Abstract
Chemoresistance of cancer cells has been a severe problem in multiple types of cancers. One possibility is to combine different drugs with chemotherapy for improved efficacy. Cyclopamine blocks Hedgehog signaling by antagonizing Smo function, which induces tumor apoptosis. Here, we show that the combined use of cyclopamine and paclitaxel (chemotherapy drugs) was able to induce breast cancer cell apoptosis both in vivo and in vitro. The results suggest that Hedgehog signaling is a prospective drug target for chemoresistant cancer cells.
Keywords: chemoresistance, Hedgehog signaling, cyclopamine, paclitaxel, cancer stem cell
Introduction
Cyclopamine blocks Hedgehog signaling by antagonizing Smo function, which leads to apoptotic cell deaths.1–5 The application of cyclopamine causes very few adverse effects in animals, and therefore demonstrates its usefulness in clinical applications. In recent years, cyclopamine has been used effectively in treatments for multiple types of cancers, both in vitro and in vivo, such as prostate cancer and pancreas cancer.6–10 Hedgehog signaling is also important for breast cancer cells,11–13 contributing to tumor stem cell maintenance and recurrence.14–16 Here, we describe the use of cyclopamine to antagonize the growth and chemoresistance of breast cancer cells. The results suggest cyclopamine as a prospective conjugate in clinical therapies.
Materials and methods
Ethics statement
This study was approved by the Animal Research Committee of Zhejiang XiaoShan Hospital (ZJXS2009-1073SJ).
Cell culture
MDA-MB-231 human breast cancer cells were purchased from Shengsheng Logistics (Shanghai, People’s Republic of China) and maintained in Roswell Park Memorial Institute 1640 medium (Life Technologies, Carlsbad, CA, USA). Paclitaxel (Life Technologies) at 50 μM was chosen as the chemotherapeutic drug, as previously described, to induce cell apoptosis.2,5 Cyclopamine at 20 μM was included to examine the effects of conjugated treatments.
Cell viability and apoptosis
Cell viability was examined with an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. The MTT assay was done with an MTT kit (EMD Millipore, Billerica, MA, USA), following the brochure carefully. Finally, each experiment was repeated at least three times.
The apoptotic cells were detected by a caspase-3 activity kit (Merck, Darmstadt, Germany) and a TUNEL (terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling) kit (Roche, Basel, Switzerland). The staining was performed as described in the kit. Then, the cells were counted for ten random sites at 40× magnification after staining. For confirmation of the results, DAPI (4′,6-diamidino-2-phenylindole; 15 μg/mL, Sigma-Aldrich, St Louis, MO, USA) was occasionally employed for nuclei staining.
Xenograft
Sixty nude mice were injected with 2 × 106 cancer cells into the flank for tumor establishment for 3 weeks. Then, the mice were subdivided into three groups: control group with saline injection (every 3 days), paclitaxel (20 mg/kg/day)-treated (every 3 days), and paclitaxel (20 mg/kg/day) plus cyclopamine (25 mg/kg/day)-treated (every 3 days). The mice were killed 6 weeks and 9 weeks after the start of cancer cell transplantation for tumor harvesting. The size of the tumors was measured. Then, the tissue was fixed in 4% paraformaldehyde for 48 hours before being processed for paraffin embedding. Then, 5 μm sections were prepared for TUNEL staining, and the number of apoptotic cells within the tumor was determined by positive cells/hematoxylin and eosin-stained cells.
Statistics
The data are presented as means ± standard deviation and were analyzed with SPSS 13.0 (IBM Corporation, Armonk, NY, USA) software. The group data were compared with analysis of variance and paired t-tests. P<0.05 was determined as statistically significant.
Results
Cyclopamine-enhanced paclitaxel-induced cell death
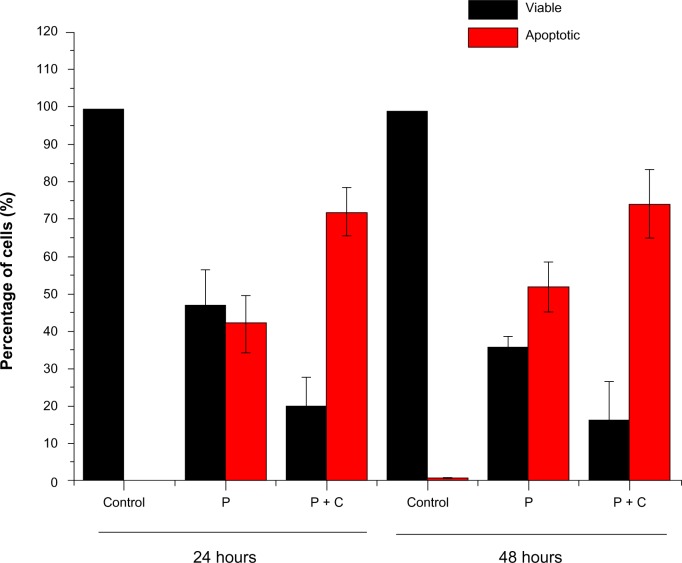
We found at both the 24-hour and 48-hour time points that the addition of cyclopamine had further enhanced paclitaxel-induced cell death, reflected by both decreased percentage of viable cells and increased percentage of apoptotic cells (P<0.05; Table 1 and Figure 1).
Table 1.
Cyclopamine enhances paclitaxel-induced cell death
| 24 hours
|
48 hours
|
|||||
|---|---|---|---|---|---|---|
| Control | Paclitaxel | +Cyclopamine | Control | Paclitaxel | +Cyclopamine | |
| Viable cells | 99.4% ± 0.1% | 47.0% ± 9.4%* | 20.1% ± 7.3%# | 98.7% ± 0.09% | 35.6% ± 2.8%* | 16.4% ± 10.2%# |
| Number of repeated experiments | 3 | 6 | 6 | 3 | 6 | 6 |
| Apoptotic cells | 0.2% ± 0.03% | 42.1% ± 7.7%* | 71.9% ± 6.4%# | 0.6% ± 0.07% | 51.8% ± 6.9%* | 74.2% ± 9.3%# |
| Number of repeated experiments | 5 | 8 | 8 | 5 | 6 | 6 |
Notes:
P<0.05 compared to control group
P<0.05 compared to paclitaxel-treated group.
Figure 1.
Cyclopamine-enhanced paclitaxel-induced cell death. Cyclopamine further enhances the cell apoptosis (red) and reduce the viable cells (black) at both the 24-hour and 48-hour time points.
Abbreviations: P, paclitaxel; C, cyclopamine.
Cyclopamine–paclitaxel combined treatment decreased tumor growth in xenograft
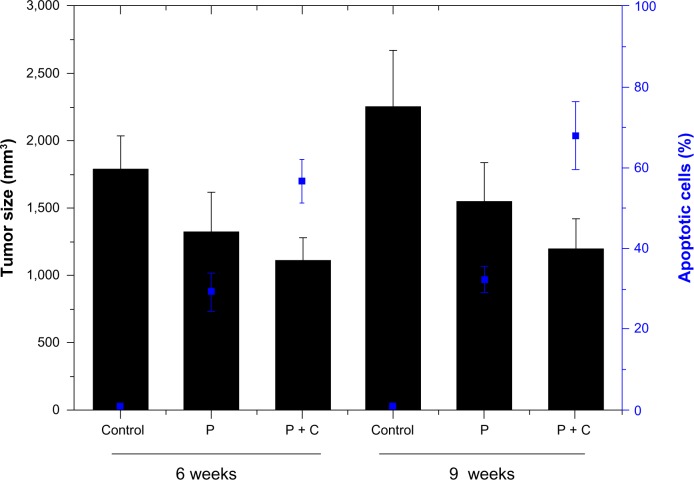
We further found that in xenograft-transplanted mice, the administration of paclitaxel reduced tumor growth and enhanced cell apoptosis significantly. Interestingly, the combined administration of cyclopamine promoted the observed antitumor effect (P<0.05; Table 2 and Figure 2).
Table 2.
Cyclopamine combined treatment decreases tumor growth in vivo
| 6 weeks
|
9 weeks
|
|||||
|---|---|---|---|---|---|---|
| Control | Paclitaxel | +Cyclopamine | Control | Paclitaxel | +Cyclopamine | |
| Tumor size (mm3) | 1,792 ± 243 | 1,329 ± 289* | 1,121 ± 163# | 2,260 ± 403 | 1,547 ± 274* | 1,196 ± 209# |
| Number of animals | 10 | 10 | 10 | 10 | 10 | 10 |
| Apoptotic cells | 1.0% ± 0.2% | 29.3% ± 4.7%* | 56.7% ± 5.4%# | 0.8% ± 0.2% | 32.4% ± 3.3%* | 67.9% ± 8.5%# |
| Number of animals | 6 | 6 | 6 | 6 | 6 | 6 |
Notes:
P<0.05 compared to control group
P<0.05 compared to paclitaxel-treated group.
Figure 2.
Cyclopamine-combined treatment decreased tumor growth in vivo. Cyclopamine further reduced the tumor size (black) and increased the cancer cell-apoptosis rate (blue), at both the 6-week and 9-week time points.
Abbreviations: P, paclitaxel; C, cyclopamine.
Discussion
Hedgehog signaling is important for breast cancer cells, contributing to tumor stem cell maintenance and recurrence in multiple models.11–13,15,17 It has been found that tumor stem cells are partly responsible for the chemoresistance of tumor cells in response to chemotherapy, which is maintained by Hedgehog-signaling pathways.4,18–22 Therefore, the antagonism of Hedgehog signaling might sensitize tumor cells to chemotherapy and reduce recurrence after surgical removal. This could be the same for breast cancer, given the fact that Hedgehog signaling has been well recognized in anti-breast cancer efforts.14,18,23,24 In addition, tumor stem cells have been suggested to promote breast cancer development and recurrence.11,18,19,25
The present study examined the chemoresistance of a common chemotherapy drug – paclitaxel. Paclitaxel as a mitotic inhibitor targets tubulin, and has been employed in different types of cancers, including breast cancer.7,26 However, the chemoresistance represents a difficulty in clinical management of single-drug chemotherapy. Here, we found that the single administration of paclitaxel could reduce tumor cell survival and growth, both in vitro and in vivo, confirming previous reports. However, we showed that the combined use of cyclopamine, which blocks Hedgehog signaling, can further induce tumor cell apoptosis. This is possibly due to the loss of tumor stem cell maintenance. In future studies, it will be interesting to isolate these chemotherapy-resistant cells specifically for pharmacological and signaling pathway dissections. It should be noted that cyclopamine might also activate the Smo signaling pathway, and therefore partly contribute to the increased apoptosis of breast cancer cells. This requires further investigation to dissect downstream-signaling cascades.
Cyclopamine has been employed in different types of diseases, with proven use of safety.27,28 In the present study, we did not observe any adverse effects after 6 weeks of cyclopamine administration (nor in important organs by histological examination; data not shown). The combined use of cyclopamine with other chemotherapy drugs, however, should still be evaluated for any potential harm.
In conclusion, this study firstly demonstrated that combined use of cyclopamine might act as the chemoresistance remover in paclitaxel administration for breast cancer. In pancreas cancer cells, the combination was found to be unique compared to the use of other combinations.26 Whether this is the case for breast cancer is yet to be investigated. If so, this might further emphasize the importance of Hedgehog signaling and cancer stem cells in breast cancer chemoresistance to paclitaxel.
Author contributions
FC, JZ, CC, PS and JS designed the study; FC, JZ, CC, SX, XC and PS performed the study; FC, JZ, CC, SX, PS and JS analyzed the data; JS provided the funding for the study; FC, SX and JS wrote the draft of the paper; all authors have drafted, revised, and approved the final version of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Eimer S, Dugay F, Airiau K, et al. Cyclopamine cooperates with EGFR inhibition to deplete stem-like cancer cells in glioblastoma-derived spheroid cultures. Neuro Oncol. 2012;14:1441–1451. doi: 10.1093/neuonc/nos266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pratap A, Singh S, Mundra V, et al. Attenuation of early liver fibrosis by pharmacological inhibition of smoothened receptor signaling. J Drug Target. 2012;20:770–782. doi: 10.3109/1061186X.2012.719900. [DOI] [PubMed] [Google Scholar]
- 3.Chitkara D, Singh S, Kumar V, et al. Micellar delivery of cyclopamine and gefitinib for treating pancreatic cancer. Mol Pharm. July2012 doi: 10.1021/mp3002792. Epub. [DOI] [PubMed] [Google Scholar]
- 4.Lin TL, Matsui W. Hedgehog pathway as a drug target: Smoothened inhibitors in development. Onco Targets Ther. 2012;5:47–58. doi: 10.2147/OTT.S21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Q, Gu D, He M, et al. Tumor shrinkage by cyclopamine tartrate through inhibiting hedgehog signaling. Chin J Cancer. 2011;30:472–481. doi: 10.5732/cjc.011.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibuki N, Ghaffari M, Pandey M, et al. TAK-441, a novel investigational smoothened antagonist, delays castration-resistant progression in prostate cancer by disrupting paracrine hedgehog signaling. Int J Cancer. 2013;133:1955–1966. doi: 10.1002/ijc.28193. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Chitkara D, Mehrazin R, Behrman SW, Wake RW, Mahato RI. Chemoresistance in prostate cancer cells is regulated by miRNAs and Hedgehog pathway. PloS One. 2012;7:e40021. doi: 10.1371/journal.pone.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Yang J, Kopeček J. Selective inhibitory effect of HPMA copolymer-cyclopamine conjugate on prostate cancer stem cells. Biomaterials. 2012;33:1863–1872. doi: 10.1016/j.biomaterials.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelleher FC. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32:445–451. doi: 10.1093/carcin/bgq280. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Tian X, Xie X, Zhuang Y, Wu W, Wang W. Expression and regulation of hedgehog signaling pathway in pancreatic cancer. Langenbecks Arch Surg. 2010;395:515–525. doi: 10.1007/s00423-009-0493-9. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad A. Pathways to breast cancer recurrence. ISRN Oncol. 2013;2013:290568. doi: 10.1155/2013/290568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Toole SA, Beith JM, Millar EK, et al. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013;66(6):530–542. doi: 10.1136/jclinpath-2012-201361. [DOI] [PubMed] [Google Scholar]
- 13.Ali SA. The hedgehog pathway in breast cancer. Chin J Cancer Res. 2012;24:261–262. doi: 10.3978/j.issn.1000-9604.2012.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui M, Cazet A, Nair R, Watkins DN, O’Toole SA, Swarbrick A. The Hedgehog signalling pathway in breast development, carcinogenesis and cancer therapy. Breast Cancer Res. 2013;15:203. doi: 10.1186/bcr3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010;2:237–250. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1:563–577. doi: 10.18632/oncotarget.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra GK, Zhao X, Band H, Band V. Shared signaling pathways in normal and breast cancer stem cells. J Carcinog. 2011;10:38. doi: 10.4103/1477-3163.91413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangopadhyay S, Nandy A, Hor P, Mukhopadhyay A. Breast cancer stem cells: a novel therapeutic target. Clin Breast Cancer. 2013;13:7–15. doi: 10.1016/j.clbc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Prud’homme GJ. Cancer stem cells and novel targets for antitumor strategies. Curr Pharm Des. 2012;18:2838–2849. doi: 10.2174/138161212800626120. [DOI] [PubMed] [Google Scholar]
- 20.Lu JT, Zhao WD, He W, Wei W. Hedgehog signaling pathway mediates invasion and metastasis of hepatocellular carcinoma via ERK pathway. Acta Pharmacol Sin. 2012;33:691–700. doi: 10.1038/aps.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Fu X, Hua Y, Han Y, Lu Y, Wang J. The side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancer cells. PloS One. 2012;7:e33358. doi: 10.1371/journal.pone.0033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Venugopal C, Manoranjan B, et al. Sonic hedgehog regulates Bmi1 in human medulloblastoma brain tumor-initiating cells. Oncogene. 2012;31:187–199. doi: 10.1038/onc.2011.232. [DOI] [PubMed] [Google Scholar]
- 23.Goel HL, Pursell B, Chang C, et al. GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med. 2013;5:488–508. doi: 10.1002/emmm.201202078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das S, Tucker JA, Khullar S, Samant RS, Shevde LA. Hedgehog signaling in tumor cells facilitates osteoblast-enhanced osteolytic metastases. PloS One. 2012;7:e34374. doi: 10.1371/journal.pone.0034374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta. 2013;1830:2481–2495. doi: 10.1016/j.bbagen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Shafaee Z, Schmidt H, Du W, Posner M, Weichselbaum R. Cyclopamine increases the cytotoxic effects of paclitaxel and radiation but not cisplatin and gemcitabine in Hedgehog expressing pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;58:765–770. doi: 10.1007/s00280-006-0227-4. [DOI] [PubMed] [Google Scholar]
- 27.Heretsch P, Tzagkaroulaki L, Giannis A. Modulators of the hedgehog signaling pathway. Bioorg Med Chem. 2010;18:6613–6624. doi: 10.1016/j.bmc.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 28.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–886. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]