Abstract
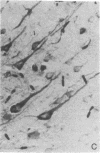
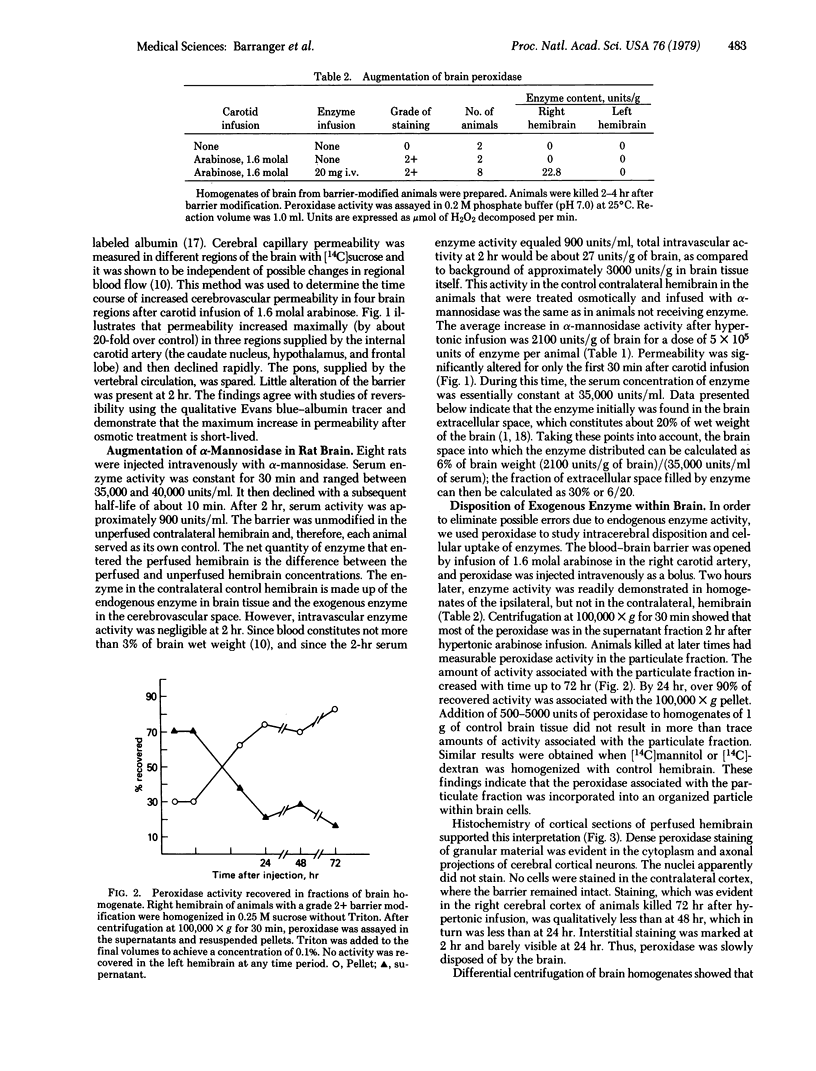
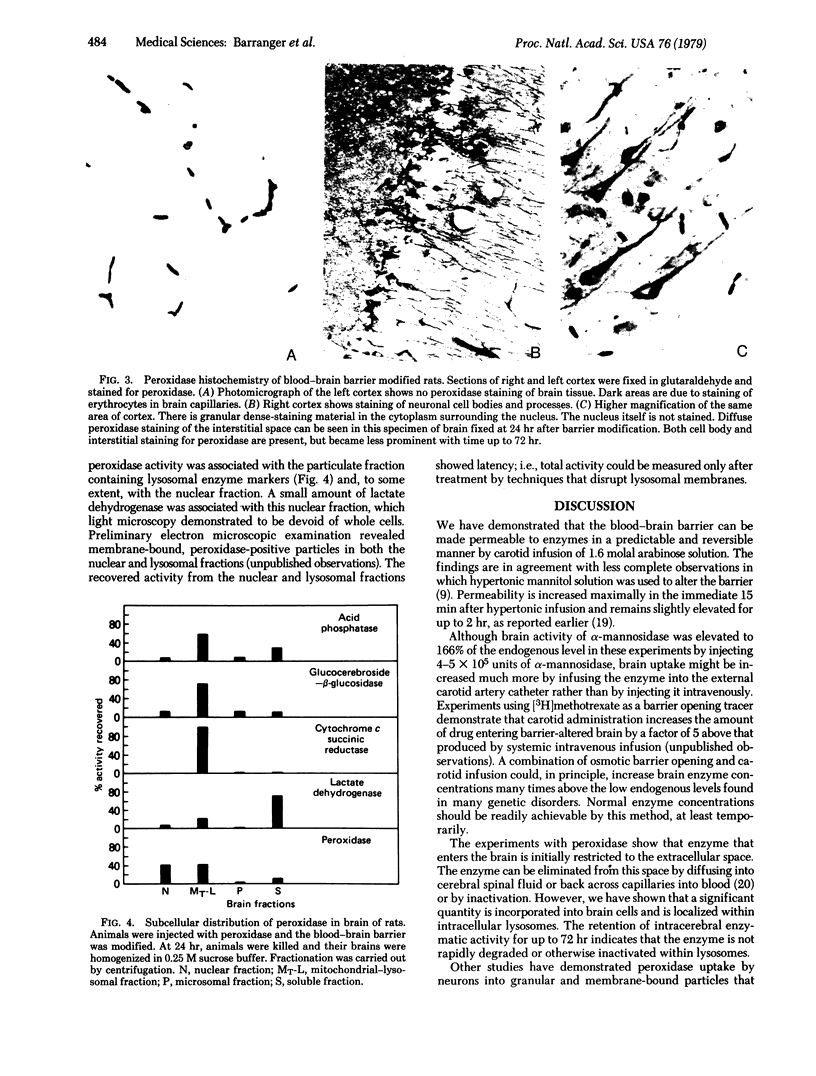
The blood-brain barrier of rats was opened reversibly by infusing a hyperosmotic solution of arabinose into the external carotid artery. Permeability was increased maximally in the first 15 min and remained slightly elevated at 1 hr. Osmotic barrier opening significantly increased brain uptake of intravenously injected alpha-mannosidase (alpha-D-mannoside mannohydrolase, EC 3.2.1.2.4) (derived from human placenta) and horseradish peroxidase (donor:hydrogen-peroxide oxidoreductase, EC 1.11.1.7). By injection of 4 X 10(5) units of alpha-mannosidase into an animal, brain activity rose to about twice the normal control activity of the enzyme. After 30 min, activity of administered enzyme in the extracellular space of the brain was calculated to be 30% of the serum concentration. Biochemical and histological studies with horseradish peroxidase showed that exogenously administered enzyme entered the brain extracellular space immediately after barrier opening and was incorporated within neuronal lysosomal packets during the next 24 hr. Measurable peroxidase activity was found in brain as much as 72 hr after osmotic treatment. The results demonstrate that the blood-brain barrier can be reversibly opened to enzymes, that a glycoprotein enzyme is incorporated into neuronal lysosomes, and that the brain may now be considered a potential target for enzyme replacement therapy in heritable metabolic disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMADOR E., DORFMAN L. E., WACKER W. E. SERUM LACTIC DEHYDROGENASE ACTIVITY: AN ANALYTICAL ASSESSMENT OF CURRENT ASSAYS. Clin Chem. 1963 Aug;12:391–399. [PubMed] [Google Scholar]
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Hori M., Rapoport S. I., Reese T. S., Westergaard E. Osmotic opening of tight junctions in cerebral endothelium. J Comp Neurol. 1973 Dec 15;152(4):317–325. doi: 10.1002/cne.901520402. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell R. D., Brightman M. W. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol. 1976 Apr 1;166(3):257–283. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- Chiueh C. C., Sun C. L., Kopin I. J., Fredericks W. R., Rapoport S. I. Entry of [3H]norepinephrine, [125I]albumin and Evans blue from blood into brain following unilateral osmotic opening of the blood-brain barrier. Brain Res. 1978 Apr 28;145(2):291–301. doi: 10.1016/0006-8993(78)90863-6. [DOI] [PubMed] [Google Scholar]
- Colman D. R., Scalia F., Cabrales E. Light and electron microscopic observations on the anterograde transport of horseradish peroxidase in the optic pathway in the mouse and rat. Brain Res. 1976 Jan 30;102(1):156–163. doi: 10.1016/0006-8993(76)90582-5. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbish F. S., Blair H. E., Shiloach J., Pentchev P. G., Brady R. O. Enzyme replacement therapy in Gaucher's disease: large-scale purification of glucocerebrosidase suitable for human administration. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3560–3563. doi: 10.1073/pnas.74.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hicks J. T., Albrecht P., Rapoport S. I. Entry of neutralizing antibody to measles into brain and cerebrospinal fluid of immunized monkeys after osmotic opening of the blood-brain barrier. Exp Neurol. 1976 Dec;53(3):768–779. doi: 10.1016/0014-4886(76)90154-0. [DOI] [PubMed] [Google Scholar]
- Itaya S. K., Williams T. H., Engel E. L. Anterograde transport of horseradish peroxidase enhanced by poly-L-ornithine. Brain Res. 1978 Jul 7;150(1):170–176. doi: 10.1016/0006-8993(78)90661-3. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Fenstermacher J. D. Measurements of dog blood-brain transfer constants by ventriculocisternal perfusion. Am J Physiol. 1975 Oct;229(4):877–884. doi: 10.1152/ajplegacy.1975.229.4.877. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Hori M., Klatzo I. Reversible osmotic opening of the blood-brain barrier. Science. 1971 Sep 10;173(4001):1026–1028. doi: 10.1126/science.173.4001.1026. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Hori M., Klatzo I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am J Physiol. 1972 Aug;223(2):323–331. doi: 10.1152/ajplegacy.1972.223.2.323. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Ohno K., Fredericks W. R., Pettigrew K. D. Regional cerebrovascular permeability to [14C]sucrose after osmotic opening of the blood-brain barrier. Brain Res. 1978 Jul 21;150(3):653–657. doi: 10.1016/0006-8993(78)90832-6. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I. Opening of the blood-brain barrier by acute hypertension. Exp Neurol. 1976 Sep;52(3):467–479. doi: 10.1016/0014-4886(76)90218-1. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Thompson H. K. Osmotic opening of the blood-brain barrier in the monkey without associated neurological deficits. Science. 1973 Jun 1;180(4089):971–971. doi: 10.1126/science.180.4089.971. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]