Abstract
Background and objective: Botulinum toxin type A (BoNT/A) is a metalloprotease that blocks synaptic transmission via the cleavage of a synaptosomal-associated protein of 25 kDa (SNAP-25). It has gained widespread use as a treatment for cerebral palsy and skeletal muscle hypertrophy. In China, Chinese botulinum toxin type A (CBTX-A), a type of BoNT/A, is in widespread clinical use. However, the changes in the morphological and biochemical properties of treated muscles and in remote muscles from the CBTX-A injection site are relatively unknown. Therefore, we investigated the changes in histomorphology and myosin heavy chain (MyHC) isoform composition and distribution in rat gastrocnemius muscles after intramuscular injection of CBTX-A. Methods: The weakness of the injected muscles was assessed periodically to identify their functional deficiency. Muscle slices were stained with hematoxylin-eosin (HE) and adenosine triphosphatase (ATPase). MyHC isoform composition was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to uncover changes in morphological and biochemical properties. Results: Our findings demonstrate that following injection of CBTX-A 5 U into rat gastrocnemius muscles, shifts in MyHC isoform composition emerged on the third day after injection and peaked in the fourth week. The composition remained distinctly different from that of the control group after the twelfth week. More specifically, there was a decrease in the proportion of the type IIb isoform and an increase in the proportions of type IIx, type IIa, and type I isoforms. Conclusions: Data revealed that CBTX-A led to a shift in MyHC composition towards slower isoforms and that the MyHC composition remained far from normal six months after a single injection. However, no noticeable remote muscle weakness was induced.
Keywords: Botulinum toxin type A (BoNT/A), Myosin heavy chain, Chemodenervation, Remote effect
1. Introduction
Botulinum toxin type A (BoNT/A) has been widely used to treat diseases that feature excessive and spastic muscle contraction. It acts selectively at the neuromuscular junction and prevents the fusion of synaptic vesicles from the nerve terminal by cleaving the synaptosomal-associated protein of 25 kDa (SNAP-25), thereby blocking neuromuscular transmission and decreasing the size and maximal strength of the target muscle (Roy et al., 2002). This action is widely known as chemodenervation. Three preparations of BoNT/A are widely used in China: Dysport (Ipsen Pharmaceuticals, Paris, France), Botox (Allergan Inc., Irvine, California, USA), and Chinese botulinum toxin type A (CBTX-A; Institute of Biological Products, Lanzhou, China). These three BoNT/A preparations differ in terms of their units of weight, chemical properties, biological activities, and mouse LD50 units (the amount of a substance that is required to kill 50% of a test population) (Tang and Wan, 2000; Dressler and Hallett, 2006).
Research findings revealed that in skeletal muscles, the use of Dysport not only weakened the force of muscle contraction, but also changed the composition of myosin heavy chain (MyHC) isoform, which plays a vital role in determining the strength and speed of muscle shortening (Dodd et al., 2005). In general, denervation causes the faster MyHC isoforms to shift towards slower isoforms (DelGaudio and Sciote, 1997; Jakubiec-Puka et al., 1999; Roy et al., 2002). However, changes in morphological and biochemical properties under the action of CBTX-A are not well understood. It is also unclear how changes in the morphologies of type I and type II MyHC isoforms occur and whether the injected muscle fibers can be restored to their original sizes.
Clinical trials of Dysport and Botox show also that local injection of BoNT/A in the target muscle using an appropriate dose results in changes in single fiber electromyography in remote muscles. This is regarded as the remote effect of BoNT/A (Olney et al., 1988; Girlanda et al., 1992; Dodd et al., 2005), but whether local intramuscular injection of CBTX-A results in obvious changes in the morphological and biochemical properties of uninjected skeletal muscles is still unknown.
Our research aimed to investigate the impact of local intramuscular injection of CBTX-A on the target muscle fibers of the gastrocnemius and on uninjected remote muscles. We measured dynamic changes in muscle weakness and morphology, the cross-sectional area of muscle fibers, and the composition of MyHC isoforms, to provide fundamental research data for the clinical applications of CBTX-A.
2. Materials and methods
Sixty adult male Sprague-Dawley rats (290 to 310 g in weight) were caged in the same room, maintained on a 12-h light/dark photoperiod, and fed standard rat chow. All rats were randomly allocated to either a sham-injected control group (normal saline) or a treatment group (CBTX-A 5 U/0.1 ml). They were temporarily anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) before injection in the right gastrocnemius. We divided the control and treatment groups into five time point subgroups. The subgroups, each comprising six rats, participated in experiments on Days 3 and 10 and in Weeks 4, 12, and 24 after intramuscular injection, in the treatment group and the control group.
2.1. Digit abduction score (DAS) for gastrocnemius muscle weakness
Local muscle weakening of the gastrocnemius was measured on Days 3 and 10, and in Weeks 4, 12, and 24 after injection using the DAS, which was scored using a five-point scale (from 0 standing for normal to 4 standing for maximal reduction in digit abduction) (Aoki, 2001). Researchers, blind to the grouping and treatment conditions, quickly lifted up the tails of the rats. The rats showed characteristic panic behavior, straightening their hind paws and stretching out their toes. Gastrocnemius muscle weakness was graded immediately, according to the DAS.
2.2. Tissue collection and processing
After induction of anesthesia, a skin incision was made on the medial surface of the right hind limb. The femoral artery was isolated from the surrounding structures and cut. Rats were sacrificed by femoral bleeding. The gastrocnemius muscle, injected with either normal saline or BoNT/A, was dissected immediately and weighed. The gastrocnemius specimens prepared for MyHC analyses were frozen in liquid nitrogen and stored at −80 °C until used.
2.3. Hematoxylin-eosin (HE) and adenosine triphosphatase (ATPase) staining
Hematoxylin stains nucleic acids a deep blue purple while eosin stains proteins non-specifically pink or red. Thus, the muscle cell nucleus is stained blue while the cytoplasm is stained pink or red. Sections of 8 μm thickness were cut using a freezing microtome (MICROM Company, HM560 model). HE staining was subsequently performed using standard protocols. ATPase staining identifies muscle fibers as type I or type II according to their color staining characteristics and the pH of the incubation solution. The base incubation solution (pH 10.4) does not develop color in type I muscle fibers but develops dark staining of type II muscle fibers. Microscopic analysis of specimens was carried out at 100× and 400× magnifications using an optical microscope (Leica Company, Germany) to compare the shape of the muscle fibers, their cross-sectional areas, and the distribution of muscle fiber types. After calibration using a scale micrometer, the Image-Pro Plus image processing system was used to measure the cross-sectional areas of type I and type II muscle fibers. Each cross-section included 200–500 fibers, and five sections of each muscle were counted for analysis. Sections were chosen at random distances from the tendon to the middle regions of the analyzed muscles.
2.4. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Protein was extracted from 200 mg of gastrocnemius tissue frozen at −80 °C. The tissue was added to liquid nitrogen in a mortar and then mashed immediately with a pestle until ground to a homogenate. All operations were performed in an ice bath. Centrifugation was carried out at 12 000 r/min at 4 °C for 10 min (Haraeus Company, Germany). The supernatant fluid was quantified using a BCA™ protein assay kit. Protein was mixed with 2× loading buffer at a proportion of 1:1 (v/v), the mixed solution was heated to 100 °C for 5 min, and 100 μg protein was loaded onto each gel. Based on a modified protocol for separating rat skeletal muscle MyHC isoforms, the stacking gels were composed of 30% glycerol (0.3 g/ml), 4% acrylamide (0.04 g/ml) including bis (50:1, w/w), 4 mmol/L ethylenediaminetetraacetic acid (EDTA), 70 mmol/L Tris (pH 6.7), and 0.4% sodium dodecyl sulfate (4 g/L). The separating gels were composed of 35% glycerol (0.35 g/ml), 8% acrylamide (0.08 g/ml) including bis (50:1, w/w), 0.1 mol/L glycine, 0.2 mol/L Tris (pH 8.8), and 0.4% sodium dodecyl sulfate (4 g/L) (Talmadge and Roy, 1993; Dodd et al., 1998). A constant voltage of 80 V was maintained for 24 h during electrophoresis at 4 °C. After completion of electrophoresis, the gel was stained with Coomassie blue (R250) for 1 to 2 h. The target protein band was scanned on the gel using a scanner after destaining (50% methanol, 10% acetic acid) over night. A gel image analytic system (Kodak digital science system DC-120) was used to analyze the gray value of the target electrophoresis band. The background was subtracted, and the density of each MyHC isoform band was expressed as a percentage of the total. The MyHC isoform composition was analyzed individually for six samples in each group.
2.5. Statistical methods
All data were processed statistically using SPSS13.0 software. A two-way analysis of variance (ANOVA) with repeated measures was used to assess DAS scores and changes in the wet weight of muscle. MyHC isoform data were assessed using a one-way ANOVA. Statistical significance was established at the level of P<0.01.
3. Results
3.1. DAS for gastrocnemius muscle weakness
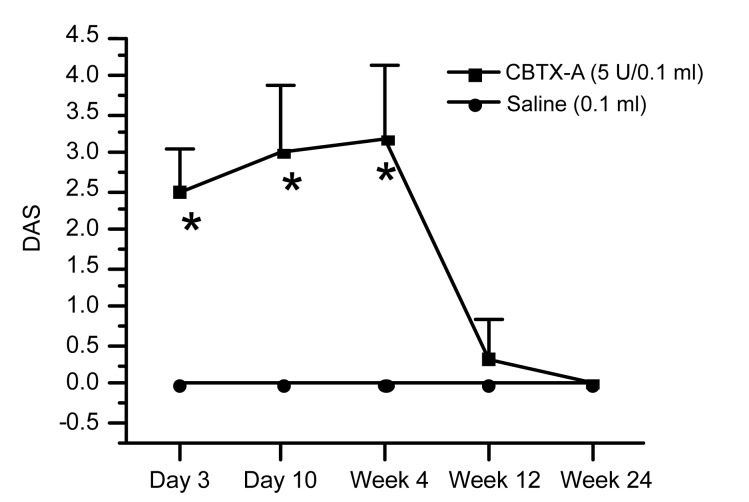
DAS results for the BoNT/A-treated gastrocnemius on Days 3 and 10, and in Weeks 4, 12, and 24 after injection were 2.50±0.54, 3.00±0.89, 3.17±0.98, 0.33±0.52, and 0, respectively. The data indicated that muscle weakening emerged on Day 3 and peaked on Day 10 and persisted for three weeks (P<0.01). Muscle strength was restored almost to the baseline level 12 weeks after injection (Fig. 1). In contrast, the results following injection of normal saline into the gastrocnemius in the control group and contralateral limbs in the two subgroups were all 0.
Fig. 1.
Digit abduction score (DAS) for the CBTX-A-injected gastrocnemius muscles
Gastrocnemius muscles were injected with 0.1 ml CBTX-A (5 U) or normal saline (NS). There were six rats in each group. Data represent mean±SD. * P<0.01, compared with the control group
3.2. Muscle wet weight
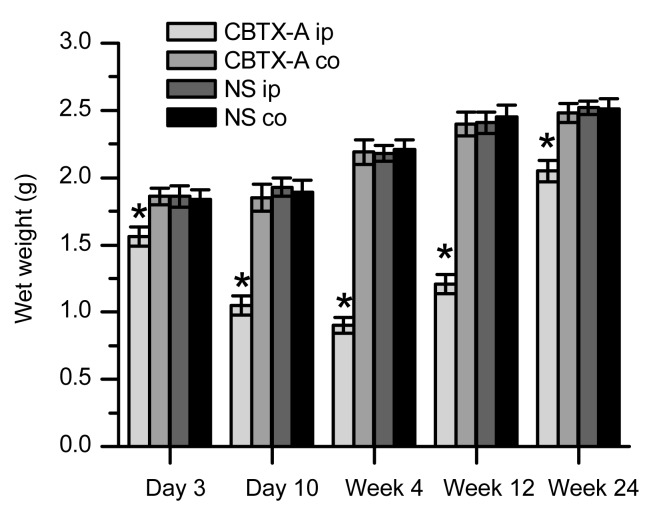
Fig. 2 illustrates the changes in the wet weight of the gastrocnemius tissue in the treatment group and the control group. The wet weight of the gastrocnemius in the treatment group was significantly reduced (by 16.1%) compared to the control group, and declined to a minimum in Week 4 (41.3%) and Week 12 (50.2%). The wet weight was restored to 81.3% by Week 24. Comparisons of the wet weight of contralateral gastrocnemius tissue in the two groups at each time point showed no significant differences (P>0.05). This suggests that after injection of CBTX-A 5 U into the gastrocnemius, CBTX-A induced local muscle atrophy, while wet weight loss in the contralateral limb (a remote site) was not detected.
Fig. 2.
Wet weight of gastrocnemius tissue from both limbs in the treatment group and the control group
The CBTX-A-injected gastrocnemius muscle (CBTX-A ip) is compared with the contralateral site of the treatment group (CBTX-A co) and with both sides of the control group (NS ip and NS co). There were six rats in each group. Data represent mean±SD. * P<0.01
3.3. HE staining of gastrocnemius muscles
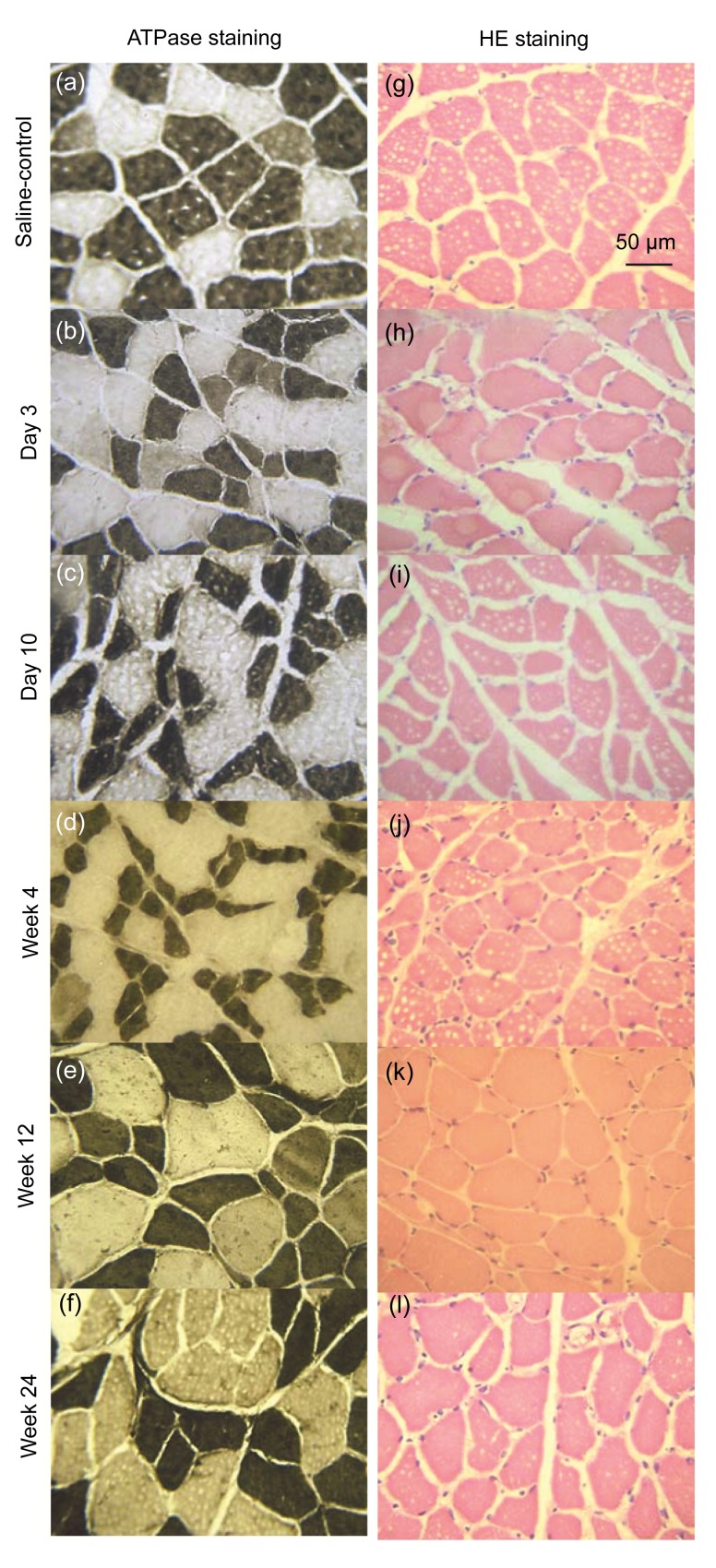
Staining of frozen sections of gastrocnemius muscle with HE stained the cell nucleus blue and the cytoplasm pink/red (Figs. 3g–3l). The cross-sectional area of muscle fibers at the injection site in the treatment group was reduced by Day 3 (Fig. 3h). The fibers had a sharp angular appearance. Fibers with these irregular patterns increased over time in association with a reduction in their cross-sectional area. Restoration of the cross-sectional area emerged gradually, starting from the Week 12 (Fig. 3k). No denaturation or necrosis was observed throughout the experiment.
Fig. 3.
ATPase enzyme and HE stainings of gastrocnemius muscles (specimen incubated at pH 10.4)
(a) ATPase enzyme staining of the uninjected gastrocnemius muscle in the control group; (b–f) ATPase enzyme staining of the CBTX-A-injected gastrocnemius muscle in the treatment group (after 3 d, 10 d, and 4 weeks, 12 weeks, and 24 weeks, respectively); (g) HE staining of the normal saline-injected gastrocnemius muscle in the control group; (h–l) HE staining of the CBTX-A-injected gastrocnemius muscle in the treatment group (after 3 d, 10 d, and 4 weeks, 12 weeks, and 24 weeks, respectively). There were six rats in each group
3.4. ATPase enzyme staining of gastrocnemius muscles
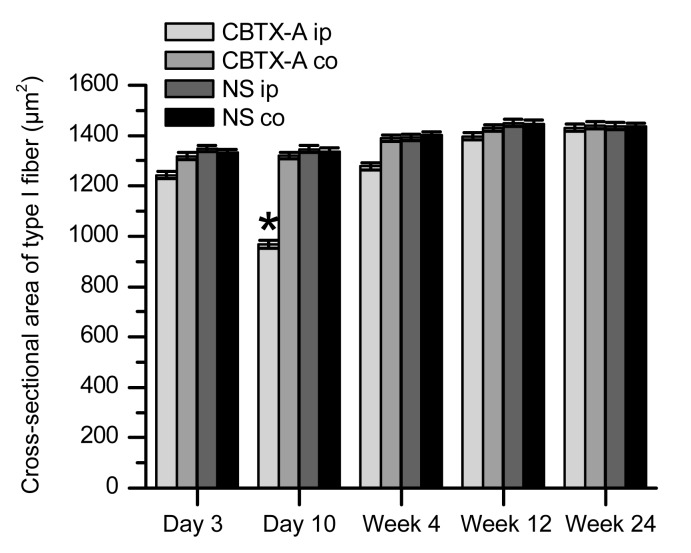
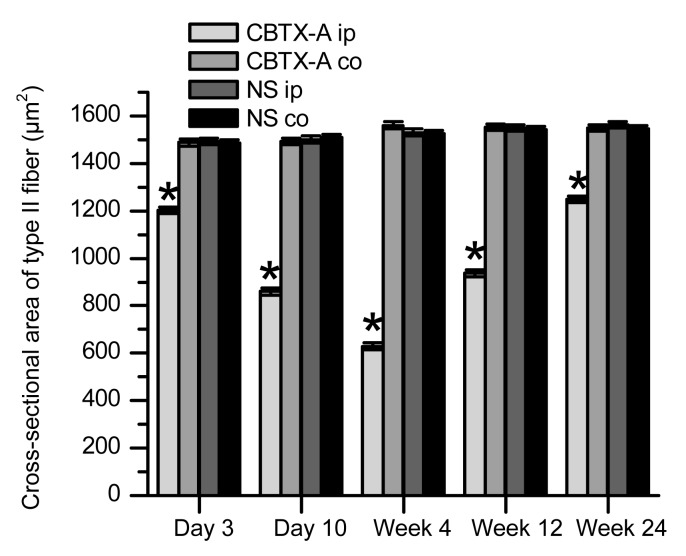
This stain identified muscle fibers as type I or type II according to the staining characteristics of the frozen sections. The basic incubation solution (pH 10.4) darkly stained the type II muscle fibers but did not color type I muscle fibers (Figs. 3a–3f). According to the features described above, we observed the two types of muscle fibers in the treatment and control groups by optical microscopy to assess how different muscle fiber types changed under the action of BoNT/A. Transverse sections of muscle fiber displayed an obtuse polygon configuration and the average cross-sectional area of type II muscle fibers was slightly larger than that of type I fibers in the control group. Using the Image-Pro Plus image processing system to measure the cross-sectional areas of the type I and type II muscle fibers (Figs. 4 and 5) revealed variation between the two fiber types in the treatment and control groups. The data showed that the cross-sectional area of type I muscle fibers of the BoNT/A-injected gastrocnemius was smaller than that of the normal saline-injected gastrocnemius (P<0.01) 10 d after injection (Figs. 3c and 4). This difference began to disappear by Week 4 (Figs. 3d and 4). The cross-sectional area of type II muscle fibers of the BoNT/A-injected gastrocnemius was also less than that of the normal saline-injected gastrocnemius (P<0.01) 3 d after injection (Figs. 3b and 5). This persisted from Day 10 to Week 4 after injection, but began to restore by Week 12 (Figs. 3c–3e and 5).
Fig. 4.
Comparison of cross-sectional areas of type I fibers between the treatment and control groups
The CBTX-A-injected gastrocnemius muscle (CBTX-A ip) is compared with the contralateral site in the treatment group (CBTX-A co) and with both sides in the control group (NS ip and NS co). There were six rats in each group. Data represent mean±SEM. * P<0.01
Fig. 5.
Comparison of cross-sectional areas of type II fibers between the treatment and control groups
The CBTX-A-injected gastrocnemius muscle (CBTX-A ip) is compared with the contralateral site in the treatment group (CBTX-A co) and with both sides in the control group (NS ip and NS co). There were six rats in each group. Data represent mean±SEM. * P<0.01
The restoration of cross-sectional area of the type II muscle fibers was less than that of the type I muscle fibers of the normal saline-injected gastrocnemius (P<0.01).
The cross-sectional areas of type I and type II muscle fibers of the uninjected gastrocnemius in the two groups were not significantly different (P>0.05). This suggests that the injection of CBTX-A 5 U in one side of the gastrocnemius in the treatment group did not cause a reduction in the cross-sectional area of the contralateral gastrocnemius (remote muscle fibers).
3.5. MyHC SDS-PAGE
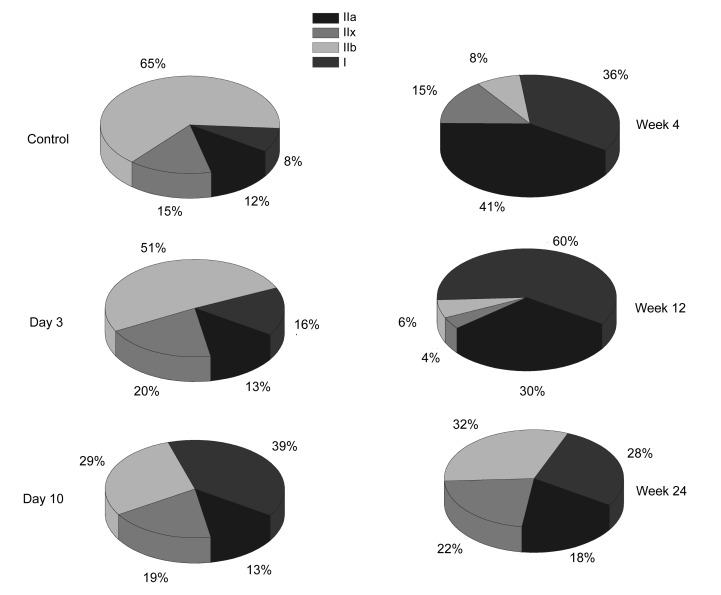
The composition of MyHC isoforms in the CBTX-A-injected gastrocnemius and in the normal saline-injected gastrocnemius was analyzed by SDS-PAGE. Four MyHC isoforms were clearly separated and identified (Fig. 6). The relative proportions of the four subtypes were estimated (Fig. 7). Data revealed that the proportion of MyHC I on Day 3 post-injection increased (16%) (P<0.01), and that this increase peaked in Week 12 (~60%) and then declined by Week 24 (28%). The proportion remained higher than that of the control group (8%) (P<0.01). The proportion of MyHC IIb declined by Day 3 (65%) (P<0.01) and reached a minimum by Week 12 (6%) (P<0.01). It then increased by Week 24 (32%), but was still lower than that of the control group (65%) (P<0.01).
Fig. 6.
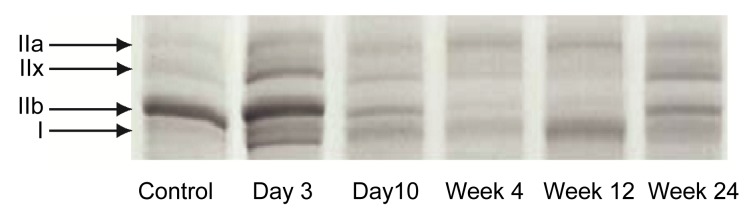
Subtypes of myosin heavy chain (MyHC) separated by SDS-PAGE from the CBTX-A-injected gastrocnemius in the treatment group after 3 d, 10 d, 4 weeks, 12 weeks, and 24 weeks, and those of the normal saline-injected gastrocnemius from the control group
MyHC isoform composition was analyzed individually for six samples from each group
Fig. 7.
Changes in the proportion of different types of muscle fibers at five time points (Day 3, Day 10, Week 4, Week 12, and Week 24) after injection of CBTX-A
The proportion of MyHC IIa increased (from 12% to a peak of 41%) until Week 4 (P<0.01), but then gradually declined from Week 12 (30%) to Week 24 (18%); the proportion of MyHC IIx increased by Day 3 (from 15% to 20%), declined from Week 4 (15%) to Week 12 (4%), but then increased again by Week 24 (22%). Overall, the MyHC subtype components showed a tendency towards an increased proportion of slow fibers under the action of BoNT/A, and a corresponding decline in the proportion of fast muscle fibers.
4. Discussion
BoNT/A blocks the fusion of synaptic vesicles with the nerve terminal and simultaneously restricts the release of acetylcholine (Schiavo et al., 2000; Turton et al., 2002). The neurotransmitter, acetylcholine, is also a vital trophic factor for muscle (Drachman and Johnston, 1975; Fu and Liu, 1997). Therefore, although the chemodenervation effect of BoNT/A is different from neurotomy, they both change muscle morphology and physiological functions. Many previous studies have focused on Dysport and Botox. Although they are the same toxin, the preparations are different in terms of unit of weight, chemical properties, biological activities, and mouse LD50 units, which results in different outcomes in clinical treatment (Dressler and Hallett, 2006; Bentivoglio et al., 2009). Tang and Wan (2000) showed some differences in the clinical use of Botox and CBTX-A, but changes in morphological and biochemical properties under the action of CBTX-A are relatively unknown. Our research suggests that CBTX-A caused muscle atrophy and that the injected muscles were unable to recover completely. Muscle atrophy appeared 3 d after injection and peaked after 4 weeks, and the muscle started to recover after 12 weeks. Type II muscle fibers were the major fiber type demonstrating atrophy. Furthermore, a single injection of CBTX-A 5 U into the gastrocnemius produced no obvious atrophic effects in the muscle fibers of remote muscle (the contralateral gastrocnemius). Finally, intramuscular injection of CBTX-A induced shifts in MyHC isoform composition, which showed a tendency towards an increased proportion of slow muscle fibers.
4.1. DAS for muscle weakness
DAS suggested that gastrocnemius muscle injected with CBTX-A showed obvious weakness 3 d after injection. The weakness peaked from Day 10 to Week 4, but normal muscle strength was almost restored by Week 12. Aoki (2001) tested two other brands and showed that the dose for the median effective dose of intramuscular (IM ED50) (DAS=2, Day 2 or 3 post-injection) was 6.2 U/kg for Dysport and 22.9 U/kg for Botox. The IM ED50 of CBTX-A (<16.7 U/kg) in our study was intermediate. Tang and Wan (2000) also found that a higher dose of the Chinese preparation was needed to produce effects similar to Botox. In contrast to previous studies, no non-target muscle weakness was observed in our experiment. Dodd et al. (2005) found that unilateral injection of 3 U (≈13.6 U/kg) Dysport into rat gastrocnemius muscle caused non-target muscle weakness. A possible reason for this discrepancy is that Dysport is more diffusible than CBTX-A. Bentivoglio et al. (2009) compared the side effects of Botox and Dysport and suggested that due to its high efficiency of diffusion, Dysport causes many more side effects than Botox in clinical treatment.
4.2. Muscle wet weight
The experimental results showed that the wet weight of the gastrocnemius muscle had declined to 83.9% of that of the control 3 d after injection. Muscle weight decreased continuously until Day 10 and then declined to the minimum weight (41.3% of the control group) by Week 4. The wet weight then increased gradually and reached 50.2% by Week 12 and 81.3% by Week 24, but was still less than the baseline weight (P<0.01). Our results agree with those of previous studies. Ma et al. (2004) injected 6 U/kg of Botox into the gastrocnemius of rats and observed that the wet weight declined 3 d after injection and peaked after two weeks. The wet weight declined by 32% in the active site compared with the contralateral site, and was restored to 92% after six months. Ahn et al. (2004) used Botox to treat benign masseter hypertrophy and observed that the masseter shrank the most in the first and second months after injection. The therapeutic effect lasted six months. Lee et al. (2004) found that the most prominent muscle atrophy of human gastrocnemius injected with Botox occurred in the first month after injection and that the effect lasted six months. We suggest that Botox and CBTX-A share the same action period. However, in agreement with the DAS results, contralateral gastrocnemius atrophy was not detectable as wet weight loss after injection of CBTX-A. Previous studies have shown that after a single intramuscular injection of Dysport (Dodd et al., 2005) or repeated intramuscular injection of Botox (Fortuna et al., 2011), non-target muscle atrophied. The probable explanations are as follows: (1) the relatively low diffusion efficiency of CBTX-A does not cause atrophy or weakness in the non-target muscle; (2) wet weight loss is not a sensitive way to detect remote atrophy under the influence of CBTX-A. Lange et al. (1991) injected a low dose of BoNT/A into patients and detected increased jitter in distant muscle using single fiber electromyography (SFEMG), before weakness occurred. Fortuna et al. (2011) demonstrated that contractile tissue is replaced to a large degree with fat, and therefore focusing on mass loss may underestimate the degree of atrophy. More research is needed to investigate the remote effects of CBTX-A.
4.3. Morphological changes
Muscle fibers of CBTX-A-injected gastrocnemius sections had reduced cross-sectional areas when observed under the optical microscope at different time points during the experiment. The reduced cross-sectional areas generally showed sharp angles. No degeneration or necrosis was observed.
Our research demonstrated that the cross-sectional area of type II muscle fibers of the CBTX-A-injected gastrocnemius was less than that of the control group after 3 d, and the reduction was the most obvious on Day 10 and by Week 4. Although the muscle had recovered partially by Weeks 12 and 24, the cross-sectional area was still smaller in the treatment group than in the control group. The cross-sectional area of type I muscle fibers had started to decrease by 10 d after injection and recovered after 4 weeks. This shows that type II muscle fibers atrophied earlier but recovered more slowly, while type I muscle fibers atrophied later but recovered faster. Thus, type II muscle fibers may be more sensitive to BoNT/A. Yaraskavitch et al. (2008) showed that muscles with different MyHC composition react differently in muscle force tests, after injection of the same dose of BoNT/A. They also concluded that BoNT/A might have a greater effect on fast fibers than on slow fibers.
4.4. Biochemical changes
Previous studies of the influence of BoNT/A found similar results for adult rat gastrocnemius (Dodd et al., 2005), laryngeal (Inagi et al., 1999), and ocular medial rectus muscle (Kranjc et al., 2001). No matter what kind of muscle was tested, the MyHC isoform tends to shift from IIb to IIa, IIx, and I. However, the data in our study did not show the same trend. We found no significant change in terms of the cross-sectional areas of slow fibers, suggesting that they maintained their sizes. Therefore, it is probably not MyHC isoform conversion, but just the proportion of these four MyHC isoforms that changes. A similar study in juvenile rat gastrocnemius showed that the MyHC isoform composition shifted from IIb to IIa and IIx, but found no effect on MyHC I (Legerlotz et al., 2009). This difference might be explained by the use of rats of a different age. Larsson and Ansved (1995) suggested that aging causes fast to slow fiber transitions. Therefore, we suggest muscle from rats of different ages may lead to different outcomes under the influence of BoNT/A.
However, studies on human muscle paralysis, induced by spinal cord injury (SCI), found that MyHC isoform composition can shift from slow to fast (Malisoux et al., 2007; Biering-Sørensen et al., 2009). The results conflict with our study and many others on muscle denervation induced by BoNT/A. The possible explanations for this contradiction are as follows: (1) Neuromuscular activity: under the condition of SCI, reduced neuromuscular activity may resulted in transitions in the slow to fast direction (Pette and Staron, 2000). However, due to the incomplete paralysis under the influence of BoNT/A, neuromuscular activity is relatively high, and BoNT/A might have a greater effect on fast fibers than on slow fibers (Yaraskavitch et al., 2008). (2) Mechanical loading and unloading: SCI-induced muscle paralysis results in mechanical unloading, which leads to slow to fast transition (Pette and Staron, 2000). In contrast, incomplete paralysis induced by BoNT/A can be regarded as a model of mechanical loading, which causes fast to slow transitions.
Previous research suggests that resistance exercise and nerve transection induce the same modification of the proportion of MyHC isoforms as observed in the present study (Jakubiec-Puka et al., 1999; Allen, 2008; Rinaldi et al., 2008). Chemodenervation by BoNT/A is considered as a reversible procedure. However, we found that the change in the proportion of MyHC isoforms could not be restored to the normal condition even six months after injection. Legerlotz et al. (2009) suggested that the application of a running exercise to a paralyzed gastrocnemius muscle did not reverse this transformation. So it is necessary to pay attention to these dramatic changes.
In conclusion, following CBTX-A 5 U injection of the target muscle, paralysis lasted at least four weeks. In terms of atrophy, type II muscle fibers were primarily affected. CBTX-A induced shifts in MyHC isoform composition, which showed a tendency towards an increased proportion of slow and a declining proportion of fast muscle fibers, which did not reverse until six months post-injection. A single injection of CBTX-A 5 U into the gastrocnemius had no obvious atrophic effects on remote muscles.
Footnotes
Project (No. 491030-w10011) supported by the Zhejiang Provincial Natural Science Foundation of China
Compliance with ethics guidelines: Bin HONG, Min CHEN, and Xing-yue HU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Ahn J, Horn C, Blitzer A. Botulinum toxin for masseter reduction in Asian patients. Arch Facial Plast Surg. 2004;6(3):188–191. doi: 10.1001/archfaci.6.3.188. [DOI] [PubMed] [Google Scholar]
- 2.Allen DL. Making sense (and antisense) of myosin heavy chain gene expression. Comments on “Intergenic bidirectional promoter and cooperative regulation of the IIx and IIb MHC genes in fast skeletal muscle” by Rinaldi et al. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R206–R207. doi: 10.1152/ajpregu.90380.2008. [DOI] [PubMed] [Google Scholar]
- 3.Aoki KR. A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon. 2001;39(12):1815–1820. doi: 10.1016/S0041-0101(01)00101-5. [DOI] [PubMed] [Google Scholar]
- 4.Bentivoglio AR, Fasano A, Ialongo T, Soleti F, Lo FS, Albanese A. Fifteen-year experience in treating blepharospasm with Botox or Dysport: same toxin, two drugs. Neurotox Res. 2009;15(3):224–231. doi: 10.1007/s12640-009-9023-3. [DOI] [PubMed] [Google Scholar]
- 5.Biering-Sørensen B, Kristensen IB, Kjaer M, Biering-Sørensen F. Muscle after spinal cord injury. Muscle Nerve. 2009;40(4):499–519. doi: 10.1002/mus.21391. [DOI] [PubMed] [Google Scholar]
- 6.DelGaudio JM, Sciote JJ. Changes in myosin expression in denervated laryngeal muscle. Ann Otol Rhinol Laryngol. 1997;106(12):1076–1081. doi: 10.1177/000348949710601212. [DOI] [PubMed] [Google Scholar]
- 7.Dodd SL, Rowell BA, Vrabas IS, Arrowsmith RJ, Weatherill PJ. A comparison of the spread of three formulations of botulinum neurotoxin A as determined by effects on muscle function. Eur J Neurol. 1998;5(2):181–186. doi: 10.1046/j.1468-1331.1998.520181.x. [DOI] [PubMed] [Google Scholar]
- 8.Dodd SL, Selsby J, Payne A, Judge A, Dott C. Botulinum neurotoxin type A causes shifts in myosin heavy chain composition in muscle. Toxicon. 2005;46(2):196–203. doi: 10.1016/j.toxicon.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Drachman DB, Johnston DM. Neurotrophic regulation of dynamic properties of skeletal muscle: effects of botulinum toxin and denervation. J Physiol. 1975;252(3):657–667. doi: 10.1113/jphysiol.1975.sp011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dressler D, Hallett M. Immunological aspects of Botox, Dysport and Myobloc/NeuroBloc. Eur J Neurol. 2006;13(s1):11–15. doi: 10.1111/j.1468-1331.2006.01439.x. [DOI] [PubMed] [Google Scholar]
- 11.Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox) J Biomech. 2011;44(1):39–44. doi: 10.1016/j.jbiomech.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Fu WM, Liu JJ. Regulation of acetylcholine release by presynaptic nicotinic receptors at developing neuromuscular synapses. Mol Pharmacol. 1997;51(3):390–398. [PubMed] [Google Scholar]
- 13.Girlanda P, Vita G, Nicolosi C, Milone S, Messina C. Botulinum toxin therapy: distant effects on neuromuscular transmission and autonomic nervous system. J Neurol Neurosurg Psychiatry. 1992;55(9):844–845. doi: 10.1136/jnnp.55.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagi K, Connor NP, Schultz E, Ford CN, Cook CH, Heisey DM. Muscle fiber-type changes induced by botulinum toxin injection in the rat larynx. Otolaryngol Head Neck Surg. 1999;120(6):876–883. doi: 10.1016/S0194-5998(99)70330-X. [DOI] [PubMed] [Google Scholar]
- 15.Jakubiec-Puka A, Ciechomska I, Morga J, Matusiak A. Contents of myosin heavy chains in denervated slow and fast rat leg muscles. Comp Biochem Physiol B Biochem Mol Biol. 1999;122(3):355–362. doi: 10.1016/S0305-0491(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 16.Kranjc BS, Sketelj J, D'Albis A, Erzen I. Long-term changes in myosin heavy chain composition after botulinum toxin a injection into rat medial rectus muscle. Invest Ophthalmol Vis Sci. 2001;42(13):3158–3164. [PubMed] [Google Scholar]
- 17.Lange DJ, Rubin M, Greene PE, Kang UJ, Moskowitz CB, Brin MF, Lovelace RE, Fahn S. Distant effects of locally injected botulinum toxin: a double-blind study of single fiber EMG changes. Muscle Nerve. 1991;14(7):672–675. doi: 10.1002/mus.880140711. [DOI] [PubMed] [Google Scholar]
- 18.Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45(5):397–458. doi: 10.1016/0301-0082(95)98601-Z. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Lee DW, Park YH, Cha MK, Kim HS, Ha SJ. Botulinum toxin A for aesthetic contouring of enlarged medial gastrocnemius muscle. Dermatol Surg. 2004;30(6):867–871. doi: 10.1111/j.1524-4725.2004.30255.x. [DOI] [PubMed] [Google Scholar]
- 20.Legerlotz K, Matthews KG, McMahon CD, Smith HK. Botulinum toxin-induced paralysis leads to slower myosin heavy chain isoform composition and reduced titin content in juvenile rat gastrocnemius muscle. Muscle Nerve. 2009;39(4):472–479. doi: 10.1002/mus.21247. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Elsaidi GA, Smith TL, Walker FO, Tan KH, Martin E, Koman LA, Smith BP. Time course of recovery of juvenile skeletal muscle after botulinum toxin A injection: an animal model study. Am J Phys Med Rehabil. 2004;83(10):774–780. doi: 10.1097/01.PHM.0000137315.17214.93. [DOI] [PubMed] [Google Scholar]
- 22.Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D. Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol. 2007;102(1):340–349. doi: 10.1152/japplphysiol.00609.2006. [DOI] [PubMed] [Google Scholar]
- 23.Olney RK, Aminoff MJ, Gelb DJ, Lowenstein DH. Neuromuscular effects distant from the site of botulinum neurotoxin injection. Neurology. 1988;38(11):1780–1783. doi: 10.1212/WNL.38.11.1780. [DOI] [PubMed] [Google Scholar]
- 24.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50(6):500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi C, Haddad F, Bodell PW, Qin AX, Jiang W, Baldwin KM. Intergenic bidirectional promoter and cooperative regulation of the IIx and IIb MHC genes in fast skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R208–R218. doi: 10.1152/ajpregu.00134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy RR, Zhong H, Monti RJ, Vallance KA, Edgerton VR. Mechanical properties of the electrically silent adult rat soleus muscle. Muscle Nerve. 2002;26(3):404–412. doi: 10.1002/mus.10219. [DOI] [PubMed] [Google Scholar]
- 27.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80(2):717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 28.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75(5):2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- 29.Tang X, Wan X. Comparison of Botox with a Chinese type A botulinum toxin. Chin Med J (Engl) 2000;113(9):794–798. [PubMed] [Google Scholar]
- 30.Turton K, Chaddock JA, Acharya KR. Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. Trends Biochem Sci. 2002;27(11):552–558. doi: 10.1016/S0968-0004(02)02177-1. [DOI] [PubMed] [Google Scholar]
- 31.Yaraskavitch M, Leonard T, Herzog W. Botox produces functional weakness in non-injected muscles adjacent to the target muscle. J Biomech. 2008;41(4):897–902. doi: 10.1016/j.jbiomech.2007.11.016. [DOI] [PubMed] [Google Scholar]