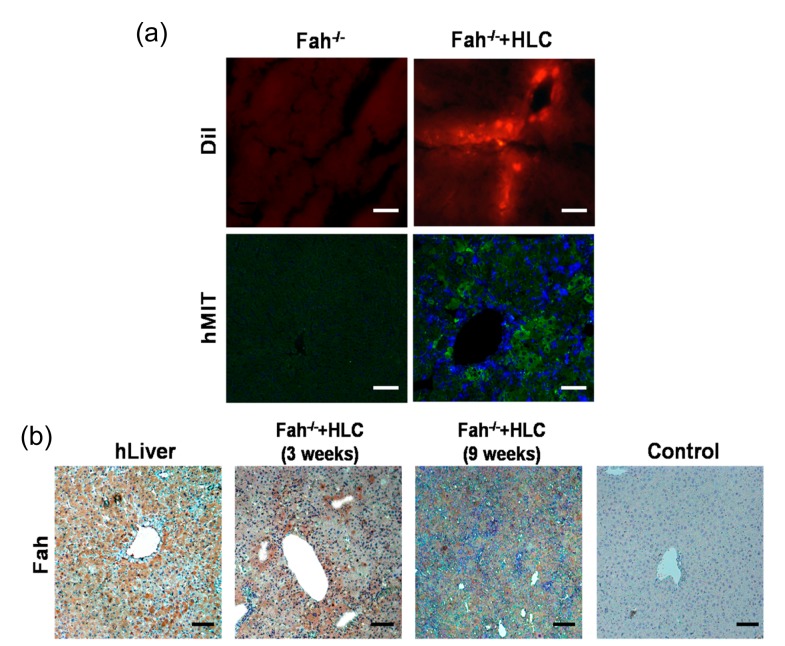
Liver injury is the most common cause of death associated with abdominal trauma (Chen et al., 2012). The 2/3 PH represents the most commonly used model for the study of liver regeneration after traumatic liver injury (Mitchell and Willenbring, 2008). We transplanted the in vitro generated hepatocytes into liver injury mice with 2/3 PH and observed direct incorporation into the liver, which was confirmed by human mitochondria and albumin immunostaining. Excitingly, the intrasplenic transplantation of MenSC-derived HLCs restored serum albumin level and significantly suppressed transaminase activity of liver injury animals, indicating that these in vitro generated hepatocytes might be useful for treatment of end-stage liver injury. Fah−/− mice defective in tyrosine metabolism require 2-(2-nitro-4-trifluoro-methylbenzyol)-1,3-cyclohexanedione (NTBC) supply for survival. After removing NTBC from the drinking water (NTBC-off), Fah−/− mice undergo liver failure and death (Huang et al., 2011). Fah−/− mice undergoing liver failure can be rescued by transplantation of wild-type primary hepatocytes and represent a useful tool to study in vivo repopulation and functions of MenSC-derived hepatocytes. In the present study, MenSC-derived hepatocytes were transplanted directly into the splenic pulp of Fah−/− mice. Three weeks after transplantation, the MenSC-derived hepatocytes were detected in the liver parenchyma by immunofluorescent methods (Fig. 5a). Incorporated cells were co-stained with 4′,6-diamidino-2-phenylindole (DAPI), indicating the injected cells remained as whole cells within the recipient livers (Fig. 5a, lower panel). Huang et al. (2011) have reported that the Fah−/− mice without transplantation were dead within 6.5 weeks after NTBC-off. Although we did not get sufficient Fah−/− mice to determine whether MenSC-derived hepatocytes could restore the liver function of recipients, the mice that received hepatocyte transplantation were alive 9 weeks after NTBC-off and showed increased body weight until scarification (data not shown). It has been shown that hepatocytes have the ability to replicate and contribute to the regeneration of the liver (Chen et al., 2012). We showed that the transplanted MenSC-derived hepatocytes had a long life span and continuously expressed hepatocyte-specific proteins including ALB and Fah in vivo (Figs. 4a and 5b), resulting in liver function restoration. Homing of the MenSC-derived hepatocytes to injured liver tissue was showed in this study, but the mechanism of attrition remains unknown. The chemokine stromal cell-derived factor-1 (SDF-1) has been found to be expressed by injured liver tissues and its receptor CXCR4 is expressed by MenSCs (Allickson et al., 2011). Whether the SDF-1/CXCR4 pathway or other migration related pathways are involved in the homing of MenSC-derived hepatocyte should be addressed in further studies.
Fig. 5.
Transplantion of MenSC-derived HLCs migrate into the liver parenchyma and expression of human Fah protein in Fah−/− mice
(a) MenSC-derived HLCs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) dye and transplanted into the spleen of Fah−/− mice. Frozen sections were made and DiI-labeled HLCs were detected in the recipient liver 3 weeks after transplantation. Immunofluorescent analysis with monoclonal antibody against human mitochondria also confirmed that transplanted HLCs integrated into the liver parenchyma. (b) Engrafted MenSC-derived HLCs expressed human Fah protein accumulating in nodules and cord in the liver parenchyma at 3 and 9 weeks after transplantation. Human liver sections and mouse liver sections were used as positive and negative controls, respectively. Scale bar 50 μm
Tumors were not found in the MenSC-derived hepatocyte transplanted mice in the present study. Allickson et al. (2011) assessed the safety of cell infusion in Harlan Sprague Dawley mice and Dunkin Hartley Albino guinea pigs. Moreover, MenSCs did not form tumors two months after subcutaneous xenograft in nude mice in our preliminary study (data not shown). These results indicate that hepatocytes differentiated from MenSCs are safe for in vivo transplantation. Another topic of interest requiring consideration is the correlation between factors such as donor age, cancer record, liver function, and the hepatogenic differentiation potential of MenSC from different females.
In summary, we have presented in vitro generation of functional and transplantable hepatocytes from MenSCs. We believe that using the MenSC-derived HLCs as a source of hepatocytes could help the development of alternative methods for treating end-stage liver diseases and toxicity screening assays for drug discovery.
Acknowledgments
We sincerely thank Ping-ping DIAO, Li YUAN, Yi-ming TANG, and Xiao-juan TONG from Zhejiang University (Hangzhou, China) for their technical support and Michael BROWNSTEIN from National Institute of Health (USA) for his critical reading of our manuscript.
Footnotes
Project supported by the National High-Tech R&D Program (863) of China (Nos. 2011AA020102 and 2012AA020905), the Key Technologies R&D Program of Zhejiang Province (Nos. 2012C13015-2 and 2011C13029-1), the Hangzhou Key Technologies R&D Program (No. 20122513A49), and the National Natural Science Foundation of China (Nos. 81201783 and 81201089)
Compliance with ethics guidelines: Xiao-zhou MOU, Jian LIN, Jing-yang CHEN, Yi-fei LI, Xiao-xing WU, Bing-yu XIANG, Cai-yun LI, Ju-ming MA, and Charlie XIANG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000(5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Allickson J, Xiang C. Human adult stem cells from menstrual blood and endometrial tissue. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2012;13(5):419–420. doi: 10.1631/jzus.B1200062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allickson JG, Sanchez A, Yefimenko N, Borlongan CV, Sanberg PR. Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. Open Stem Cell J. 2011;3(2011):4–10. doi: 10.2174/1876893801103010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 4.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22(4):625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 5.Borlongan C, Kaneko VY, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19(4):439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007;102(1):52–63. doi: 10.1002/jcb.21275. [DOI] [PubMed] [Google Scholar]
- 7.Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55(4):1193–1203. doi: 10.1002/hep.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien CC, Yen BL, Lee FK, Lai TH, Chen YC, Chan SH, Huang HI. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 2006;24(7):1759–1768. doi: 10.1634/stemcells.2005-0521. [DOI] [PubMed] [Google Scholar]
- 9.Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T, Umezawa A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18(5):1586–1594. doi: 10.1091/mbc.E06-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, Arya R, Wade JJ, Verma A, Heaton ND, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78(12):1812–1814. doi: 10.1097/01.TP.0000146386.77076.47. [DOI] [PubMed] [Google Scholar]
- 12.Fox IJ, Chowdhury JR. Hepatocyte transplantation. J Hepatol. 2004;40(6):878–886. doi: 10.1016/j.jhep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338(20):1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 14.Gunsalus JR, Brady DA, Coulter SM, Gray BM, Edge AS. Reduction of serum cholesterol in Watanabe rabbits by xenogeneic hepatocellular transplantation. Nat Med. 1997;3(1):48–53. doi: 10.1038/nm0197-48. [DOI] [PubMed] [Google Scholar]
- 15.Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26(7):1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 16.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami Y, Miyoshi S, Nishiyama N, Hida N, Okamoto K, Miyado K, Segawa K, Ogawa S, Umezawa A. Serum-independent cardiomyogenic transdifferentiation in human endometrium-derived mesenchymal cells. Artif Organs. 2010;34(4):280–288. doi: 10.1111/j.1525-1594.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40(6):1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 19.Li HY, Chen YJ, Chen SJ, Kao CL, Tseng LM, Lo WL, Chang CM, Yang DM, Ku HH, Twu NF, et al. Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J Pharmacol Exp Ther. 2010;335(3):817–829. doi: 10.1124/jpet.110.169284. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Tao R, Wu W, Cao H, Xin J, Guo J, Jiang L, Hong X, Demetriou AA, Farkas D, et al. Transcriptional profiling and hepatogenic potential of acute hepatic failure-derived bone marrow mesenchymal stem cells. Differentiation. 2010;80(2-3):166–174. doi: 10.1016/j.diff.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Xiang D, Zhang JL, Allickson J, Xiang C. Plasticity of human menstrual blood stem cells derived from the endometrium. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(5):372–380. doi: 10.1631/jzus.B1100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3(7):1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 24.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359(9303):317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 25.Murphy MP, Wang H, Patel AN, Kambhampati S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE, et al. Allogeneic endometrial regenerative cells: an “off the shelf solution” for critical limb ischemia? J Transl Med. 2008;6(1):45. doi: 10.1186/1479-5876-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12(3):266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 27.Park SM, Vo K, Lallier M, Cloutier AS, Brochu P, Alvarez F, Martin SR. Hepatocyte transplantation in the Long Evans Cinnamon rat model of Wilson’s disease. Cell Transplant. 2006;15(1):13–22. doi: 10.3727/000000006783982188. [DOI] [PubMed] [Google Scholar]
- 28.Patel AN, Silva F. Menstrual blood stromal cells: the potential for regenerative medicine. Regen Med. 2008;3(4):443–444. doi: 10.2217/17460751.3.4.443. [DOI] [PubMed] [Google Scholar]
- 29.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17(3):303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 30.Peleman RR, Gavaler JS, van Thiel DH, Esquivel C, Gordon R, Iwatsuki S, Starzl TE. Orthotopic liver transplantation for acute and subacute hepatic failure in adults. Hepatology. 1987;7(3):484–489. doi: 10.1002/hep.1840070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phuc PV, Lam DH, Ngoc VB, Thu DT, Nguyet NT, Ngoc PK. Production of functional dendritic cells from menstrual blood—a new dendritic cell source for immune therapy. In Vitro Cell Dev Biol Anim. 2011;47(5-6):368–375. doi: 10.1007/s11626-011-9399-2. [DOI] [PubMed] [Google Scholar]
- 32.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 33.Sanberg PR, Eve DJ, Willing AE, Garbuzova-Davis S, Tan J, Sanberg CD, Allickson JG, Cruz LE, Borlongan CV. The treatment of neurodegenerative disorders using umbilical cord blood and menstrual blood-derived stem cells. Cell Transplant. 2011;20(1):85–94. doi: 10.3727/096368910X532855. [DOI] [PubMed] [Google Scholar]
- 34.Santamaria X, Massasa EE, Feng Y, Wolff E, Taylor HS. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther. 2011;19(11):2065–2071. doi: 10.1038/mt.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109(10):1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328(1):258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 38.Snykers S, Vanhaecke T, Papeleu P, Luttun A, Jiang Y, van der Heyden Y, Verfaillie C, Rogiers V. Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci. 2006;94(2):330–341. doi: 10.1093/toxsci/kfl058. [DOI] [PubMed] [Google Scholar]
- 39.Stephenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130(4):1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Toyoda M, Cui C, Umezawa A. Myogenic transdifferentiation of menstrual blood-derived cells. Acta Myol. 2007;26(3):176–178. [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, Ochiya T. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37(5):983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- 42.Yu J, Yang J, Pan Q, Ma J, Li J, Li Y, Cao H, Wang Y, Li L. In vivo hepatic differentiation of mesenchymal stem cells from human umbilical cord blood after transplantation into mice with liver injury. Biochem Biophys Res Commun. 2012;422(4):539–545. doi: 10.1016/j.bbrc.2012.04.156. [DOI] [PubMed] [Google Scholar]